Solid-State Solar Cells Based on TiO2 Nanowires and CH3NH3PbI3 Perovskite
Abstract
1. Introduction
2. Methodology
2.1. Experimental Details
2.2. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bach, U.; Lupo, D.; Comte, P.; E Moser, J.; Weissörtel, F.; Salbeck, J.; Spreitzer, H.; Gratzel, M. Solid-State Dye-Sensitized Mesoporous Tio2 Solar Cells with High Photon-to-Electron Conversion Efficiencies. Nature 1998, 395, 583–585. [Google Scholar] [CrossRef]
- Hodes, G.; Cahen, D. All-Solid-State, Semiconductor-Sensitized Nanoporous Solar Cells. Acc. Chem. Res. 2012, 45, 705–713. [Google Scholar] [CrossRef]
- Snaith, H.J.; Petrozza, A.; Ito, S.; Miura, H.; Grätzel, M. Charge Generation and Photovoltaic Operation of Solid-State Dye-Sensitized Solar Cells Incorporating a High Extinction Coefficient Indolene-Based Sensitizer. Adv. Funct. Mater. 2009, 19, 1810–1818. [Google Scholar] [CrossRef]
- Plass, R.; Pelet, S.; Krueger, J.; Grätzel, M.; Bach, U. Quantum Dot Sensitization of Organic−Inorganic Hybrid Solar Cells. J. Phys. Chem. B 2002, 106, 7578–7580. [Google Scholar] [CrossRef]
- Lee, H.; Leventis, S.M.; Haque, T.T.; Zakeeruddin, M.G.; Nazeeruddin, M.K. Pbs and Cds Quantum Dot-Sensitized Solid-State Solar Cells: Old Concepts, New Results. Adv. Funct. Mater. 2009, 19, 2735–2742. [Google Scholar] [CrossRef]
- Moon, S.; Zakeeruddin, G.H.; Grätzel, M. Sb2s3-Based Mesoscopic Solar Cell Using an Organic Hole Conductor. J. Phys. Chem. Lett. 2010, 1, 1524–1527. [Google Scholar] [CrossRef]
- Chang, J.A.; Rhee, J.H.; Im, S.H.; Lee, Y.H.; Kim, H.-J.; Seok, S.I.; Nazeeruddin, M.K.; Gratzel, M. High-Performance Nanostructured Inorganic-Organic Heterojunction Solar Cells. Nano Lett. 2010, 10, 2609–2612. [Google Scholar] [CrossRef] [PubMed]
- Snaith, H.; Moule, C.K.; Grätzel, M. Efficiency Enhancements in Solid-State Hybrid Solar Cells Via Reduced Charge Recombination and Increased Light Capture. Nano Lett. 2007, 7, 3372–3376. [Google Scholar] [CrossRef]
