High-Performance Graphene Coating on Titanium Bipolar Plates in Fuel Cells via Cathodic Electrophoretic Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Sample Preparation
2.3. Characterization and Electrochemical Corrosion Tests
2.4. ICR Measurements
3. Results and Discussion
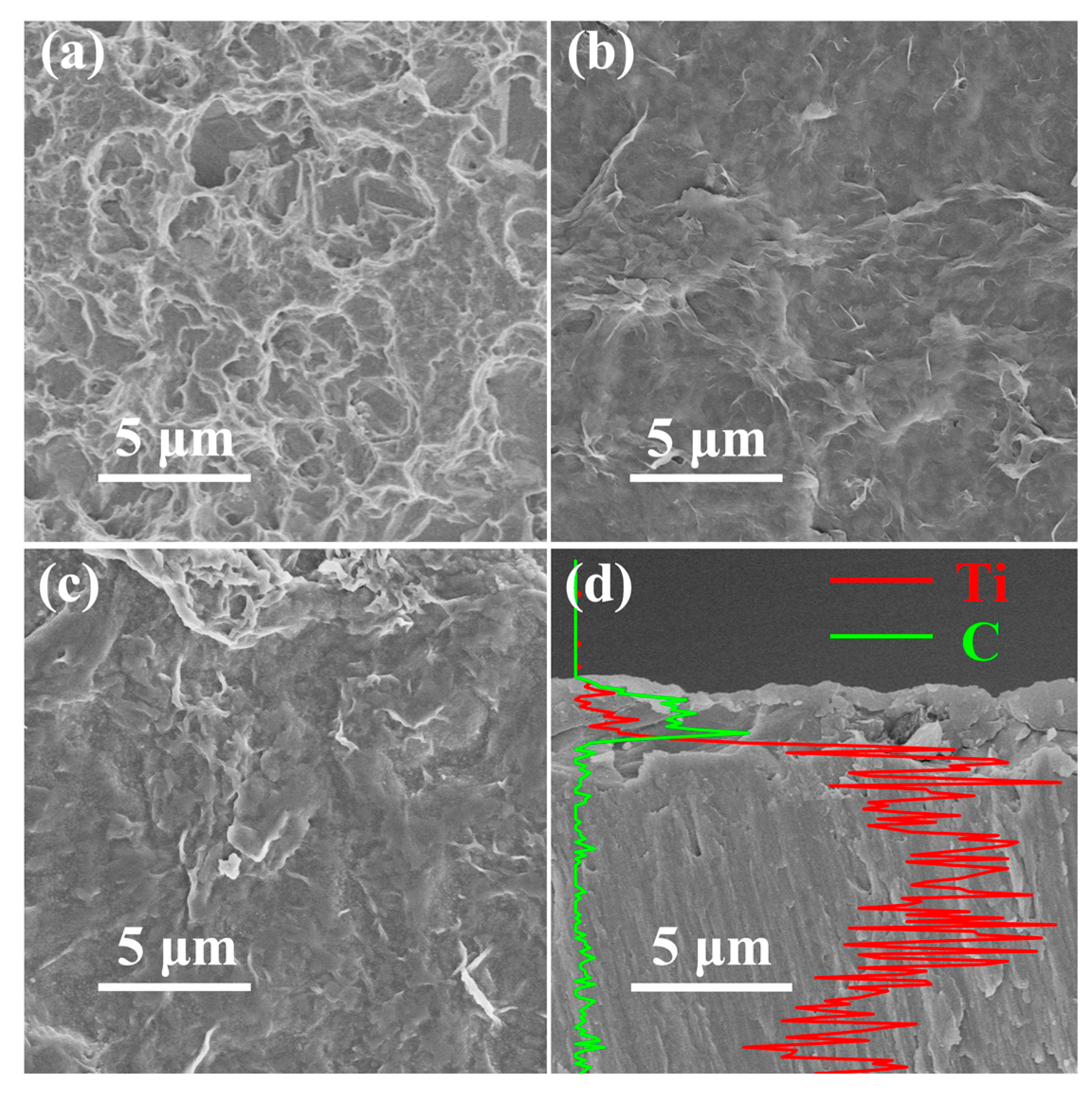
3.1. Characterization
3.2. Electrochemical Corrosion Tests
3.3. ICR Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agarwal, H.; Pandey, R.; Bhat, S.D. Improved polymer electrolyte fuel cell performance with membrane electrode assemblies using modified metallic plate: Comparative study on impact of various coatings. Int. J. Hydrogen Energy 2020, 45, 18731–18742. [Google Scholar] [CrossRef]
- Madadi, F.; Rezaeian, A.; Edris, H.; Zhiani, M. Improving performance in PEMFC by applying different coatings to metallic bipolar plates. Mater. Chem. Phys. 2019, 238, 121911. [Google Scholar] [CrossRef]
- Alnegren, P.; Grolig, J.G.; Ekberg, J.; Goransson, G.; Svensson, J.-E. Metallic Bipolar Plates for High Temperature Polymer Electrolyte Membrane Fuel Cells. Fuel Cells 2016, 16, 39–45. [Google Scholar] [CrossRef]
- Abeyrathna, B.; Zhang, P.; Pereira, M.P.; Wilkosz, D.; Weiss, M. Micro-roll forming of stainless steel bipolar plates for fuel cells. Int. J. Hydrogen Energy 2019, 44, 3861–3875. [Google Scholar] [CrossRef]
- Wilberforce, T.; Ijaodola, O.; Ogungbemi, E.; Khatib, F.; Leslie, T.; El-Hassan, Z.; Thomposon, J.; Olabi, A. Technical evaluation of proton exchange membrane (PEM) fuel cell performance—A review of the effects of bipolar plates coating. Renew. Sustain. Energy Rev. 2019, 113. [Google Scholar] [CrossRef]
- Bhosale, A.C.; Rengaswamy, R. Interfacial contact resistance in polymer electrolyte membrane fuel cells: Recent developments and challenges. Renew. Sustain. Energy Rev. 2019, 115, 109351. [Google Scholar] [CrossRef]
- Hermann, A.; Chaudhuri, T.; Spagnol, P. Bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2005, 30, 1297–1302. [Google Scholar] [CrossRef]
- Antunes, R.A.; Oliveira, M.C.L.; Ett, G.; Ett, V. Corrosion of metal bipolar plates for PEM fuel cells: A review. Int. J. Hydrogen Energy 2010, 35, 3632–3647. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, W.; Mu, Y.; Fang, F.; Huang, C.; Wang, Y. Facile fabrication of holey graphene oxide paper bonded with sulfonic acid for highly efficient proton conduction. Ionics 2018, 25, 573–581. [Google Scholar] [CrossRef]
- Wang, J.; Min, L.; Fang, F.; Zhang, W.; Wang, Y. Electrodeposition of graphene nano-thick coating for highly enhanced performance of titanium bipolar plates in fuel cells. Int. J. Hydrogen Energy 2019, 44, 16909–16917. [Google Scholar] [CrossRef]
