Simulating the Growth of Dual-Phase Boride Layer on AISI M2 Steel by Two Kinetic Approaches
Abstract
:1. Introduction
2. Diffusion Models
2.1. Integral Diffusion Model
2.2. Dybkov Model
3. Calculation Results and Discussion
3.1. Assessment of Boron Diffusivities in FeB and Fe2B with the Integral Method
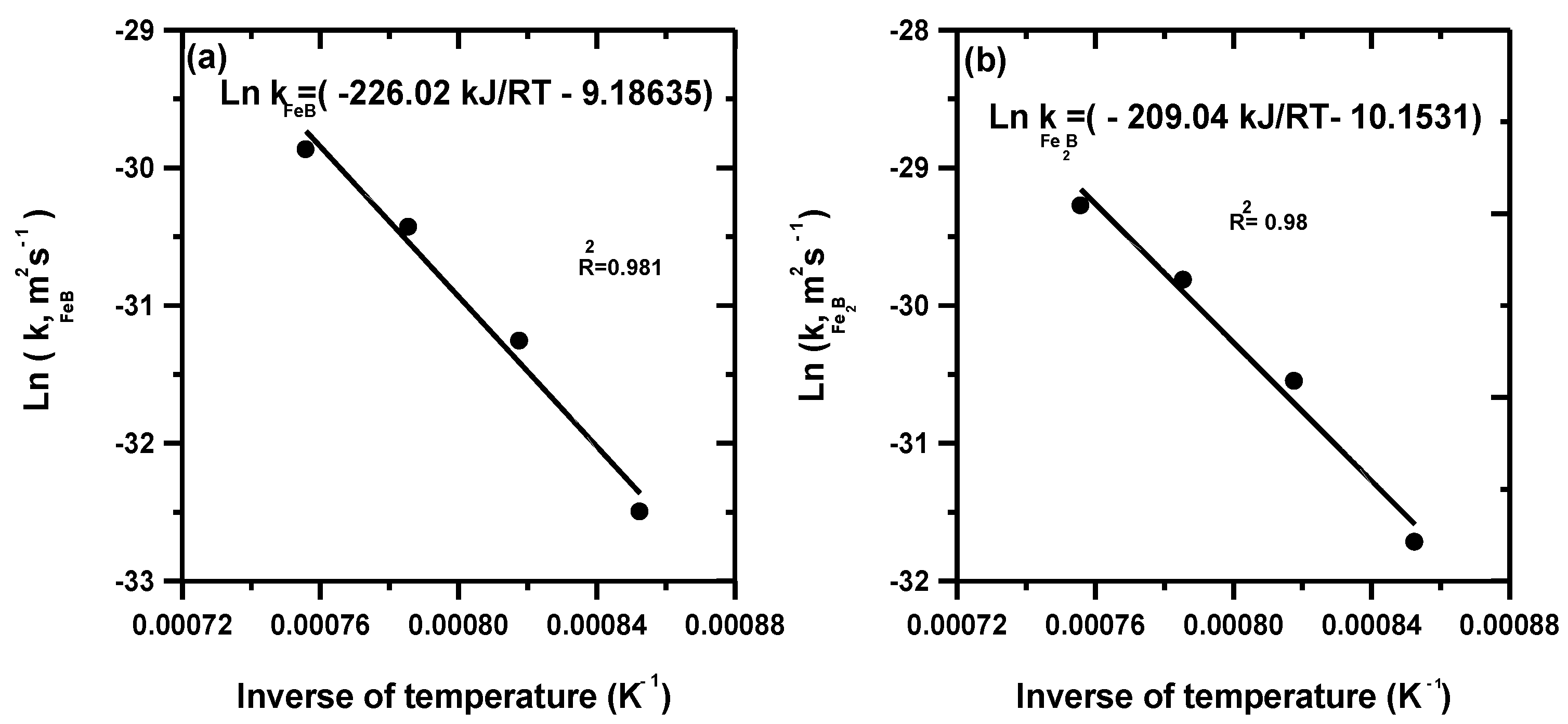
3.2. Estimation of the Two Temperature-Dependent Parameters kFeB and kFe2B Using the Dybkov Model
3.3. Experimental Verification of Both Kinetics Approaches
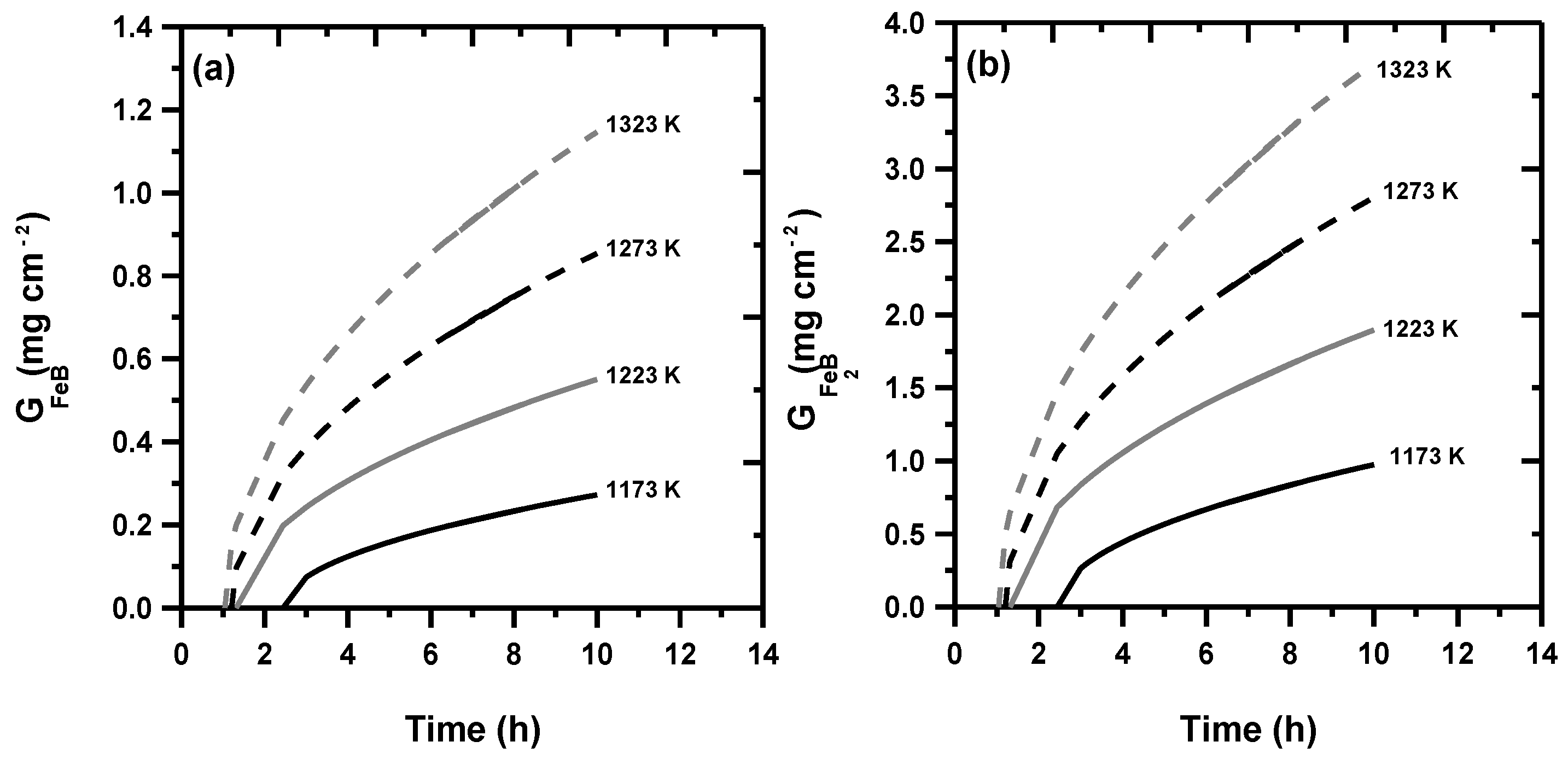
3.4. Assessment of Mass Gain for FeB and Fe2B
4. Conclusions
- (1)
- A peculiar solution of the obtained DAE system was suggested in order to assess the boron diffusivities in FeB and Fe2B for a maximum boron content in FeB of 16.40 wt.%.
- (2)
- The two fitting parameters and for the Dybkov model were estimated as a function of process temperature by utilizing the experimental data of reference [23].
- (3)
- The estimated values of activation energies for boron diffusion in FeB and Fe2B were 206.41 and 216.18 kJ mol−1, respectively, with the integral method while with the Dybkov model, the corresponding values were equal to 226.02 and 209.04 kJ mol−1 (for FeB and Fe2B).
- (4)
- The integral method and Dybkov model have been experimentally verified for four additional boriding conditions.
