Diffusion Aluminide Coatings for Hot Corrosion and Oxidation Protection of Nickel-Based Superalloys: Effect of Fluoride-Based Activator Salts
Abstract
:1. Introduction
2. Experimental
2.1. Coatings Deposition
2.2. Isothermal Oxidation and Hot Corrosion Tests
2.3. Coatings Characterization
3. Results and Discussion
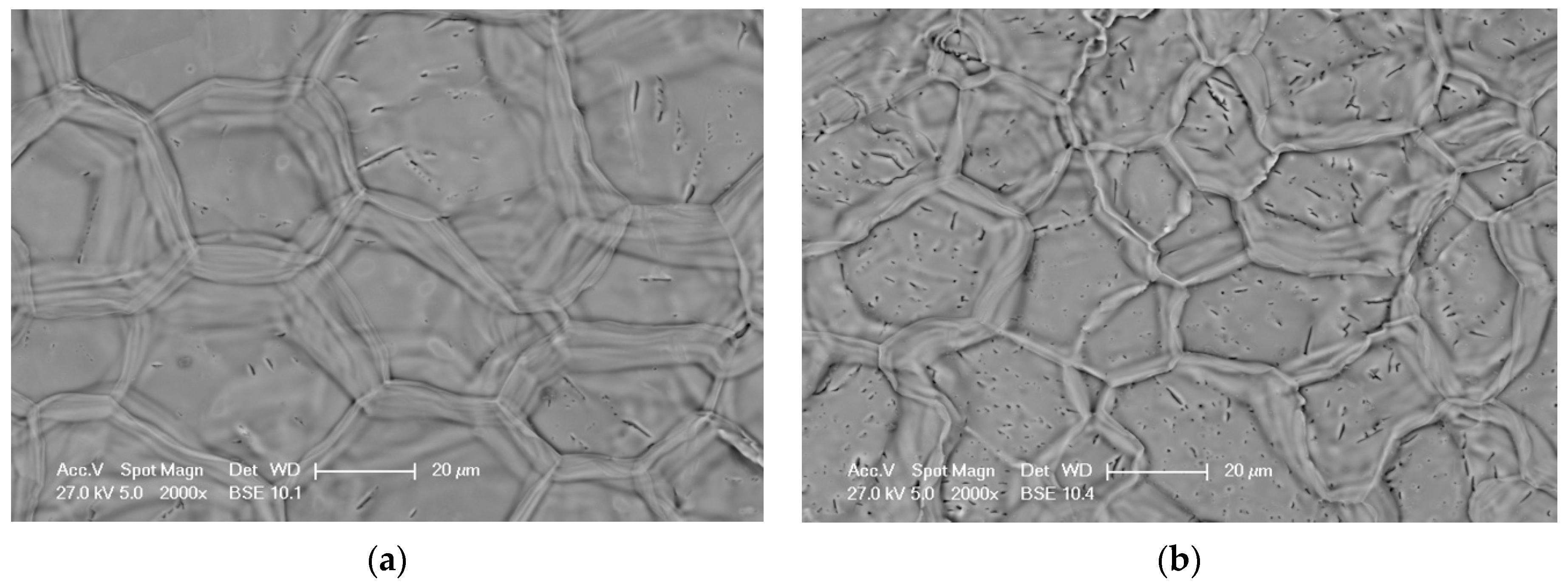
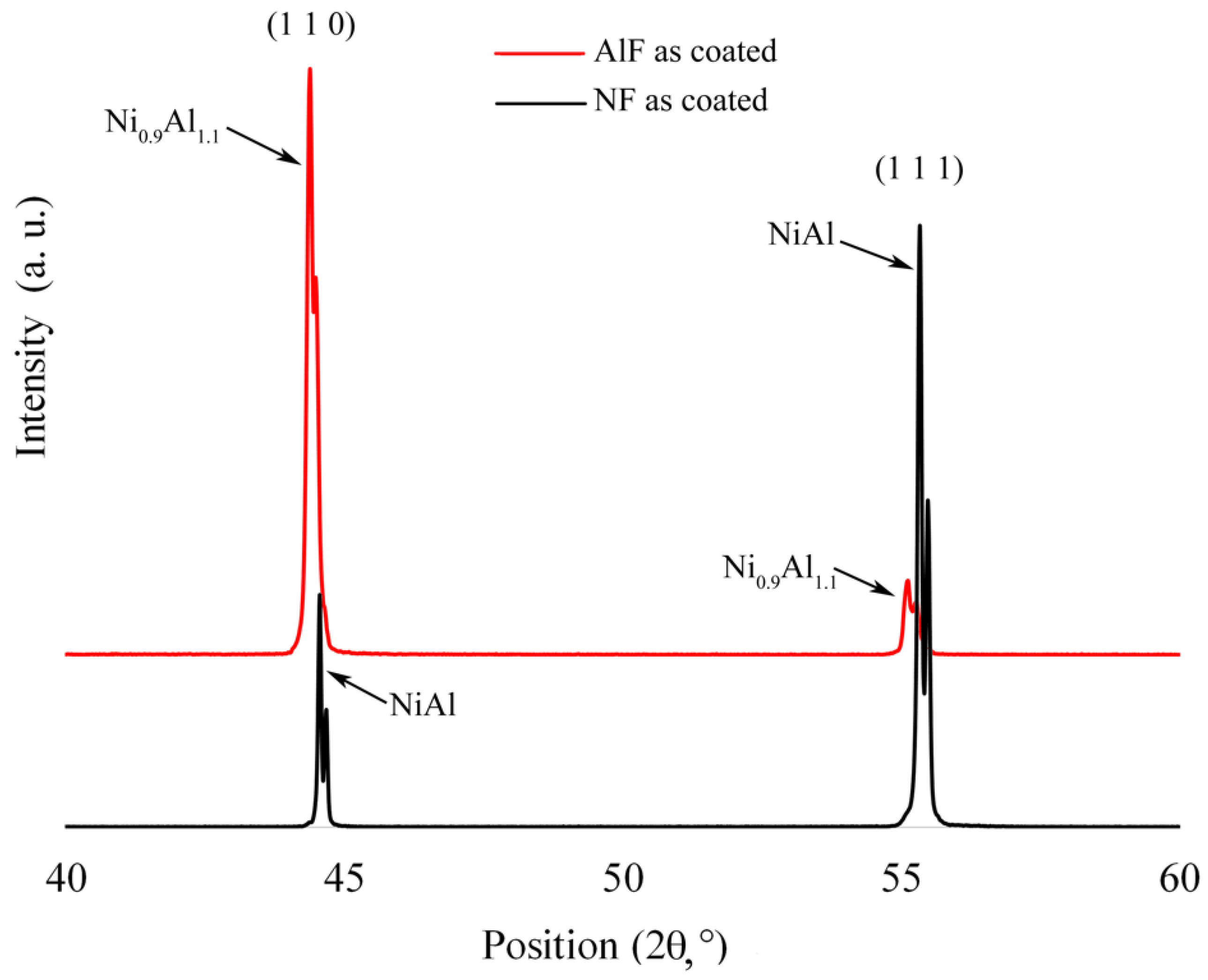
3.1. Surface Microstructure of AS-Deposited Aluminide Coatings
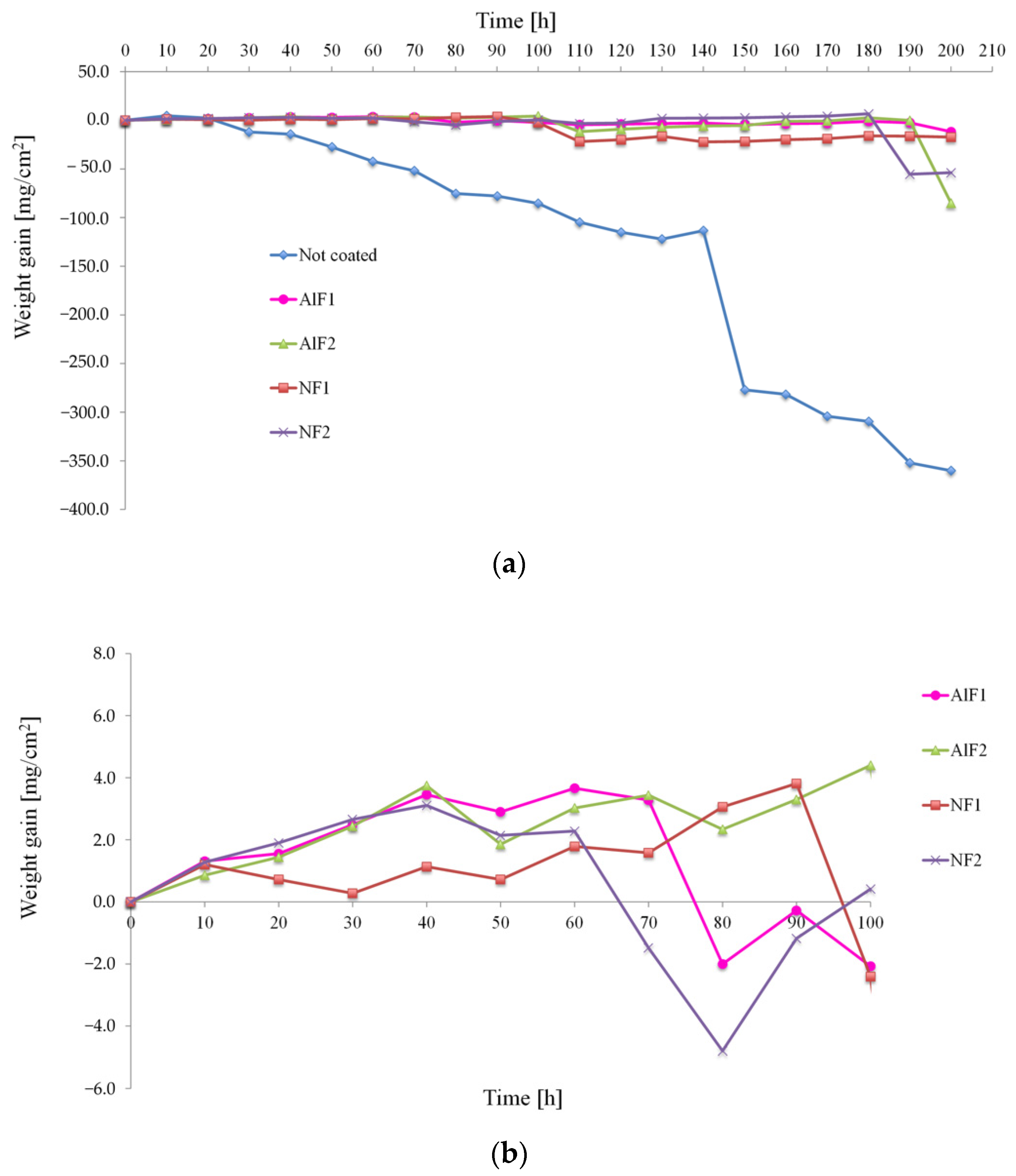
3.2. Comparison of Oxidation and Hot Corrosion Kinetics