- Allahkaram, S.R.; Mohammadi, N. Corrosion behavior of two candidate PEMFC’s bipolar plate materials. Anti-Corros. Methods Mater. 2017, 64, 293–298. [Google Scholar]
- Zhang, W.; Yi, P.; Peng, L.; Lai, X. Strategy of alternating bias voltage on corrosion resistance and interfacial conductivity enhancement of TiCx/a-C coatings on metallic bipolar plates in PEMFCs. Energy 2018, 162, 933–943. [Google Scholar] [CrossRef]
- Tawfik, H.; Hung, Y.; Mahajan, D. Metal bipolar plates for PEM fuel cell—A review. J. Power Sources 2007, 163, 755–767. [Google Scholar] [CrossRef]
- Asri, N.F.; Husaini, T.; Sulong, A.B.; Majlan, E.H.; Daud, W.R.W. Coating of stainless steel and titanium bipolar plates for anticorrosion in PEMFC: A review. Int. J. Hydrogen Energy 2017, 42, 9135–9148. [Google Scholar] [CrossRef]
- Saadat, N.; Dhakal, H.N.; Jaffer, S.; Tjong, J.; Yang, W.; Tan, J.; Sain, M. Expanded and nano-structured carbonaceous graphite for high performance anisotropic fuel cell polymer composites. Compos. Sci. Technol. 2021, 207, 108654. [Google Scholar] [CrossRef]
- Weissbecker, V.; Wippermann, K.; Lehnert, W. Electrochemical Corrosion Study of Metallic Materials in Phosphoric Acid as Bipolar Plates for HT-PEFCs. J. Electrochem. Soc. 2014, 161, F1437–F1447. [Google Scholar] [CrossRef]
- Husby, H.; Kongstein, O.E.; Oedegaard, A.; Seland, F. Carbon-polymer composite coatings for PEM fuel cell bipolar plates. Int. J. Hydrogen Energy 2014, 39, 951–957. [Google Scholar] [CrossRef]
- Sadeghian, Z.; Hadidi, M.R.; Salehzadeh, D.; Nemati, A. Hydrophobic octadecylamine-functionalized graphene/TiO2 hybrid coating for corrosion protection of copper bipolar plates in simulated proton exchange membrane fuel cell environment. Int. J. Hydrogen Energy 2020, 45, 15380–15389. [Google Scholar] [CrossRef]
- Jiang, L.; Syed, J.A.; Zhang, G.; Ma, Y.; Ma, J.; Lu, H.; Meng, X. Enhanced anticorrosion performance of PPY-graphene oxide/PPY-camphorsulfonic acid composite coating for 304SS bipolar plates in proton exchange membrane fuel cell. J. Ind. Eng. Chem. 2019, 80, 497–507. [Google Scholar] [CrossRef]
- Alavijeh, M.S.; Kefayati, H.; Golikand, A.N.; Shariati, S. Synthesis and characterization of epoxy/graphite/nano-copper nanocomposite for the fabrication of bipolar plate for PEMFCs. J. Nanostruct. Chem. 2019, 9, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.H.; Noh, S.; Lee, J.-H.; Chun, S.-H.; Cha, S.W.; Chang, I. Durable graphene-coated bipolar plates for polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2017, 42, 27350–27353. [Google Scholar] [CrossRef]
- Chen, P.; Fang, F.; Zhang, Z.; Zhang, W.; Wang, Y. Self-assembled graphene film to enable highly conductive and corrosion resistant aluminum bipolar plates in fuel cells. Int. J. Hydrogen Energy 2017, 42, 12593–12600. [Google Scholar] [CrossRef]