- (5)
- Simple equations derived from the integral method were used to determine the layers’ thicknesses of FeB and Fe2B.
- (6)
- For both approaches, the predicted layers’ thicknesses were in line with the experimental data.
- (7)
- The values of mass gain within FeB and Fe2B were determined as a function of treatment time by considering the presence of boride incubation periods.
- (8)
- As prospects, both kinetics approaches can be employed to simulate the diffusion kinetics of intestinal elements in a multiphase system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinha, A.K. Boriding (Boronizing) of steels. J. Heat Treat. 1991, 4, 437–447. [Google Scholar]
- Kulka, M. Engineering Materials. Current Trends in Boriding, 1st ed.; Springer Nature Switzerland AG 2019; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Keddam, M.; Hudáková, M.; Ptačinová, J.; Moravčík, R.; Gogola, P.; Gabalcová, Z.; Jurči, P. Characterization of boronized layers on vanadis 6 tool steel. Surface Eng. 2020, 37, 445–454. [Google Scholar] [CrossRef]
- Okamoto, H. B-Fe (boron-iron). J. Phase Equilibria Diffus. 2004, 25, 297–298. [Google Scholar] [CrossRef]
- Kapfenberger, C.; Albert, B.; Pöttgen, R.; Huppertz, H. Structure refinements of iron borides Fe2B and FeB. Z. Krist. Cryst. Mater. 2006, 221, 477–481. [Google Scholar] [CrossRef]
- Skugorova, L.P.; Nechaev, A.I. Investigation of the gas boriding process. Met. Sci. Heat Treat. 1973, 15, 989–990. [Google Scholar] [CrossRef]
- Smol’nikov, E.A.; Sarmanova, L.M. Study of the possibility of liquid boriding of high-speed steels. Met. Sci. Heat Treat. 1982, 24, 785–788. [Google Scholar] [CrossRef]
- Segers, L.; Fontana, A.; Winand, R. Electrochemical boriding of iron in molten salts. Electrochim. Acta. 1991, 36, 41–47. [Google Scholar] [CrossRef]
- Campos, I.; Torres, R.; Bautista, O.; Ramírez, G.; Zúñiga, L. Effect of boron paste thickness on the growth kinetics of polyphase boride coatings during the boriding process. Appl. Surf. Sci. 2006, 252, 2396–2403. [Google Scholar] [CrossRef]
- Calik, A.; Ucar, N.; Karakas, M.S.; Tanis, H. Pack-boriding of pure iron with powder mixtures containing ZrB2. High Temp. Mater. Process. 2019, 38, 342–346. [Google Scholar] [CrossRef] [Green Version]
- Anthymidis, K.G.; Stergioudis, E.; Tsipas, D.N. Boriding in a fluidized bed reactor. Mater. Lett. 2001, 51, 156–160. [Google Scholar] [CrossRef]
- Rodrıguez Cabeo, E.; Laudien, G.; Biemer, S.; Rie, K.T.; Hoppe, S. Plasma-assisted boriding of industrial components in a pulsed d.c. glow discharge. Surf. Coat. Technol. 1999, 116–119, 229–233. [Google Scholar] [CrossRef]
- Gunes, I.; Taktak, S.; Bindal, C.; Yalcin, Y.; Ulker, S.; Kayali, Y. Investigation of diffusion kinetics of plasma paste borided AISI 8620 steel using a mixture of B2O3 paste and B4C/SiC. Sadhana 2013, 38, 513–526. [Google Scholar] [CrossRef] [Green Version]
- Jain, V.; Sundararajan, G. Influence of the pack thickness of the boronizing mixture on the boriding of steel. Surf. Coat. Technol. 2002, 149, 21–26. [Google Scholar] [CrossRef]
- Campos, I.; Oseguera, J.; Figueroa, U.; Garcia, J.; Bautista, O.; Kelemenis, G. Kinetic study of boron diffusion in the paste-boriding process. Mater. Sci. Eng. A. 2003, 352, 261–265. [Google Scholar] [CrossRef]