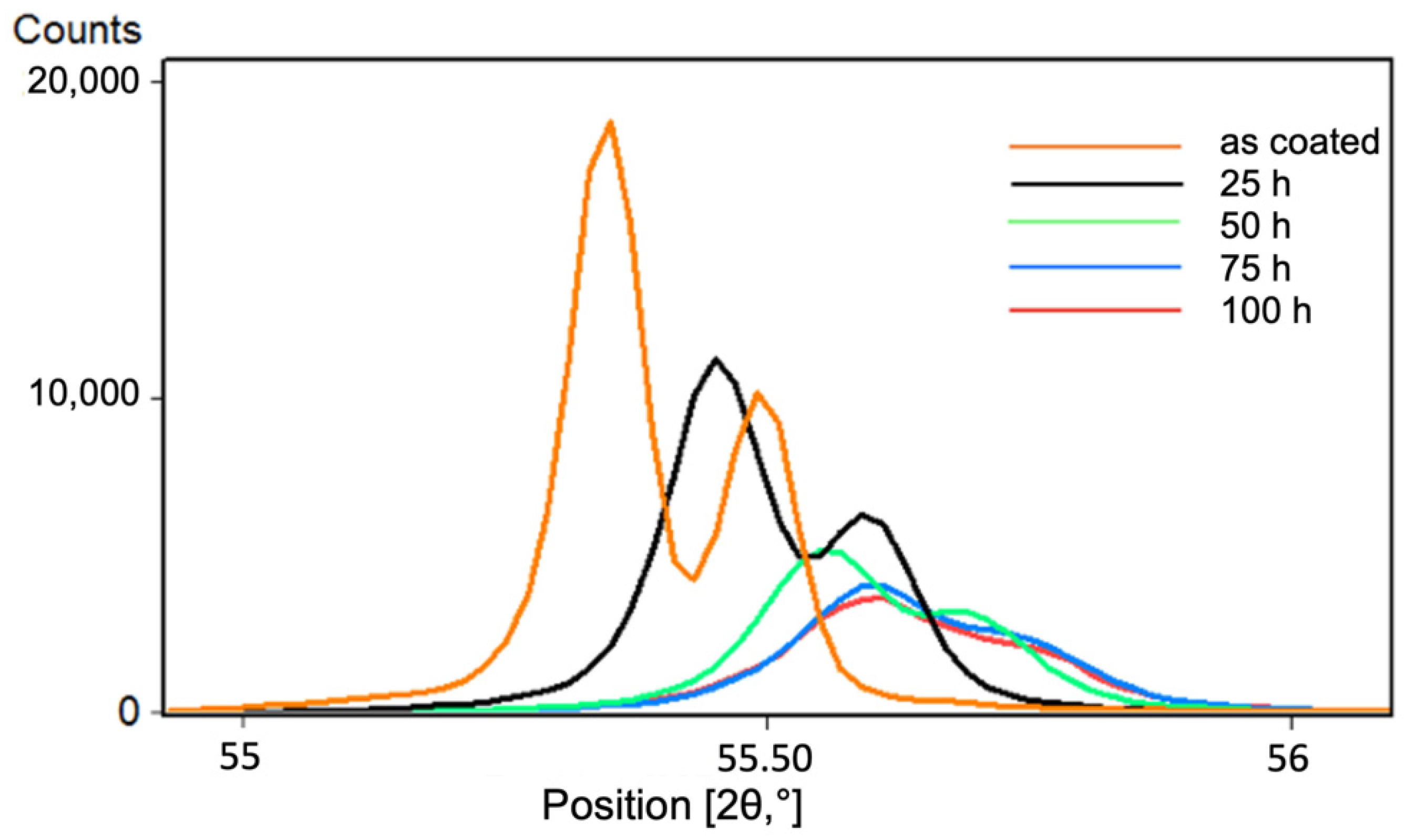
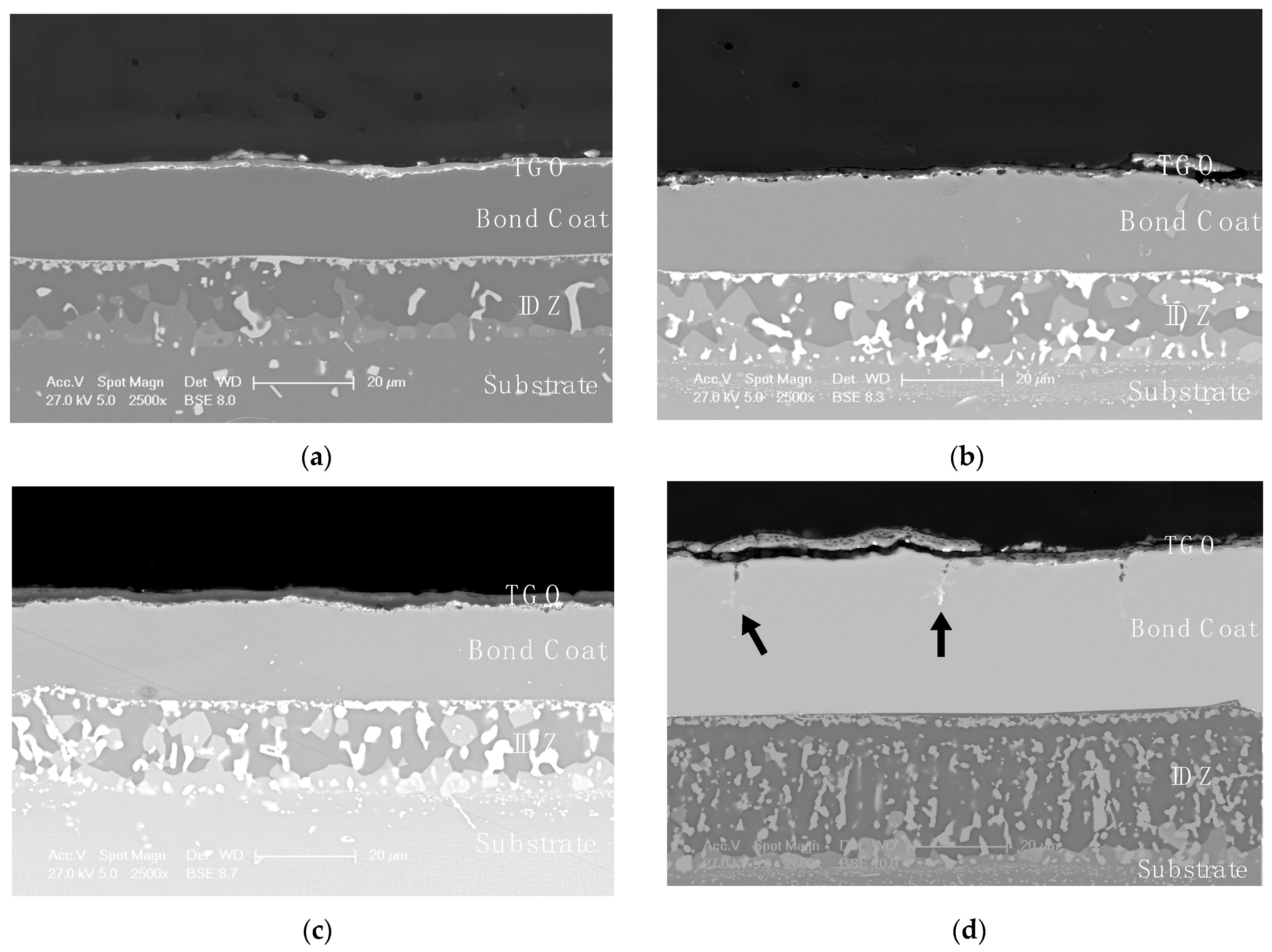
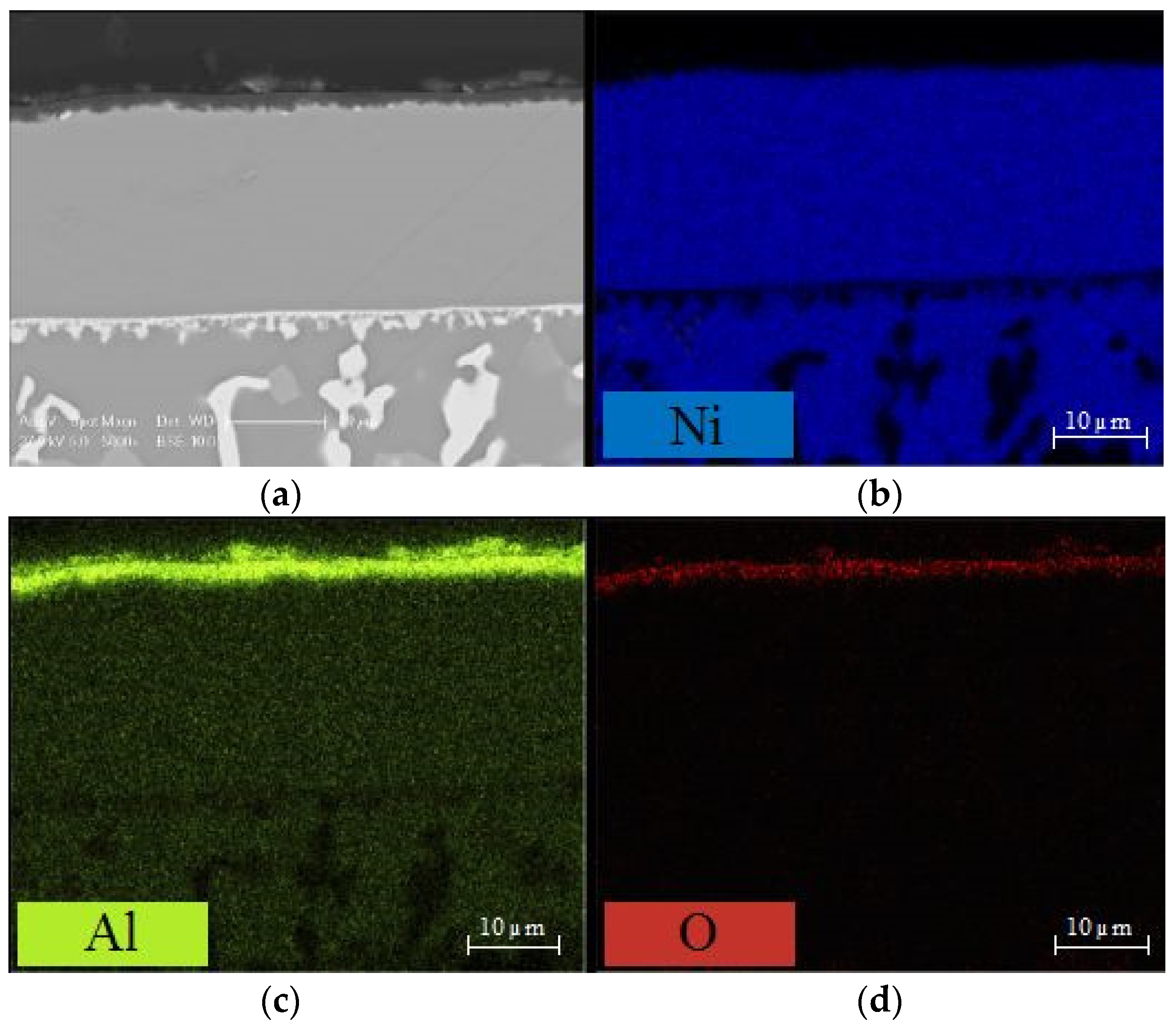
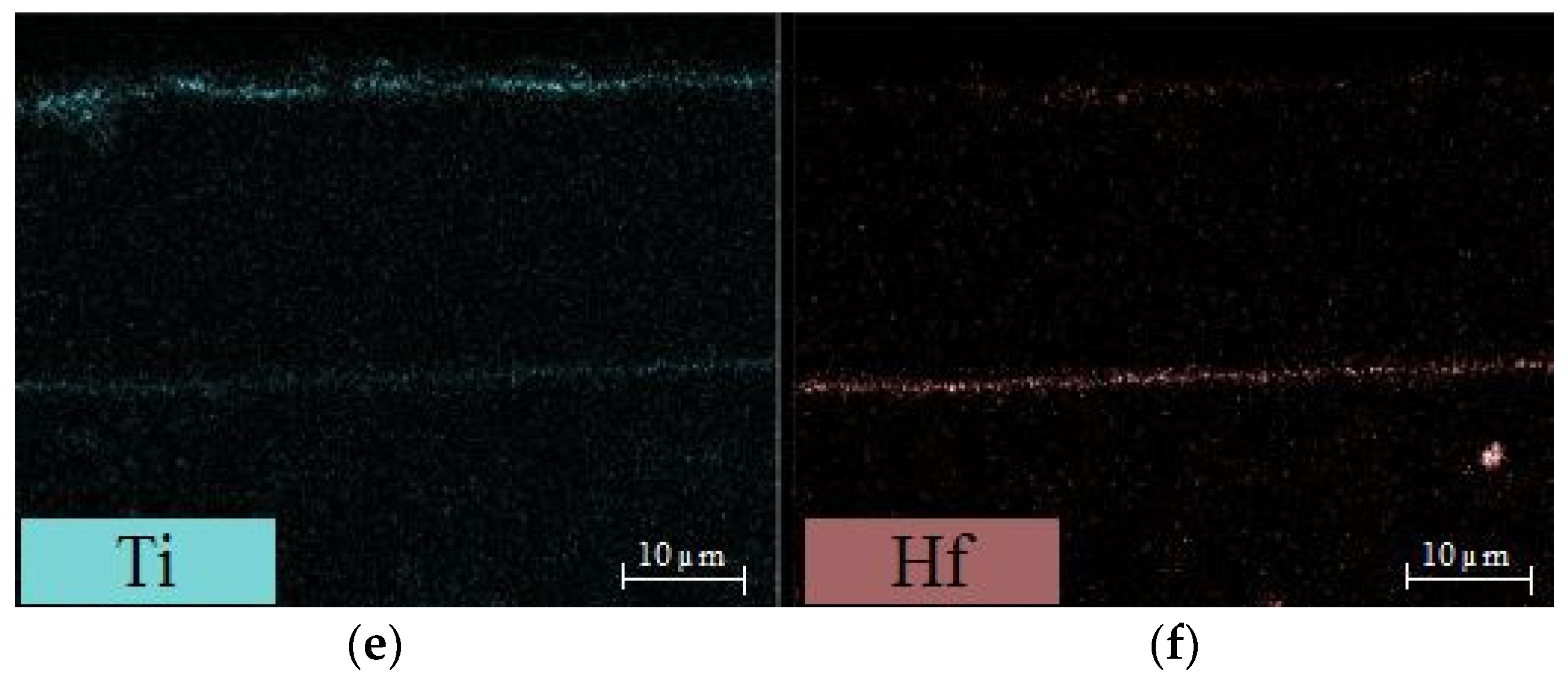
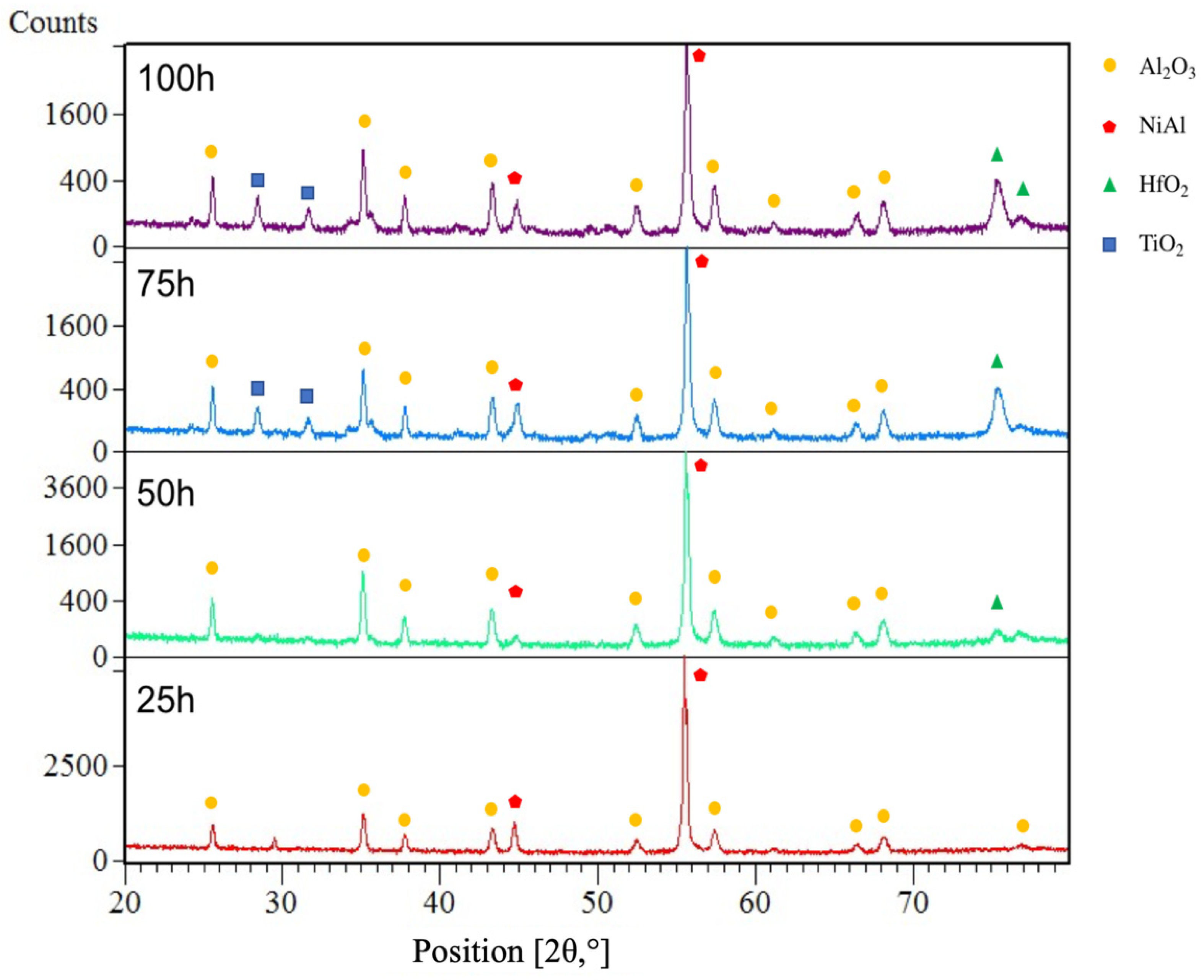
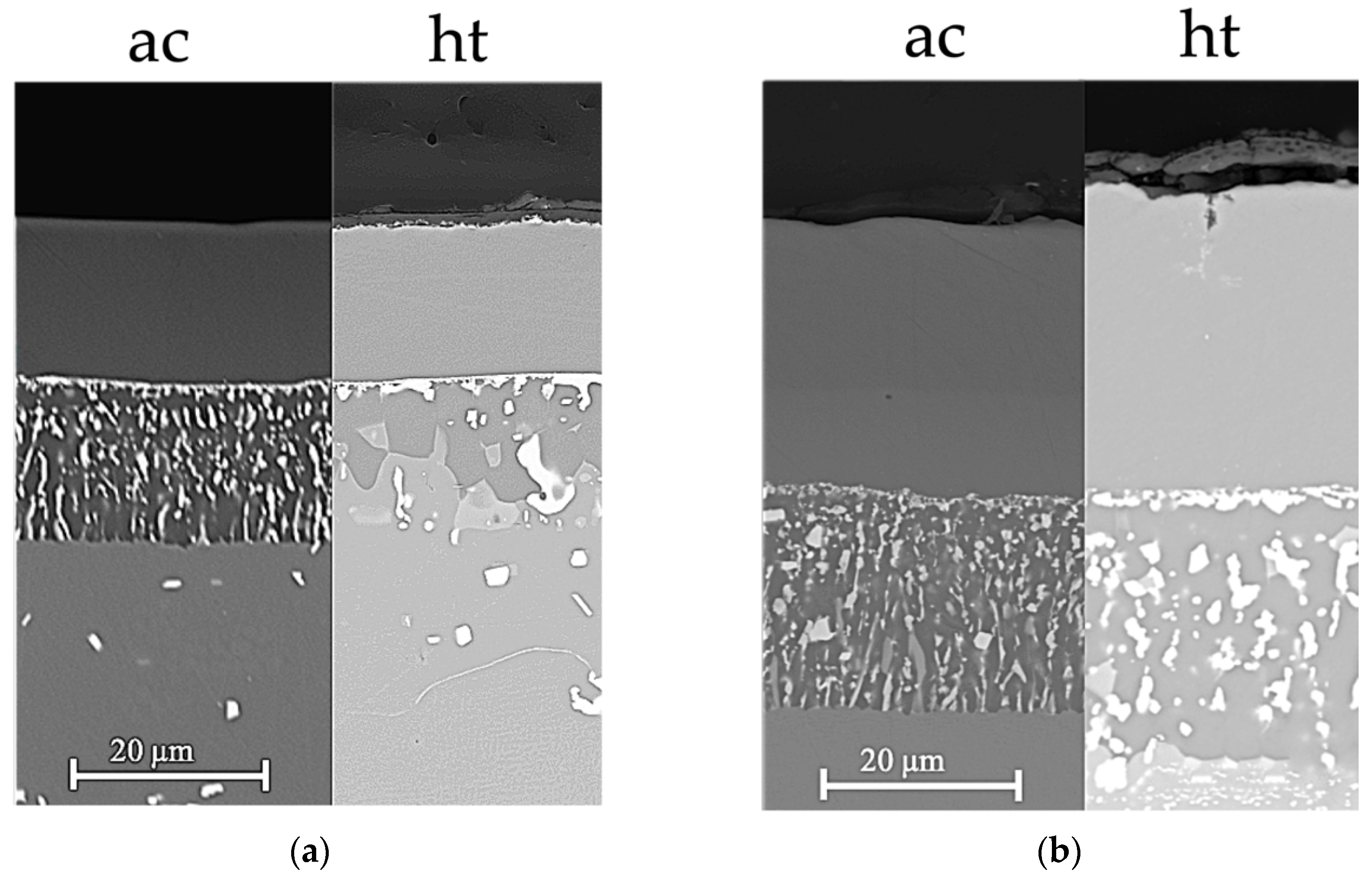