- Sim, Y.; Kwak, J.; Kim, S.-Y.; Jo, Y.; Kim, S.; Kim, S.Y.; Kim, J.H.; Lee, C.-S.; Jo, J.H.; Kwon, S.-Y. Formation of 3D graphene–Ni foam heterostructures with enhanced performance and durability for bipolar plates in a polymer electrolyte membrane fuel cell. J. Mater. Chem. A 2017, 6, 1504–1512. [Google Scholar] [CrossRef]
- López-Oyama, A.; Domínguez-Crespo, M.; Torres-Huerta, A.; Onofre-Bustamante, E.; Gámez-Corrales, R.; Cayetano-Castro, N. Electrochemical alternative to obtain reduced graphene oxide by pulse potential: Effect of synthesis parameters and study of corrosion properties. Diam. Relat. Mater. 2018, 88, 167–188. [Google Scholar] [CrossRef]
- Stoot, A.C.; Camilli, L.; Spiegelhauer, S.-A.; Yu, F.; Bøggild, P. Multilayer graphene for long-term corrosion protection of stainless steel bipolar plates for polymer electrolyte membrane fuel cell. J. Power Sources 2015, 293, 846–851. [Google Scholar] [CrossRef]
- Weissbecker, V.; Lehnert, W.; Reimer, U. Bipolar Plate for Electrochemical Cells and Method for the Production Thereof. U.S. Patent 10,418,643 B2, 17 September 2019. [Google Scholar]
- Stein, T.; Ein-Eli, Y. Challenges and perspectives of metal-based proton exchange membrane’s bipolar plates: Exploring durability and longevity. Energy Technol. 2020, 8, 1–13. [Google Scholar] [CrossRef]
- Elyasi, M.; Khatir, F.A.; Hosseinzadeh, M. Manufacturing metallic bipolar plate fuel cells through rubber pad forming process. Int. J. Adv. Manuf. Technol. 2017, 89, 3257–3269. [Google Scholar] [CrossRef]
- Weissbecker, V.; Reimer, U.; Wippermann, K.; Lehnert, W. A Comprehensive Corrosion Study on Metallic Materials for HT-PEFC Application. ECS Trans. 2013, 58, 693–704. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Guo, P.; Zhang, D.; Liu, L.; Wang, Z.; Ma, G.; Xin, Y.; Ke, P.; Saito, H.; Wang, A. Interface-induced degradation of amorphous carbon films/stainless steel bipolar plates in proton exchange membrane fuel cells. J. Power Sources 2020, 469, 228269. [Google Scholar] [CrossRef]
- Cooper, L.; El-Kharouf, A. Titanium Nitride Polyaniline Bilayer Coating for Metallic Bipolar Plates used in Polymer Electrolyte Fuel Cells. Fuel Cells 2020, 20, 453–460. [Google Scholar] [CrossRef]
- Khan, M.F.; Adesina, A.Y.; Gasem, Z.M. Electrochemical and electrical resistance behavior of cathodic arc PVD TiN, CrN, AlCrN, and AlTiN coatings in simulated proton exchange membrane fuel cell environment. Mater. Corros. 2019, 70, 281–292. [Google Scholar] [CrossRef]