- Chi, C.F.; Chen, P.; Lee, Y.L.; Liu, I.P.; Chou, S.C.; Zhang, X.L.; Bach, U. Surface Modifications of Cds/Cdse Co-Sensitized Tio2 Photoelectrodes for Solid-State Quantum-Dot-Sensitized Solar Cells. J. Mater. Chem. 2011, 21, 17534. [Google Scholar] [CrossRef]
- Tvrdy, K.; Frantsuzov, P.A.; Kamat, P.V. Photoinduced Electron Transfer from Semiconductor Quantum Dots to Metal Oxide Nanoparticles. Proc. Natl. Acad. Sci. USA 2011, 108, 29–34. [Google Scholar] [CrossRef]
- Zhu, R.; Jiang, C.-Y.; Liu, B.; Ramakrishna, S. Highly Efficient Nanoporous Tio2-Polythiophene Hybrid Solar Cells Based on Interfacial Modification Using a Metal-Free Organic Dye. Adv. Mater. 2009, 21, 994–1000. [Google Scholar] [CrossRef]
- Cai, N.; Moon, S.-J.; Cevey-Ha, L.; Moehl, T.; Humphry-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Gratzel, M. An Organic D-Pi-a Dye for Record Efficiency Solid-State Sensitized Heterojunction Solar Cells. Nano Lett. 2011, 11, 1452–1456. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; Grätzel, M. Tio2 Pore-Filling and Its Effect on the Efficiency of Solid-State Dye-Sensitized Solar Cells. Thin Solid Film. 2006, 500, 296–301. [Google Scholar] [CrossRef]
- Snaith, H.J.; Humphry-Baker, R.; Chen, P.; Cesar, I.; Zakeeruddin, S.M.; Grätzel, M. Charge Collection and Pore Filling in Solid-State Dye-Sensitized Solar Cells. Nanotechnology 2008, 19, 424003. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient Planar Heterojunction Perovskite Solar Cells by Vapour Deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Burschka, J.; Pellet, S.J.; Moon, R.H.; Gao, M.K. Nazeeruddin, and M. Gratzel. Sequential Deposition as a Route to High-Performance Perovskite-Sensitized Solar Cells. Nature 2013, 499, 316–319. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, W.; Li, Y.; Ye, S.; Rao, H.; Gu, F.; Liu, Z.; Bian, Z.; Huang, C. Simplification of Device Structures for Low-Cost, High-Efficiency Perovskite Solar Cells. J. Mater. Chem. A 2017, 5, 4756–4773. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.; Grancini, C.; Menelaou, M.J.; Alcocer, T.; Leijtens, L.M.; Herz, A.P.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Kazim, S.; Nazeeruddin, M.G.; Ahmad, S. Perovskite as Light Harvester: A Game Changer in Photovoltaics. Angew. Chem. Int. Ed. Engl. 2014, 53, 2812–2824. [Google Scholar] [CrossRef]
- Rong, Y.; Ku, A.; Mei, T.; Liu, M.; Xu, S.; Ko, X.L.; Han, H. Hole-Conductor-Free Mesoscopic Tio2/Ch3nh3pbi3 Heterojunction Solar Cells Based on Anatase Nanosheets and Carbon Counter Electrodes. J. Phys. Chem. Lett. 2014, 5, 2160–2164. [Google Scholar] [CrossRef]
- Yella, A.; Heiniger, P.; Gao, M.K.N.; Gratzel, M. Nanocrystalline Rutile Electron Extraction Layer Enables Low-Temperature Solution Processed Perovskite Photovoltaics with 13.7% Efficiency. Nano Lett. 2014, 14, 2591–2596. [Google Scholar] [CrossRef]
- Melas-Kyriazi, J.; Hardin, G.; Burkhard, N.T.; Michael, D.M. The Effect of Hole Transport Material Pore Filling on Photovoltaic Performance in Solid-State Dye-Sensitized Solar Cells. Adv. Energy Mater. 2011, 1, 407–414. [Google Scholar] [CrossRef]
- Chandiran, A.K.; Yella, M.T.; Mayer, P.; Gao, M.K.N.; Gratzel, M. Sub-Nanometer Conformal Tio2 Blocking Layer for High Efficiency Solid-State Perovskite Absorber Solar Cells. Adv. Mater. 2014, 26, 4309–4312. [Google Scholar] [CrossRef]
- Hao, F.; Stoumpos, D.H.C.; Mercouri, G.K. Lead-Free Solid-State Organic–Inorganic Halide Perovskite Solar Cells. Nat. Photonics 2014, 8, 489–494. [Google Scholar] [CrossRef]
- Qin, P.; Paek, M.I.; Dar, N.; Pellet, J.; Ko, M.G.; Nazeeruddin, M.K. Perovskite Solar Cells with 12.8% Efficiency by Using Conjugated Quinolizino Acridine Based Hole Transporting Material. J. Am. Chem. Soc. 2014, 136, 8516–8519. [Google Scholar] [CrossRef]
