Influence of Microdefect Size on Corrosion Behavior of Epoxy-Coated Rebar for Application in Seawater-Mixed Concrete
Abstract
1. Introduction
2. Experimental
2.1. Materials and Sample Preparation
2.2. Experimental Condition
2.3. Electrochemical Measurements
2.4. LEIS and SVET Measurements
2.5. Microstructure and Chemical Analyses
3. Results and Discussion
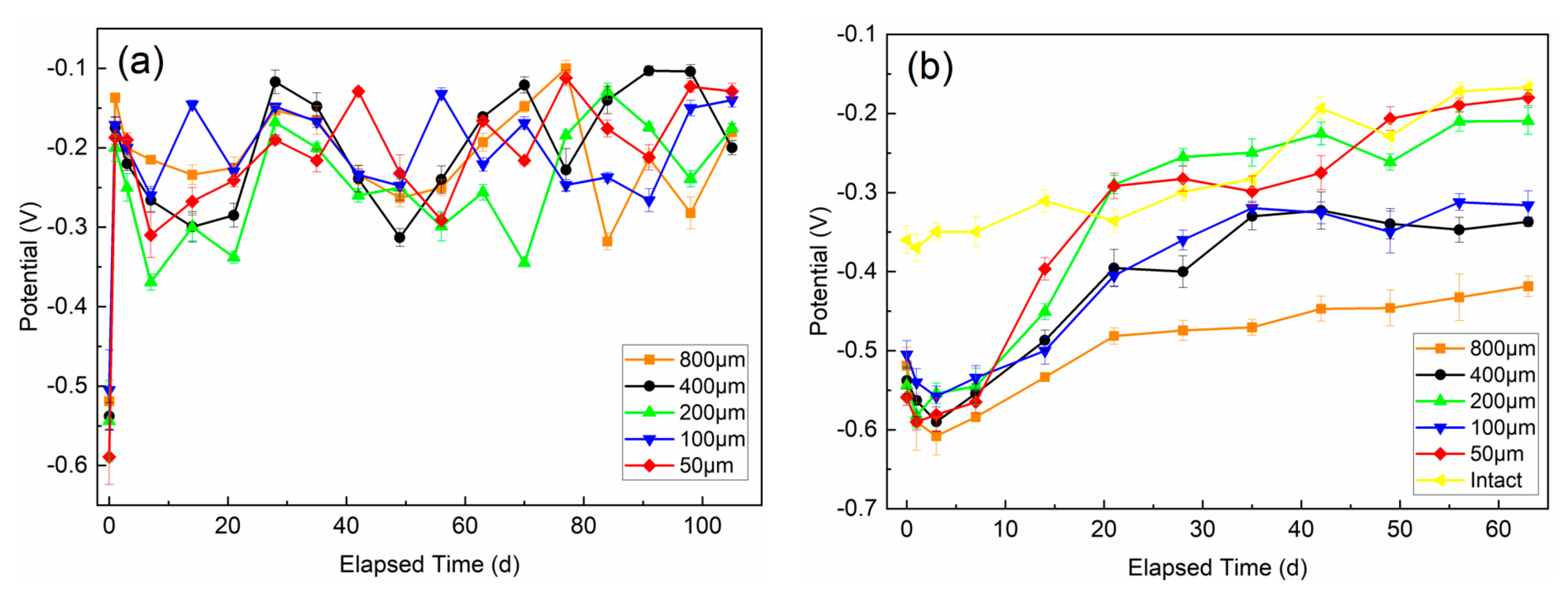
3.1. Electrochemical Properties