- Marzo, F.; Alberro, M.; Manso, A.; Garikano, X.; Alegre, C.; Montiel, M.; Lozano, A.; Barreras, F. Evaluation of the corrosion resistance of Ni(P)Cr coatings for bipolar plates by electrochemical impedance spectroscopy. Int. J. Hydrogen Energy 2020, 45, 20632–20646. [Google Scholar] [CrossRef]
- Zhang, P.; Han, Y.T.; Shi, J.F.; Li, T.; Wang, H.Y.; Sun, J.C. ZrC Coating Modified Ti Bipolar Plate for Proton Exchange Membrane Fuel Cell. Fuel Cells 2020, 20, 540–546. [Google Scholar] [CrossRef]
- Gao, P.; Xie, Z.; Wu, X.; Ouyang, C.; Lei, T.; Yang, P.; Liu, C.; Wang, J.; Ouyang, T.; Huang, Q. Development of Ti bipolar plates with carbon/PTFE/TiN composites coating for PEMFCs. Int. J. Hydrogen Energy 2018, 43, 20947–20958. [Google Scholar] [CrossRef]
- Xu, J.; Huang, H.J.; Li, Z.; Xu, S.; Tao, H.; Munroe, P.; Xie, Z.-H. Corrosion behavior of a ZrCN coated Ti alloy with potential application as a bipolar plate for proton exchange membrane fuel cell. J. Alloys Compd. 2016, 663, 718–730. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.-J.; Kim, M.-G.; Kim, S.; Kim, Y.C.; Seo, H.W.; Cho, J.K.; Park, I.-K.; Suhr, J.; Moon, H.; Koo, J.C.; et al. Cathodic electrophoretic deposition (EPD) of phenylenediamine-modified graphene oxide (GO) for anti-corrosion protection of metal surfaces. Carbon 2019, 142, 68–77. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Yu, P.; Ma, Y. Stable dispersions of graphene and highly conducting graphene films: A new approach to creating colloids of graphene monolayers. Chem. Commun. 2009, 4527–4529. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.-L.; Zhang, H.-B.; Hu, Q.-H.; Li, W.-J.; Jiang, Z.-G.; Yu, Z.-Z.; Dasari, A. Functionalization and Reduction of Graphene Oxide with p-Phenylene Diamine for Electrically Conductive and Thermally Stable Polystyrene Composites. ACS Appl. Mater. Interfaces 2012, 4, 1948–1953. [Google Scholar] [CrossRef]
- Li, M.-J.; Liu, C.-M.; Xie, Y.-B.; Cao, H.-B.; Zhao, H.; Zhang, Y. The evolution of surface charge on graphene oxide during the reduction and its application in electroanalysis. Carbon 2014, 66, 302–311. [Google Scholar] [CrossRef]
- Baskoro, F.; Wong, C.-B.; Kumar, S.R.; Chang, C.-W.; Chen, C.-H.; Chen, D.W.; Lue, S.J. Graphene oxide-cation interaction: Inter-layer spacing and zeta potential changes in response to various salt solutions. J. Membr. Sci. 2018, 554, 253–263. [Google Scholar] [CrossRef]
- Feng, J.; Ye, Y.; Xiao, M.; Wu, G.; Ke, Y. Synthetic routes of the reduced graphene oxide. Chem. Pap. 2020, 74, 3767–3783. [Google Scholar] [CrossRef]
- Everett, D.H. Basic Principles of Colloid Science; Royal Society of Chemistry (RSC): London, UK, 2007; pp. 9–15. [Google Scholar]
- Wang, C. Novel Structured Metal Bipolar Plates for Low Cost Manufacturing; US Department of Energy Hydrogen and Fuel Cells Program 2015: Anaheim, CA, USA, 2015.