3.3. Comparison of Phase Structure and Microstructure of Coatings after Exposure Tests
3.3.1. Isothermal Oxidation
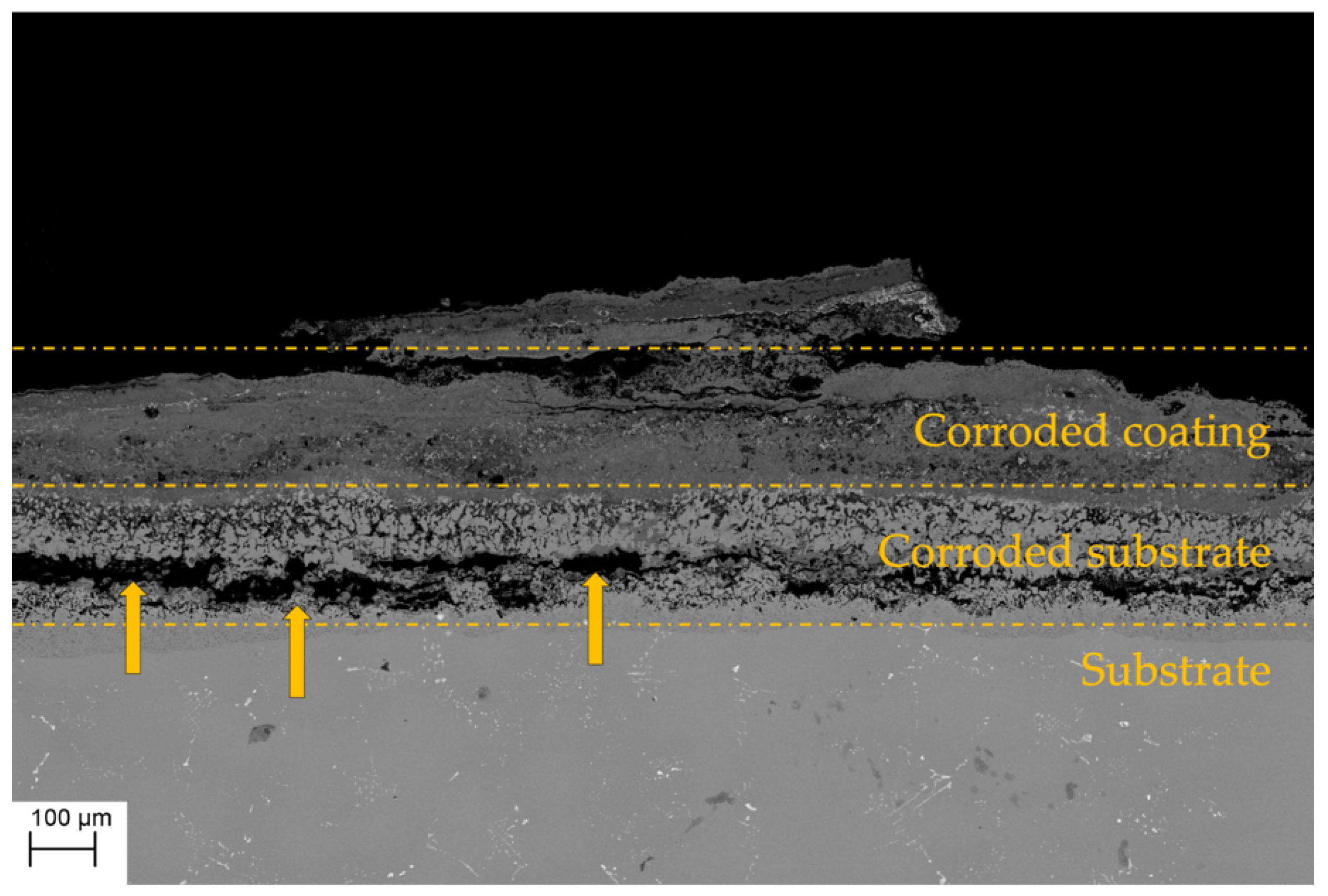
3.3.2. Hot Corrosion
- The mechanism of penetration of sulfur within the scale can cause a more aggressive oxidation, also leading to internal corrosion phenomena (as reported in Figure 10).
- the scale dissolution reaction consumes the protective layer, therefore exposing the metal to further corrosion.
- The thicker bond coat involves a longer path for sulfur to reach the substrate.
- The outward diffusion of Ni observed during the high temperature exposure in the AlF samples (leading to the further growth of the NiAl layer) could oppose to the sulfur inward diffusion within the bond coat, thus reducing the sulfur (in the form of SO3) penetration kinetics.