- Zhu, Z.; Ma, Z.; Wang, C.; Mu, Z.; Fan, L.; Du, Y.; Bai, L.; Fan, H.; Yan, D.L.P.; Yang, S. Efficiency Enhancement of Perovskite Solar Cells through Fast Electron Extraction: The Role of Graphene Quantum Dots. J. Am. Chem. Soc. 2014, 136, 3760–3763. [Google Scholar] [CrossRef] [PubMed]
- Lan, Y.; Lu, Y.; Ren, Z. Mini Review on Photocatalysis of Titanium Dioxide Nanoparticles and Their Solar Applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Y.; Jia, X.; Chen, H.H.; Titanium, C.L. Dioxide-Based Nanomaterials for Photocatalytic Fuel Generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef] [PubMed]
- Maijenburg, A.W.; Veerbeek, J.; Veldhuis, S.A.; Zoontjes, M.G.C.; Mul, G.; Montero-Moreno, J.M.; De Putter, R.; Nielsch, K.; Schäfer, H.; Steinhart, M.; et al. Electrochemical Synthesis of Coaxial TiO2–Ag Nanowires and Their Application in Photocatalytic Water Splitting. J. Mater. Chem. A 2014, 2, 2648–2656. [Google Scholar] [CrossRef]
- Xie, S.; Zhai, T.; Li, W.; Yu, M.; Liang, C.; Gan, J.; Lu, X.; Tong, Y. Hydrogen Production from Solar Driven Glucose Oxidation over Ni(Oh)2 Functionalized Electroreduced-Tio2 Nanowire Arrays. Green Chem. 2013, 15, 2434. [Google Scholar] [CrossRef]
- Chen, F.; Fang, P.; Liu, Z.; Gao, Y.; Liu, Y.; Dai, Y.; Luo, H.; Feng, J. Dimensionality-Dependent Photocatalytic Activity of Tio2-Based Nanostructures: Nanosheets with a Superior Catalytic Property. J. Mater. Sci. 2013, 48, 5171–5179. [Google Scholar] [CrossRef]
- Tu, W.; Zhou, Y.; Liu, Q.; Yan, S.; Bao, S.; Wang, X.; Xiao, M.; Zou, Z. An in Situ Simultaneous Reduction-Hydrolysis Technique for Fabrication of Tio2-Graphene 2d Sandwich-Like Hybrid Nanosheets: Graphene-Promoted Selectivity of Photocatalytic-Driven Hydrogenation and Coupling of Co2into Methane and Ethane. Adv. Funct. Mater. 2013, 23, 1743–1749. [Google Scholar] [CrossRef]