3.1.1. Potentiodynamic Polarization Curves
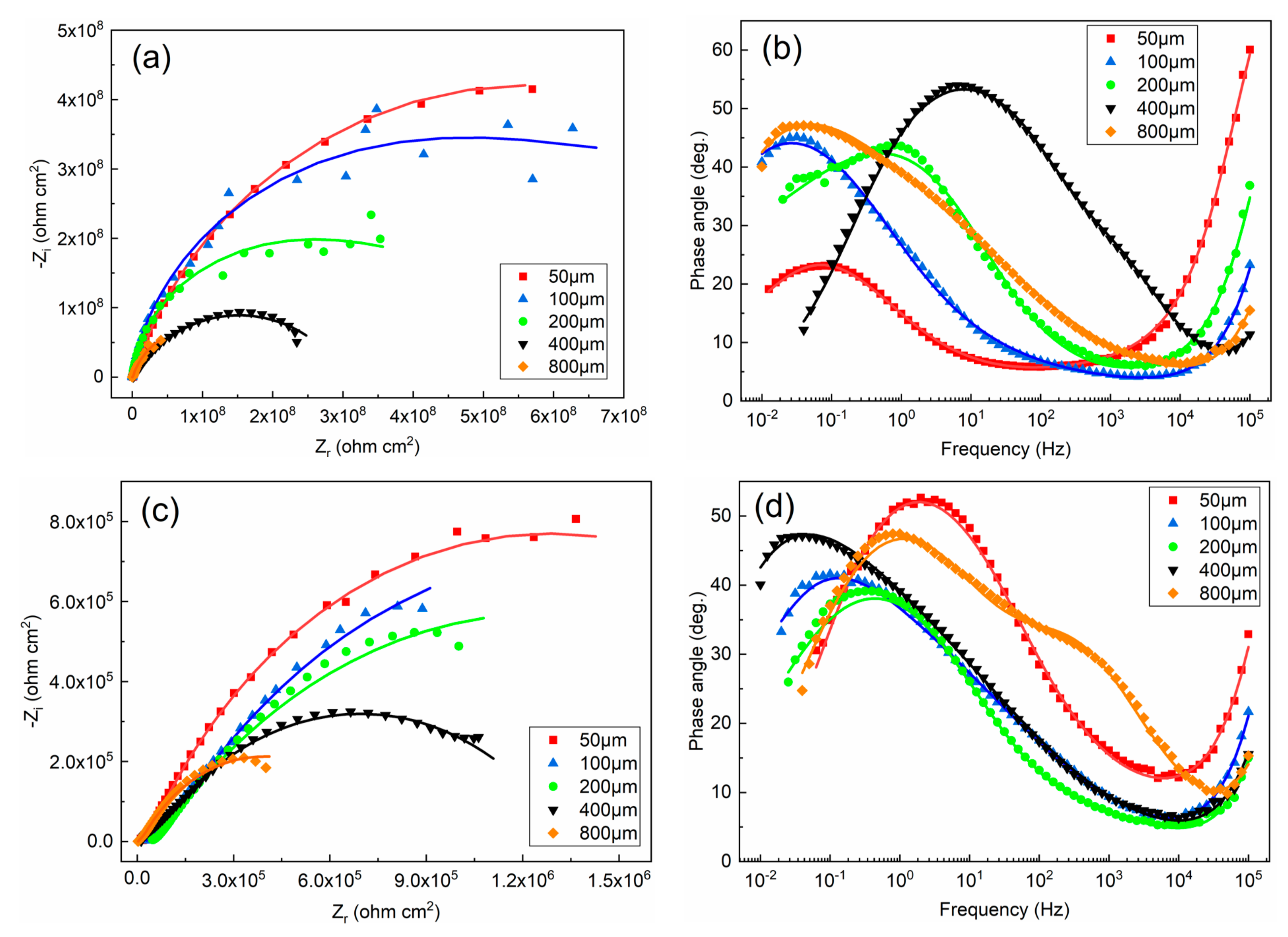