4. Conclusions
- the use of AlF3 allows the growth of a thicker diffusion coating. Due to the higher concentration in the vapour phase of Al, originating both from the thermal decomposition of the activator salt and from the reaction with the aluminium pack, thicker Al-rich β-NiAl and IDZ layers grow on the Ni-based alloy surface;
- the coatings obtained with NH4F show a higher resistance to the isothermal oxidation than the coatings produced with AlF3; on the other hand, coatings obtained using AlF3 activator show improved hot corrosion resistance after 100 h due to the higher thickness of the β-NiAl coating. This result has been ascribed to the unstable β-NiAl (Al-rich) phase obtained via AlF3 pack cementation with the higher concentration of activator salt. The high temperature exposure caused a further diffusion of the Al excess present in the outer layer of coatings produced with AlF3, and this phenomenon is likely to be responsible for the reduced oxidation resistance, as further growth of the NiAl layer probably affects the thermally grown oxide formation mechanisms. At the same time, it was proved to increase the hot corrosion resistance because the outward diffusion of Ni and the thicker NiAl layer may hinder the penetration of sulfur.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brotzu, A.; Felli, F.; Marra, F.; Pilone, D.; Pulci, G. Mechanical properties of a TiAl-based alloy at room and high temperatures. Mater. Sci. Technol. 2018, 34, 1847–1853. [Google Scholar] [CrossRef]
- Pilone, D.; Pulci, G.; Paglia, L.; Mondal, A.; Marra, F.; Felli, F.; Brotzu, A. Mechanical behaviour of an Al2O3 dispersion strengthened γTiAl alloy produced by centrifugal casting. Metals 2020, 10, 1457. [Google Scholar] [CrossRef]
- Beranoagirre, A.; Urbikain, G.; Calleja, A.; de Lacalle, L.N.L. Drilling process in γ-TiAl intermetallic alloys. Materials 2018, 11, 2379. [Google Scholar] [CrossRef] [Green Version]
- Boissonnet, G.; Grégoire, B.; Bonnet, G.; Pedraza, F. Development of thermal barrier coating systems from Al microparticles. Part I: Influence of processing conditions on the mechanisms of formation. Surf. Coat. Technol. 2019, 380, 125085. [Google Scholar] [CrossRef]
- Grégoire, B.; Bonnet, G.; Pedraza, F. Development of a new slurry coating design for the surface protection of gas turbine components. Surf. Coat. Technol. 2019, 374, 521–530. [Google Scholar] [CrossRef]
- Pedraza, F.; Mollard, M.; Rannou, B.; Balmain, J.; Bouchaud, B.; Bonnet, G. Potential thermal barrier coating systems from Al microparticles. Mechanisms of coating formation on pure nickel. Mater. Chem. Phys. 2012, 134, 700–705. [Google Scholar] [CrossRef]
- Baiamonte, L.; Marra, F.; Pulci, G.; Tirillò, J.; Sarasini, F.; Bartuli, C.; Valente, T. High temperature mechanical characterization of plasma-sprayed zirconia-yttria from conventional and nanostructured powders. Surf. Coat. Technol. 2015, 277, 289–298. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Marra, F.; Blasi, C.; Schioppa, M.; Pulci, G.; Serra, E.; Valente, T. High-temperature mechanical behavior of plasma sprayed lanthanum zirconate coatings. Ceram. Int. 2014, 40, 11433–11436. [Google Scholar] [CrossRef]
- Pulci, G.; Tirillò, J.; Marra, F.; Sarasini, F.; Bellucci, A.; Valente, T.; Bartuli, C. High temperature oxidation and microstructural evolution of modified MCrAlY coatings. Met. Mater. Trans. A Phys. Met. Mater. Sci. 2014, 45, 1401–1408. [Google Scholar] [CrossRef]
- Pomeroy, M.J. Coatings for gas turbine materials and long term stability issues. Mater. Des. 2005, 26, 223–231. [Google Scholar] [CrossRef]
- Di Girolamo, G.; Marra, F.; Schioppa, M.; Blasi, C.; Pulci, G.; Valente, T. Evolution of microstructural and mechanical properties of lanthanum zirconate thermal barrier coatings at high temperature. Surf. Coat. Technol. 2015, 268, 298–302. [Google Scholar] [CrossRef]
- Pulci, G.; Tirillò, J.; Marra, F.; Sarasini, F.; Bellucci, A.; Valente, T.; Bartuli, C. High temperature oxidation of MCrAlY coatings modified by Al2O3 PVD overlay. Surf. Coat. Technol. 2015, 268, 198–204. [Google Scholar] [CrossRef]
- Zagula-Yavorska, M.; Romanowska, J.; Sieniawski, J.; Wierzbińska, M. Hafnium modified aluminide coatings obtained by the CVD and PVD methods. Solid State Phenom. 2015, 227, 353–356. [Google Scholar] [CrossRef]
- Warnes, B.M. Improved aluminide/MCrAlX coating systems for super alloys using CVD low activity aluminizing. Surf. Coat. Technol. 2003, 163, 106–111. [Google Scholar] [CrossRef]
- Voudouris, N.; Christoglou, C.; Angelopoulos, G.N. Formation of aluminide coatings on nickel by a fluidised bed CVD process. Surf. Coat. Technol. 2001, 141, 275–282. [Google Scholar] [CrossRef]
- Levine, S.R.; Caves, R.M. Thermodynamics and kinetics of pack aluminide coating formation on IN-100. J. Electrochem. Soc. 1974, 121, 1051. [Google Scholar] [CrossRef] [Green Version]
- Rakov, E.G.; Mel’nichenko, E.I. The properties and reactions of ammonium fluorides. Russ. Chem. Rev. 1984, 53, 851–869. [Google Scholar] [CrossRef]
- Menz, D.-H.; Zacharias, A.; Kolditz, L. A comparison of the thermal behaviour of α-AIF3 and aluminium fluoride hydrates. J. Therm. Anal. Calorim. 1988, 33, 811–815. [Google Scholar] [CrossRef]
- Nowak, W.J.; Ochał, K.; Wierzba, P.; Gancarczyk, K.; Wierzba, B. Effect of substrate roughness on oxidation resistance of an aluminized Ni-base superalloy. Metals 2019, 9, 782. [Google Scholar] [CrossRef] [Green Version]
- Bozza, F.; Bolelli, G.; Giolli, C.; Giorgetti, A.; Lusvarghi, L.; Sassatelli, P.; Scrivani, A.; Candeli, A.; Thoma, M. Diffusion mechanisms and microstructure development in pack aluminizing of Ni-based alloys. Surf. Coat. Technol. 2014, 239, 147–159. [Google Scholar] [CrossRef]
- Lu, J.; Zhu, S.; Wang, F. Cyclic oxidation and hot corrosion behavior of Y/Cr-modified aluminide coatings prepared by a hybrid slurry/pack cementation process. Oxid. Met. 2011, 76, 67–82. [Google Scholar] [CrossRef]
- Warnes, B.M.; Punola, D.C. Clean diffusion coatings by chemical vapor deposition. Surf. Coat. Technol. 1997, 94, 1–6. [Google Scholar] [CrossRef]