- Keddam, M.; Kulka, M. Mean diffusion coefficient method in studying armco iron boriding kinetics. Met. Sci. Heat Treat. 2020, 62, 326–330. [Google Scholar] [CrossRef]
- Campos, I.; Islas, M.; Ramírez, G.; VillaVelázquez, C.; Mota, C. Growth kinetics of borided layers: Artificial neural network and least square approaches. Appl. Surf. Sci. 2007, 253, 6226–6231. [Google Scholar] [CrossRef]
- Ortiz-Domínguez, M.; Keddam, M.; Elias-Espinosa, M.; Damián-Mejía, O.; Flores-Rentería, M.A.; Arenas-Flores, A.; Hernández-Ávila, J. Investigation of boriding kinetics of AISI D2 steel. Surf. Eng. 2014, 30, 490–497. [Google Scholar] [CrossRef]
- Campos, I.; Islas, M.; González, E.; Ponce, P.; Ramírez, G. Use of fuzzy logic for modelling the growth of Fe2B boride layers during boronizing. Surf. Coat. Technol. 2006, 201, 2717–2723. [Google Scholar] [CrossRef]
- Ramdan, R.D.; Takaki, T.; Tomita, Y. Free energy problem for the simulations of the growth of Fe2B phase using phase-field method. Mater. Trans. 2008, 49, 2625–2631. [Google Scholar] [CrossRef] [Green Version]
- Campos-Silva, I.; López-Perrusquia, N.; Ortiz-Domínguez, M.; Figueroa-López, U.; Gómez-Vargas, O.A.; Meneses-Amador, A.; Rodríguez-Castro, G. Characterization of boride layers formed at the surface of gray cast irons. Kov. Mater. 2009, 47, 75–81. [Google Scholar]
- Pablo, A.; Ruiz-Trabolsi, P.A.; Cesar Velázquez, J.; Orozco-Álvarez, C.; Carrera-Espinoza, R.; Yescas-Hernández, J.A.; González-Arévalo, N.E.; Hernández-Sánchez, E. Kinetics of the boride layers obtained on AISI 1018 steel by considering the amount of matter involved. Coatings 2021, 11, 259. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Ortiz-Dominguez, M.; Tapia-Quintero, C.; Rodríguez-Castro, G.; Jiménez-Reyes, M.Y.; Chávez-Gutiérrez, E. Kinetics and boron diffusion in the FeB/Fe2B layers formed at the surface of borided high-alloy steel. J. Mater. Eng. Perform. 2012, 21, 1714–1723. [Google Scholar] [CrossRef]
- Keddam, M.; Kulka, M. Boriding kinetics of AISI D2 steel by using two different approaches. Met. Sci. Heat Treat. 2020, 61, 756–763. [Google Scholar] [CrossRef]
- Keddam, M.; Kulka, M. Modeling of the kinetics of boron diffusion in dehydrated paste pack-borided AISI M2 steel based on two mathematical approaches. Mater. Perform. Charact. 2020, 9, 303–314. [Google Scholar] [CrossRef]
- Zouzou, C.; Keddam, M. Boriding kinetics of FeB and Fe2B layers on AISI M2 steel by the integral diffusion model. Ann. Chim. Sci. Mater. 2019, 43, 159–164. [Google Scholar] [CrossRef]
- Yu, L.G.; Chen, X.J.; Khor, A.K.; Sundararajan, G. FeB/Fe2B phase transformation during SPS pack-boriding: Boride layer growth kinetics. Acta Mater. 2005, 53, 2361–2368. [Google Scholar] [CrossRef]
- Dybkov, V.I.; Goncharuk, L.V.; Khoruzha, V.G.; Meleshevich, K.A.; Samelyuk, A.V.; Sidorko, V.R. Diffusional growth kinetics of boride layers on iron-chromium alloys. Solid State Phenomena. 2008, 138, 181–188. [Google Scholar] [CrossRef]
- Delai, O.; Xia, C.; Shiqiang, L. Growth kinetics of the FeB/Fe2B boride layer on the surface of 4Cr5MoSiV1 steel: Experiments and modelling. J. Mater. Res. Technol. 2021, 11, 1272–1280. [Google Scholar] [CrossRef]