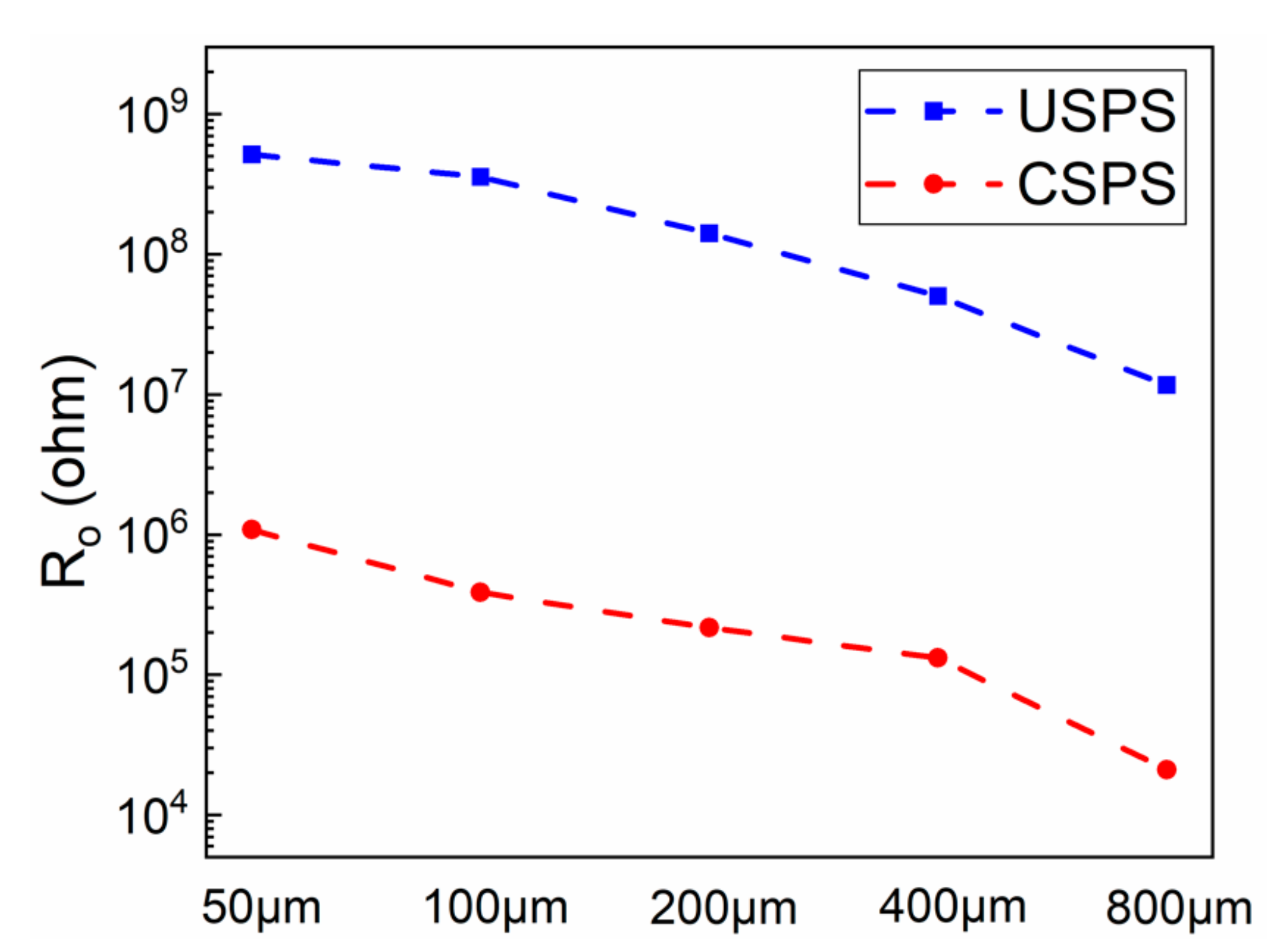
3.1.2. EIS Test Results
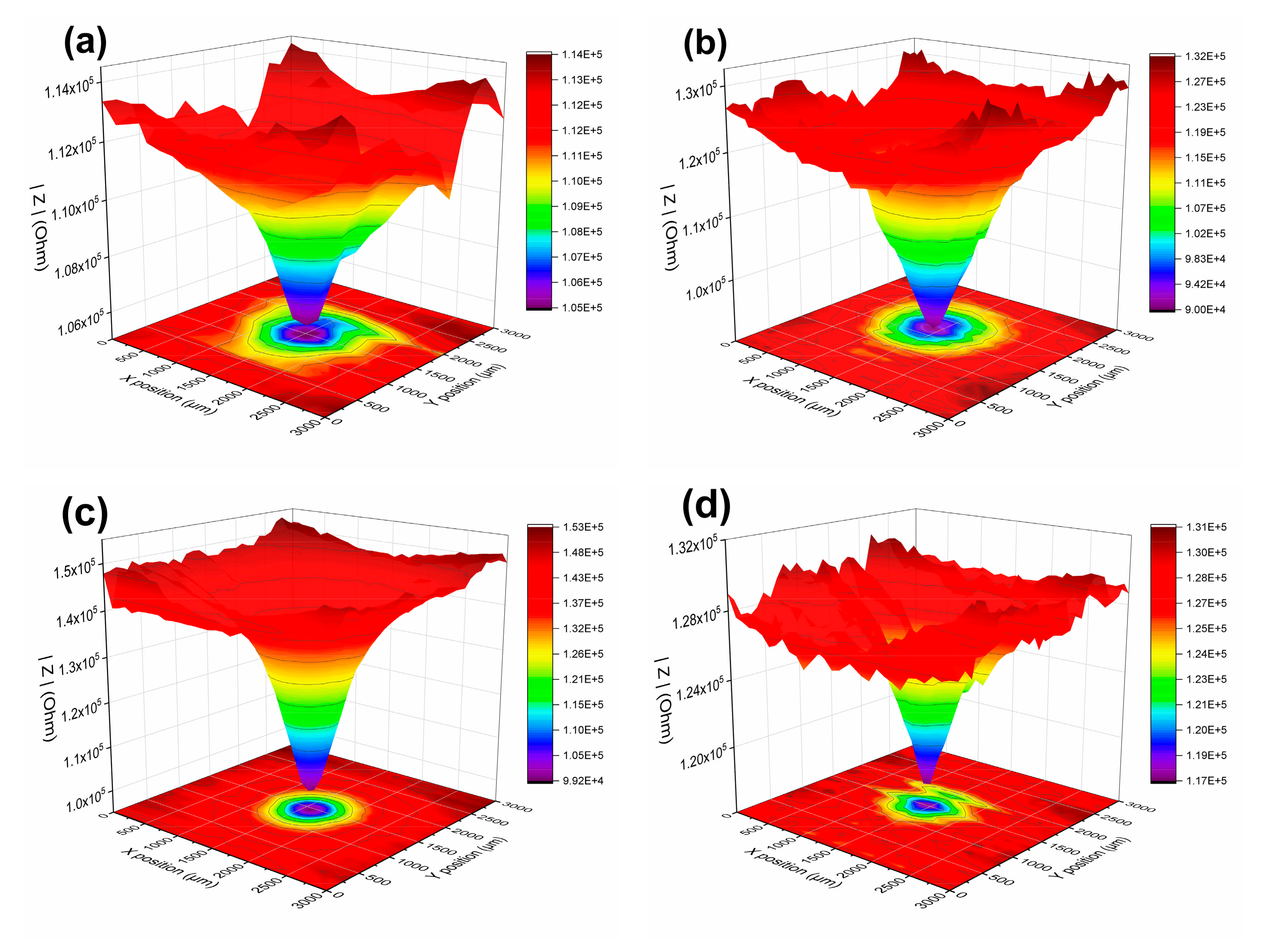