- Zeng, Y.; He, Z.; Hua, Q.; Xu, Q.; Min, Y. Polyacrylonitrile Infused in a Modified Honeycomb Aluminum Alloy Bipolar Plate and Its Acid Corrosion Resistance. ACS Omega 2020, 5, 16976–16985. [Google Scholar] [CrossRef] [PubMed]
- Gago, A.; Ansar, S.; Saruhan, B.; Schulz, U.; Lettenmeier, P.; Cañas, N.A.; Gazdzicki, P.; Morawietz, T.; Hiesgen, R.; Arnold, J.; et al. Protective coatings on stainless steel bipolar plates for proton exchange membrane (PEM) electrolysers. J. Power Sources 2016, 307, 815–825. [Google Scholar] [CrossRef] [Green Version]
- Haynes, W. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2014; pp. 12–235. [Google Scholar]



| Samples | Anode | Cathode | ||
|---|---|---|---|---|
| Icorr (A·cm−2) | Ecorr (V) | Icorr (A·cm−2) | Ecorr (V) | |
| Ti-Unpretreated | 9.78 × 10−6 | −0.496 | 4.95 × 10−6 | −0.128 |
| Ti-Pretreated | 1.88 × 10−5 | −0.561 | 2.91 × 10−5 | −0.295 |
| MGO@Ti | 3.37 × 10−6 | −0.333 | 1.58 × 10−6 | 0.067 |
| RMGO@Ti | 7.55 × 10−7 | −0.177 | 7.52 × 10−7 | 0.019 |
| Samples | Icorr (A·cm−2) | |
|---|---|---|
| Anode | Cathode | |
| Ti-Unpretreated | 7.41 × 10−6 | 4.30 × 10−6 |
| Ti-Pretreated | 2.03 × 10−5 | 1.66 × 10−5 |
| MGO@Ti | 1.22 × 10−6 | 2.09 × 10−6 |
| RMGO@Ti | 2.64 × 10−7 | 2.94 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Min, L.; Zhang, W.; Wang, Y. High-Performance Graphene Coating on Titanium Bipolar Plates in Fuel Cells via Cathodic Electrophoretic Deposition. Coatings 2021, 11, 437. https://doi.org/10.3390/coatings11040437
Liu Y, Min L, Zhang W, Wang Y. High-Performance Graphene Coating on Titanium Bipolar Plates in Fuel Cells via Cathodic Electrophoretic Deposition. Coatings. 2021; 11(4):437. https://doi.org/10.3390/coatings11040437
Chicago/Turabian StyleLiu, Yi, Luofu Min, Wen Zhang, and Yuxin Wang. 2021. "High-Performance Graphene Coating on Titanium Bipolar Plates in Fuel Cells via Cathodic Electrophoretic Deposition" Coatings 11, no. 4: 437. https://doi.org/10.3390/coatings11040437
APA StyleLiu, Y., Min, L., Zhang, W., & Wang, Y. (2021). High-Performance Graphene Coating on Titanium Bipolar Plates in Fuel Cells via Cathodic Electrophoretic Deposition. Coatings, 11(4), 437. https://doi.org/10.3390/coatings11040437