- Xu, X.; Fang, T.; Zhai, H.; Zeng, B.; Liu, X.; Hu, Y.B.; Golberg, D. Tube-in-Tube TiO2 Nanotubes with Porous Walls: Fabrication, Formation Mechanism, and Photocatalytic Properties. Small 2011, 7, 445–449. [Google Scholar] [CrossRef]
- Liu, S.; Tang, Y.; Sun, J.C.C.; Xu, Y.J. One-Dimension-Based Spatially Ordered Architectures for Solar Energy Conversion. Chem. Soc. Rev. 2015, 44, 5053–5075. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Shih, Y.T.; Tsay, C. Synthesizing and Comparing the Photocatalytic Activities of Single-Crystalline Tio[Sub 2] Rutile Nanowires and Mesoporous Anatase Paste. J. Electrochem. Soc. 2007, 154, H157. [Google Scholar] [CrossRef]
- Sahaym, U.; Norton, M.G. Advances in the Application of Nanotechnology in Enabling a ‘Hydrogen Economy’. J. Mater. Sci. 2008, 43, 5395–5429. [Google Scholar] [CrossRef]
- Wu, W.Q.; Lei, H.S.; Rao, Y.F.; Xu, Y.F.; Wang, C.Y.S.; Kuang, D.B. Hydrothermal Fabrication of Hierarchically Anatase Tio2 Nanowire Arrays on Fto Glass for Dye-Sensitized Solar Cells. Sci. Rep. 2013, 3, 1352. [Google Scholar] [CrossRef]
- Qiu, J.; Qiu, K.; Yan, M.; Zhong, C.; Mu, H.Y.; Yang, S. All-Solid-State Hybrid Solar Cells Based on a New Organometal Halide Perovskite Sensitizer and One-Dimensional Tio2 Nanowire Arrays. Nanoscale 2013, 5, 3245–3248. [Google Scholar] [CrossRef] [PubMed]
- Mali, S.S.; Shim, C.S.; Park, H.K.; Heo, J.; Patil, P.S.; Hong, C.K. Ultrathin Atomic Layer Deposited TiO2 for Surface Passivation of Hydrothermally Grown 1d Tio2 Nanorod Arrays for Efficient Solid-State Perovskite Solar Cells. Chem. Mater. 2015, 27, 1541–1551. [Google Scholar] [CrossRef]
- Almora, O.; Aranda, C.; Mas-Marzá, E.; Garcia-Belmonte, G. On Mott-Schottky Analysis Interpretation of Capacitance Measurements in Organometal Perovskite Solar Cells. Appl. Phys. Lett. 2016, 109, 173903. [Google Scholar] [CrossRef]