- Campos-Silva, I.; Ortiz-Domínguez, M.; Bravo-Bárcenas, O.; Doñu-Ruiz, M.A.; Bravo-Bárcenas, D.; Tapia-Quintero, C.; Jiménez-Reyes, M.Y. Formation and kinetics of FeB/Fe2B layers and diffusion zone at the surface of AISI 316 borided steels. Surf. Coat. Technol. 2010, 205, 403–412. [Google Scholar] [CrossRef]
- Brakman, C.M.; Gommers, A.W.J.; Mittemeijer, E.J. Boriding of Fe and Fe–C, Fe–Cr, and Fe–Ni alloys; Boride-layer growth kinetics. J. Mater. Res. 1989, 4, 1354–1370. [Google Scholar] [CrossRef]
- Nait Abdellah, Z.; Keddam, M. Estimation of the boron diffusion coefficients in the FeB and Fe2B layers during the pack-boriding of a high-alloy steel. Mater. Tehnol. 2014, 48, 237–242. [Google Scholar]
- VillaVelázquez-Mendoza, C.I.; Rodríguez-Mendoza, J.L.; Ibarra-Galván, V.; Hodgkins, R.P.; López-Valdivieso, A.; Serrato-Palacios, L.L.; Leal-Cruz, A.L.; Ibarra-Junquera, V. Effect of substrate roughness, time and temperature on the processing of iron boride coatings: Experimental and statistical approaches. Int. J. Surf. Sci. Eng. 2014, 8, 71–91. [Google Scholar] [CrossRef]
- Belguendouz, O.; Mebarek, B.; Keddam, M.; El Guerri, Y. Diffusion model for simulating the kinetics of boronizing process in the case of FeB/Fe2B bilayer configuration. Ann. Chim. Sci. Mater. 2020, 44, 191–197. [Google Scholar] [CrossRef]
- Zouzou, C.; Keddam, M. Application of integral method for investigating the boriding kinetics of AISI 316 steel. Metall. Res. Technol. 2020, 117, 202. [Google Scholar] [CrossRef] [Green Version]
- Goodman, T.R. Application of integral methods to transient nonlinear heat transfer. Adv. Heat Transfer. 1964, 1, 51–122. [Google Scholar]
- Press, W.H.; Flannery, B.P.; Teukolsky, S.A. Numerical Recipes in Pascal: The Art of Scientific Computing; Cambridge University Press: New York, NY, USA, 1989. [Google Scholar]
- Keddam, M.; Chegroune, R.; Kulka, M.; Makuch, N.; Panfil, D.; Siwak, P.; Taktak, S. Characterization, tribological and mechanical properties of plasma paste borided AISI 316 steel. Trans. Indian Inst. Met. 2018, 71, 79–90. [Google Scholar] [CrossRef]
- Yu, L.G.; Khor, K.A.; Sundararajan, G. Boriding of mild steel using the spark plasma sintering (SPS) technique. Surf. Coat. Technol. 2002, 157, 226–230. [Google Scholar] [CrossRef]
- Kartal, G.; Eryilmaz, O.L.; Krumdick, G.; Erdemir, A.; Timur, S. Kinetics of electrochemical boriding of low carbon steel. Appl. Surf. Sci. 2011, 257, 6928–6934. [Google Scholar] [CrossRef]
- Sen, S.; Sen, U.; Bindal, C. An approach to kinetic study of borided steels. Surf. Coat. Technol. 2005, 191, 274–285. [Google Scholar] [CrossRef]
- Genel, K.; Ozbek, I.; Bindal, C. Kinetics of boriding of AISI W1 steel. Mater. Sci. Eng. A. 2003, 347, 311–314. [Google Scholar] [CrossRef]
- Genel, K. Boriding kinetics of H13 steel. Vacuum 2006, 80, 451–457. [Google Scholar] [CrossRef]
- Doñu Ruiz, M.A.; López Perrusquia, N.; Sánchez Huerta, D.; Torres San Miguel, C.R.; Urriolagoitia Calderón, G.M.; Cerillo Moreno, E.A.; Cortes Suarez, J.V. Growth kinetics of boride coatings formed at the surface AISI M2 during dehydrated paste pack boriding. Thin Solid Films 2015, 596, 147–154. [Google Scholar] [CrossRef]


| T (K) | (s) | |||
|---|---|---|---|---|
| 1173 | 0.065 | 10131 | 0.168 | 8806.2 |
| 1223 | 0.121 | 6085.7 | 0.305 | 4729 |
| 1273 | 0.179 | 4347.8 | 0.448 | 4323 |
| 1323 | 0.238 | 3815.5 | 0.5891 | 3742.7 |