- Goward, G.W.; Boone, D.H. Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Yang, Y.F.; Jiang, C.Y.; Bao, Z.B.; Zhu, S.L.; Wang, F.H. Effect of aluminisation characteristics on the microstructure of single phase β-(Ni,Pt)Al coating and the isothermal oxidation behaviour. Corros. Sci. 2016, 106, 43–54. [Google Scholar] [CrossRef]
- Warnes, B.M. Reactive element modified chemical vapor deposition low activity platinum aluminide coatings. Surf. Coat. Technol. 2001, 146, 7–12. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Burnell-Gray, J.S.; Datta, P.K. Aluminide coating formation on nickel-base superalloys by pack cementation process. J. Mater. Sci. 2001, 36, 5673–5682. [Google Scholar] [CrossRef]
- Naveos, S.; Oberlaender, G.; Cadoret, Y.; Josso, P.; Bacos, M.P. Zirconium modified aluminide by a vapour pack cementation process for thermal barrier applications: Formation mechanisms and properties. Mater. Sci. Forum. 2004, 461, 375–382. [Google Scholar] [CrossRef]
- Baiamonte, L.; Marra, F.; Gazzola, S.; Giovanetto, P.; Bartuli, C.; Valente, T.; Pulci, G. Thermal sprayed coatings for hot corrosion protection of exhaust valves in naval diesel engines. Surf. Coat. Technol. 2016, 295, 78–87. [Google Scholar] [CrossRef]
- Chatha, S.S.; Sidhu, H.S.; Sidhu, B.S. High temperature hot corrosion behaviour of NiCr and Cr3C2-NiCr coatings on T91 boiler steel in an aggressive environment at 750 °C. Surf. Coat. Technol. 2012, 206, 3839–3850. [Google Scholar] [CrossRef]
- Li, C.; Xu, X.; Wang, S.; Tabie, V.M.; Yang, S.; Zhang, T.; Liu, Y. High-temperature oxidation and hot corrosion behavior of the Cr-modified aluminide coating obtained by a thermal diffusion process. Mater. Res. Express. 2019, 6, 086444. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, M.M.; Liu, S.L.; Sun, C.; Wang, F.H. Hot corrosion behaviour of a cobalt-base super-alloy K40S with and without NiCrAlYSi coating. Corros. Sci. 2011, 53, 1990–1998. [Google Scholar] [CrossRef]
- Tolpygo, V.K.; Clarke, D.R. Rumpling of CVD (Ni,Pt)Al diffusion coatings under intermediate temperature cycling. Surf. Coat. Technol. 2009, 203, 3278–3285. [Google Scholar] [CrossRef]
- Zielińska, M.; Zagula-Yavorska, M.; Sieniawski, J.; Filip, R. Microstructure and oxidation resistance of an aluminide coating on the nickel based superalloy Mar M247 deposited by the CVD aluminizing process. Arch. Met. Mater. 2013, 58, 697–701. [Google Scholar] [CrossRef]
- Xiang, Z.D.; Datta, P.K. Pack cementation process for the formation of refractory metal modified aluminide coatings on nickel-base superalloys. J. Mater. Sci. 2003, 38, 3721–3728. [Google Scholar] [CrossRef]
- Bianco, R.; Rapp, R.A. Pack cementation diffusion coatings. In Metallurgical and Ceramic Protective Coatings; Stern, K.H., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; pp. 236–260. [Google Scholar]
- Yamai, I.; Saito, H. Vapor phase growth of alumina whiskers by hydrolysis of aluminum fluoride. J. Cryst. Growth 1978, 45, 511–516. [Google Scholar] [CrossRef]
- Riello, D.; Zetterström, C.; Parr, C.; Braulio MA, L.; Moreira, M.; Gallo, J.B.; Pandolfelli, V.C. AlF3 reaction mechanism and its influence on α-Al2O3 mineralization. Ceram. Int. 2016, 42, 9804–9814. [Google Scholar] [CrossRef]
- Monceau, D.; Pieraggi, B. Determination of parabolic rate constants from a local analysis of mass-gain curves. Oxid. Met. 1998, 50, 477–493. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.; Doyle, N.J. Further studies on the nickel–aluminium system. I. β-NiAl and δ-Ni2Al3 phase fields. J. Appl. Cryst. 1972, 5, 201–209. [Google Scholar] [CrossRef]
- Prakash, S. Hot corrosion of alloys and coatings. In Developments in High Temperature Corrosion and Protection of Materials; Woodhead Publishing: Cambridge, UK, 2008; pp. 164–191. [Google Scholar] [CrossRef]
- Liu, G.; Li, M.; Zhu, M.; Zhou, Y. Transient of alumina oxide scale on β-NiAl coated on M38G alloy at 950 °C. Intermetallics 2007, 15, 1285–1290. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S.; Ma, Y.; Gong, S. Study on behavior of NiAl coating with different Ni/Al ratios. Vaccum 2013, 93, 37–44. [Google Scholar] [CrossRef]
- Fan, Q.X.; Jiang, S.M.; Wu, D.L.; Gong, J.; Sun, C. Preparation and hot corrosion behaviour of two Co modified NiAl coatings on a Ni-based superalloy. Corros. Sci. 2013, 76, 373–381. [Google Scholar] [CrossRef]








| Alloy | Ni | Cr | Co | Al | Ti | Mo | W | Hf | Ta |
|---|---|---|---|---|---|---|---|---|---|
| René 108 DS | Bal. | 8.4 | 9.5 | 5.5 | 0.7 | 0.5 | 9.5 | 1.5 | 3.0 |
| Step | Temperature (°C) | Time (h) | Note |
|---|---|---|---|
| 1 | 100 | 0.5 | Water traces removal |
| 2 | 150 | 1 | Thermal decomposition of NH4F [14] |
| 3 | 600 | 2 | Formation of AlFx-g |
| 4 | 1000 | 8 | Diffusion of Al |
| Coating Name | Activator Salt Type | Activator Salt Concentration (wt.%) | Moles of Activator Salt |
|---|---|---|---|
| NF1 | NH4F | 0.050 | 0.0135 |
| AlF1 | AlF3 | 0.100 | 0.0135 |
| NF2 | NH4F | 0.075 | 0.0202 |
| AlF2 | AlF3 | 0.170 | 0.0202 |
| Sample Name | β-NiAl Thickness (µm) | IDZ Thickness (µm) |
|---|---|---|
| NF1 | 23 ± 1.0 | 20 ± 0.9 |
| AlF1 | 27 ± 1.2 | 24 ± 0.9 |
| NF2 | 20 ± 0.6 | 19 ± 0.7 |
| AlF2 | 30 ± 0.9 | 25 ± 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Genova, V.; Paglia, L.; Pulci, G.; Bartuli, C.; Marra, F. Diffusion Aluminide Coatings for Hot Corrosion and Oxidation Protection of Nickel-Based Superalloys: Effect of Fluoride-Based Activator Salts. Coatings 2021, 11, 412. https://doi.org/10.3390/coatings11040412
Genova V, Paglia L, Pulci G, Bartuli C, Marra F. Diffusion Aluminide Coatings for Hot Corrosion and Oxidation Protection of Nickel-Based Superalloys: Effect of Fluoride-Based Activator Salts. Coatings. 2021; 11(4):412. https://doi.org/10.3390/coatings11040412
Chicago/Turabian StyleGenova, Virgilio, Laura Paglia, Giovanni Pulci, Cecilia Bartuli, and Francesco Marra. 2021. "Diffusion Aluminide Coatings for Hot Corrosion and Oxidation Protection of Nickel-Based Superalloys: Effect of Fluoride-Based Activator Salts" Coatings 11, no. 4: 412. https://doi.org/10.3390/coatings11040412
APA StyleGenova, V., Paglia, L., Pulci, G., Bartuli, C., & Marra, F. (2021). Diffusion Aluminide Coatings for Hot Corrosion and Oxidation Protection of Nickel-Based Superalloys: Effect of Fluoride-Based Activator Salts. Coatings, 11(4), 412. https://doi.org/10.3390/coatings11040412