- Willis, K.J.; Ayubi-Moak, J.S.; Hagness, S.C.; Knezevic, I. Global Modeling of Carrier-Field Dynamics in Semiconductors Using Emc–Fdtd. J. Comput. Electron. 2009, 8, 153–171. [Google Scholar] [CrossRef]
- Wang, K.X.; Yu, V.; Liu, Y.C.; Fan, S. Absorption Enhancement in Ultrathin Crystalline Silicon Solar Cells with Antireflection and Light-Trapping Nanocone Gratings. Nano Lett. 2012, 12, 1616–1619. [Google Scholar] [CrossRef] [PubMed]

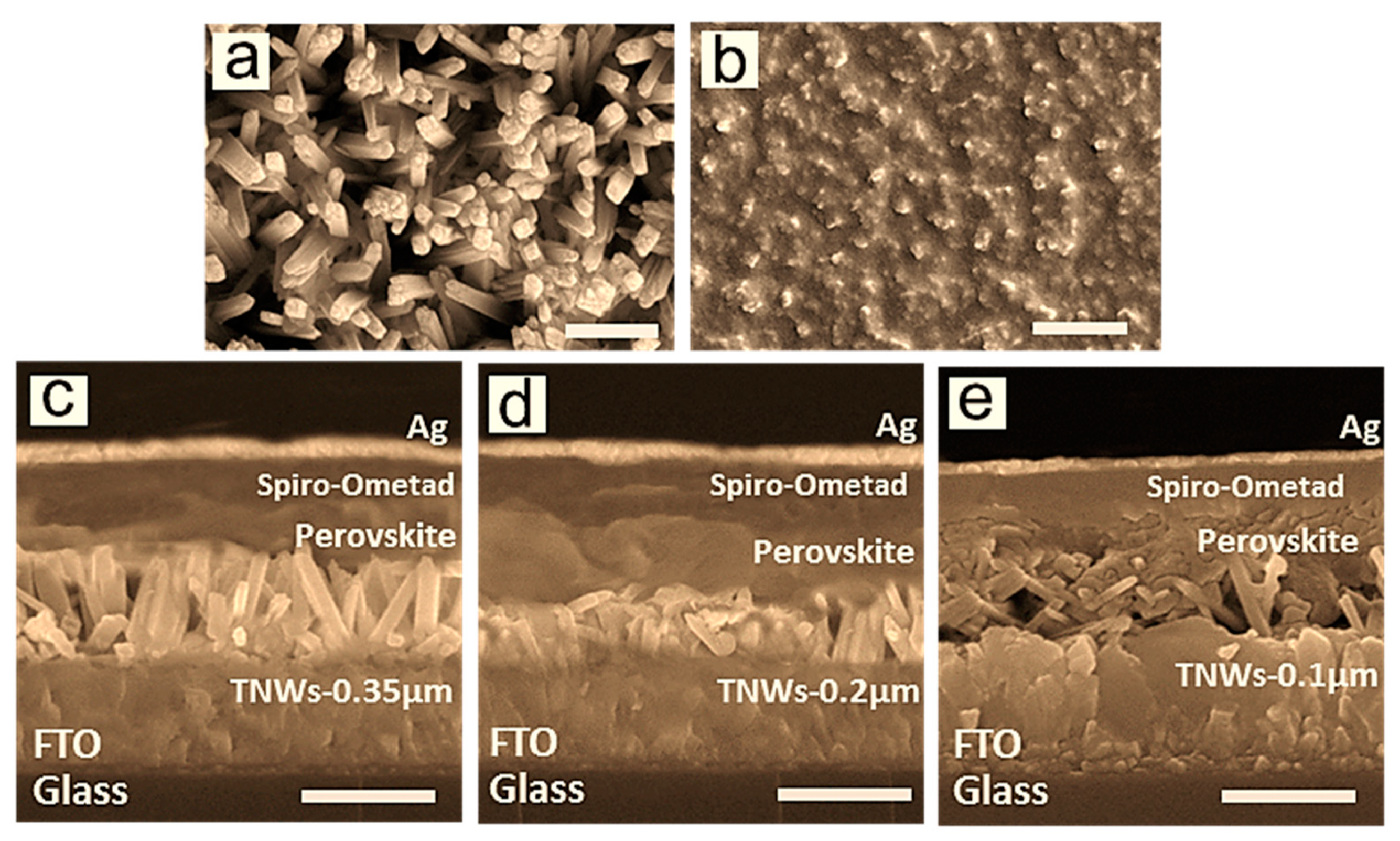
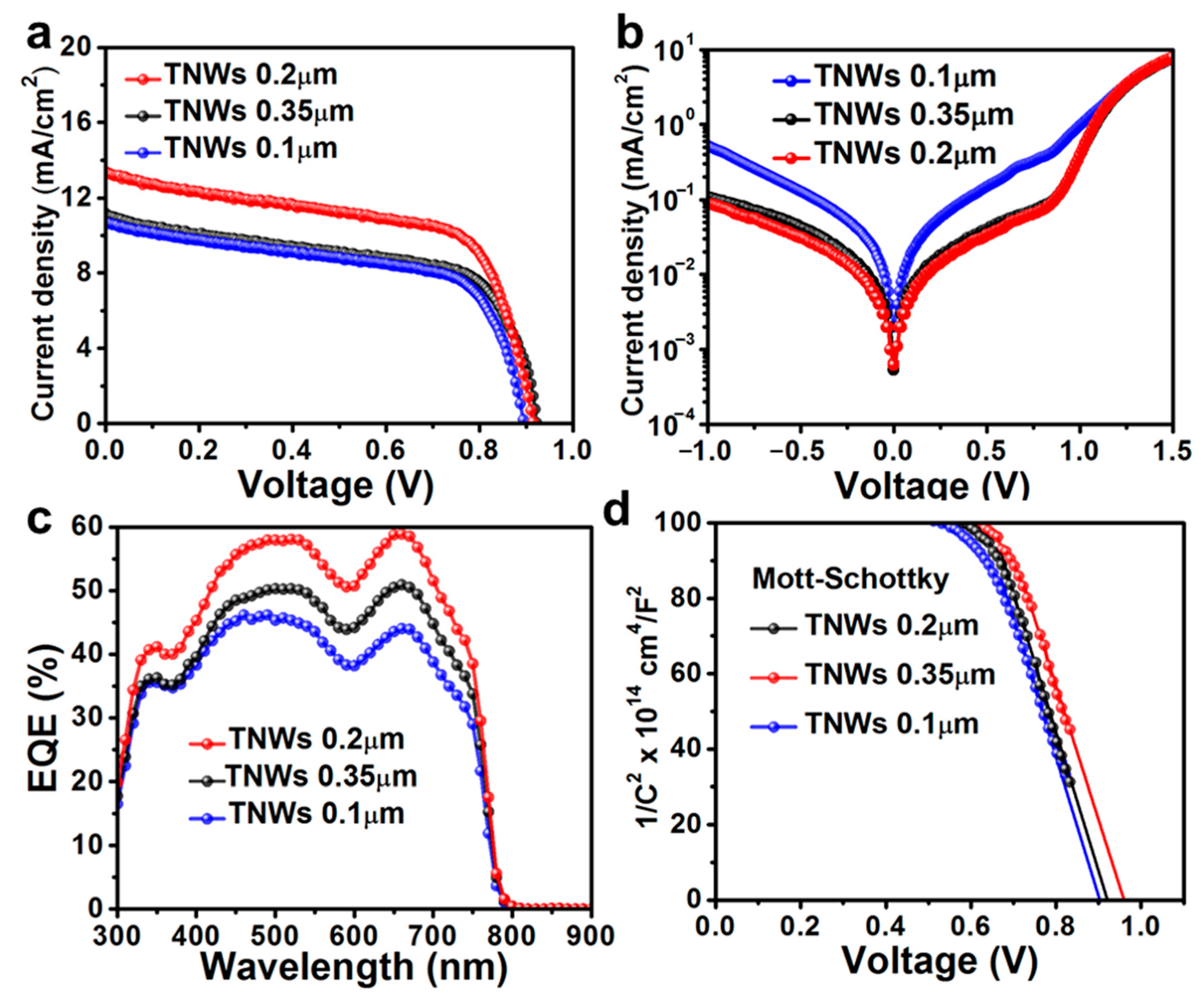


| Solid-State Perovskite Devices | Jsc (mA/cm2) | Voc (V) | FF (%) | CE (%) |
|---|---|---|---|---|
| CH3NH3PbI3/TNWs (0.2 µm) | 13.06 (±0.02) | 0.91(±0.06) | 63.2 (±0.1) | 7.40 (±0.04) |
| CH3NH3PbI3/TNWs (0.35 µm) | 11.11 (±0.05) | 0.92 (±0.03) | 60.1 (±0.1) | 6.13 (±0.07) |
| CH3NH3PbI3/TNWs (0.1 µm) | 10.66 (±0.07) | 0.89 (±0.02) | 61.1 (±0.1) | 5.80 (±0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sami, A.; Ansari, A.; Idrees, M.D.; Alam, M.M.; Imtiaz, J. Solid-State Solar Cells Based on TiO2 Nanowires and CH3NH3PbI3 Perovskite. Coatings 2021, 11, 404. https://doi.org/10.3390/coatings11040404
Sami A, Ansari A, Idrees MD, Alam MM, Imtiaz J. Solid-State Solar Cells Based on TiO2 Nanowires and CH3NH3PbI3 Perovskite. Coatings. 2021; 11(4):404. https://doi.org/10.3390/coatings11040404
Chicago/Turabian StyleSami, Abdul, Arsalan Ansari, Muhammad Dawood Idrees, Muhammad Musharraf Alam, and Junaid Imtiaz. 2021. "Solid-State Solar Cells Based on TiO2 Nanowires and CH3NH3PbI3 Perovskite" Coatings 11, no. 4: 404. https://doi.org/10.3390/coatings11040404
APA StyleSami, A., Ansari, A., Idrees, M. D., Alam, M. M., & Imtiaz, J. (2021). Solid-State Solar Cells Based on TiO2 Nanowires and CH3NH3PbI3 Perovskite. Coatings, 11(4), 404. https://doi.org/10.3390/coatings11040404