| T (K) | ||
|---|---|---|
| 1173 | 0.0615 | 8806.2 |
| 1223 | 0.1160 | 4729.0 |
| 1273 | 0.1788 | 4323.0 |
| 1323 | 0.2380 | 3742.7 |
| T (K) | (m2 s−1) | |||
|---|---|---|---|---|
| 1173 | 0.23 | 0.33 | 0.06388 | 0.09314 |
| 1223 | 0.80 | 1.06 | 0.06461 | 0.0916 |
| 1273 | 1.86 | 2.25 | 0.06553 | 0.08958 |
| 1323 | 2.44 | 4.14 | 0.06173 | 0.09735 |
| T (K) | ||
|---|---|---|
| 1173 | 0.0077 | 0.01680 |
| 1223 | 0.0266 | 0.0539 |
| 1273 | 0.0608 | 0.1125 |
| 1323 | 0.1067 | 0.1929 |
| Steel | Boriding Method | Temperature Range (K) | Activation Energy (kJmol−1) | Approach Used | Refs. |
|---|---|---|---|---|---|
| AISI M2 | Paste | 1223–1273 | 283 (FeB) 239.4 (Fe2B) | A modified version of Brakman model | [9] |
| AISI 8620 | Plasma paste | 973–1073 | 124.7–138.5 (FeB + Fe2B) | Parabolic growth law | [13] |
| AISI D2 | Powder | 1123–1273 | 201.5 (Fe2B) | Diffusion model | [18] |
| AISI 1018 | Powder | 1173–1273 | 93.8–155.22 (FeB + Fe2B) | Parabolic growth law | [22] |
| AISI M2 | Powder | 1173–1323 | 223.0 (FeB) 207.0 (Fe2B) | Kinetic model | [23] |
| AISI D2 | Powder | 1223–1273 | 208.04 (FeB) 197.46 (Fe2B) 207.84 (FeB) 197.04 (Fe2B) | Integral method Dybkov model | [24] |
| AISI M2 | Powder | 1173–1323 | 218.06 (FeB) 212.1 (Fe2B) | Integral method | [26] |
| AISI M2 | Powder | 1173–1323 | 220.2 (FeB) 213.0 (Fe2B) | Diffusion model | [32] |
| AISI 316 | Plasma paste boriding | 973–1073 | 118.12 (FeB + Fe2B) | Empirical law | [38] |
| Mild steel | Spark Plasma Sintering (SPS) | 973–1273 | 145.84 (FeB + Fe2B) | Parabolic growth law | [39] |
| Low carbon steel | Electro-Chemical | 1123–1273 | 172.75 (FeB + Fe2B) | Parabolic growth law | [40] |
| AISI D2 | Salt bath | 1073–1273 | 170 (FeB + Fe2B) | Parabolic growth law | [41] |
| AISI W1 | Powder | 1123–1323 | 171.2 ± 16.6 (FeB + Fe2B) | Parabolic growth law | [42] |
| AISI H13 | Powder | 1073–1273 | 186.2 (FeB + Fe2B) | Parabolic growth law | [43] |
| AISI M2 | Dehydrated Paste pack | 1173–1273 | 233.42 (FeB) 211.89 (Fe2B) | Diffusion model | [44] |
| AISI M2 | Powder | 1173–1323 | 206.41 (FeB) 216.18 (Fe2B) 226.02 (FeB) 209.04 (Fe2B) | Integral method Dybkov model | This work |
| T (K) | uexp (μm) | usim (μm) by the Integral Method | usim (μm) by the Dybkov Model |
|---|---|---|---|
| 1173 | 10.17 | 10.06 | 10.85 |
| 1223 | 20.98 | 16.70 | 18.98 |
| 1273 | 28.30 | 25.14 | 29.96 |
| 1323 | 40.24 | 36.80 | 45.02 |
| T (K) | lexp (μm) | lsim (μm) by the Integral Method | lsim (μm) by the Dybkov Model |
|---|---|---|---|
| 1173 | 19.66 | 18.61 | 18.71 |
| 1223 | 32.81 | 31.4 | 30.8 |
| 1273 | 51.83 | 47.98 | 45.96 |
| 1323 | 72.28 | 71.22 | 66.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keddam, M.; Jurči, P. Simulating the Growth of Dual-Phase Boride Layer on AISI M2 Steel by Two Kinetic Approaches. Coatings 2021, 11, 433. https://doi.org/10.3390/coatings11040433
Keddam M, Jurči P. Simulating the Growth of Dual-Phase Boride Layer on AISI M2 Steel by Two Kinetic Approaches. Coatings. 2021; 11(4):433. https://doi.org/10.3390/coatings11040433
Chicago/Turabian StyleKeddam, Mourad, and Peter Jurči. 2021. "Simulating the Growth of Dual-Phase Boride Layer on AISI M2 Steel by Two Kinetic Approaches" Coatings 11, no. 4: 433. https://doi.org/10.3390/coatings11040433