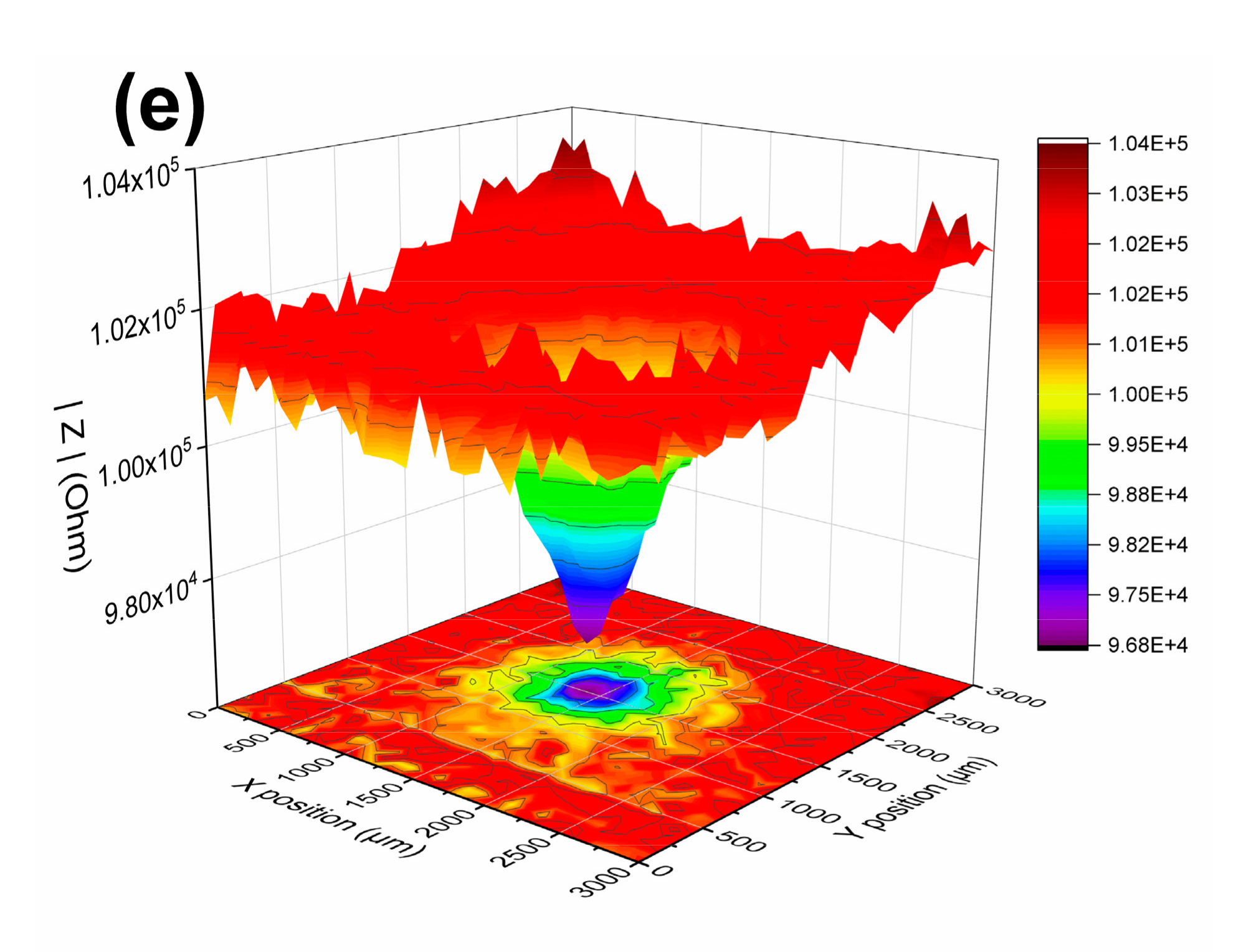
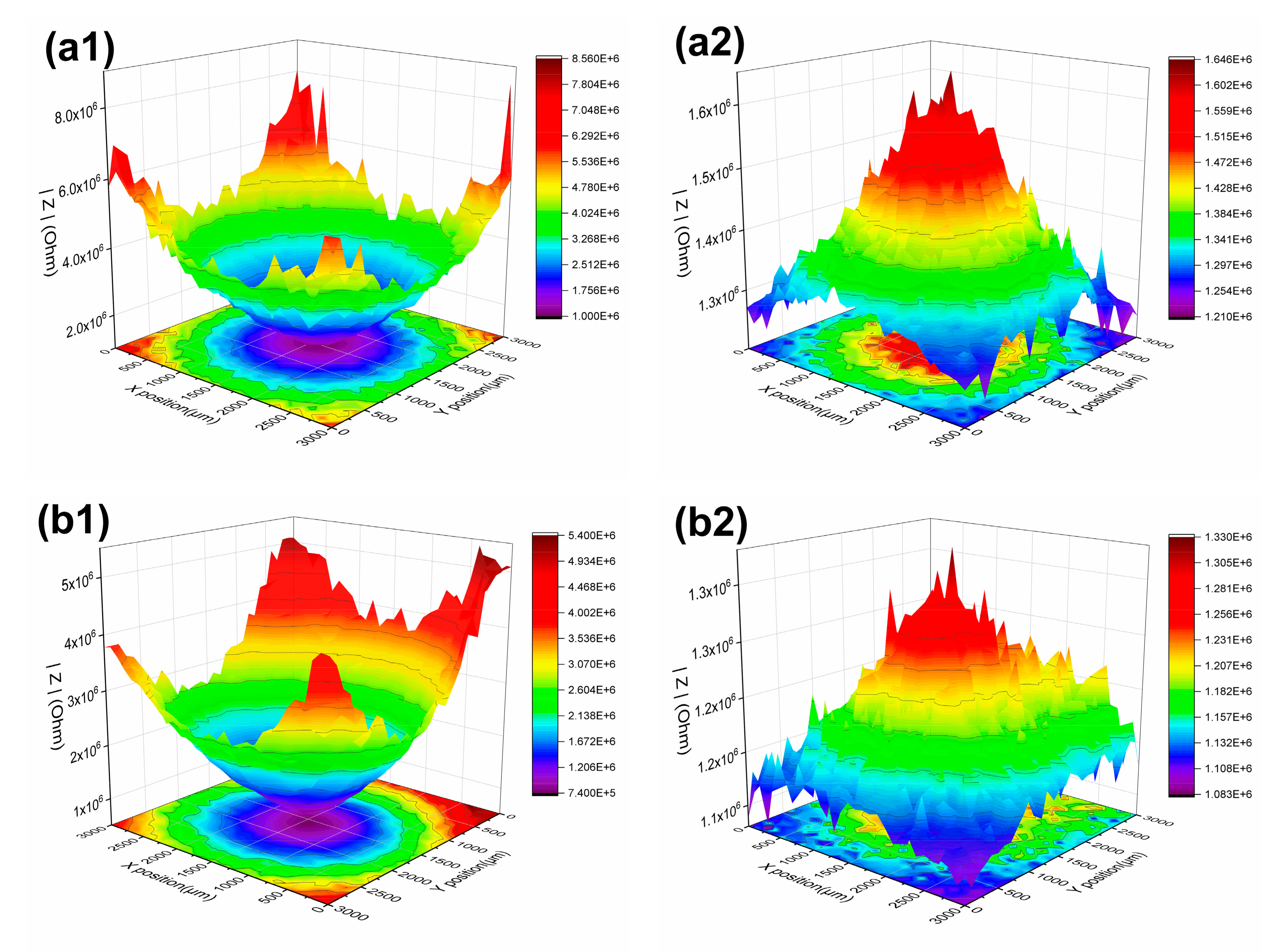
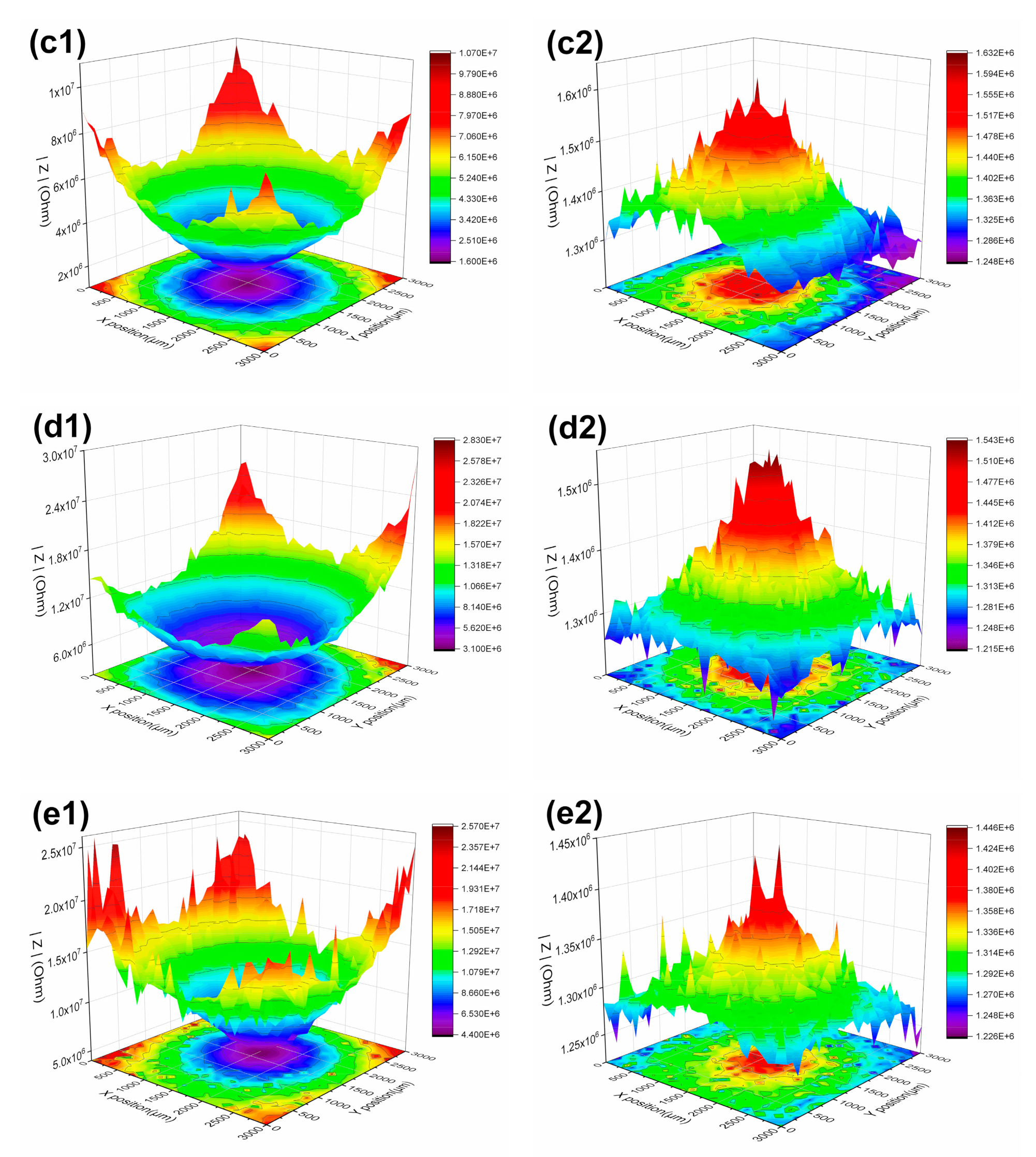
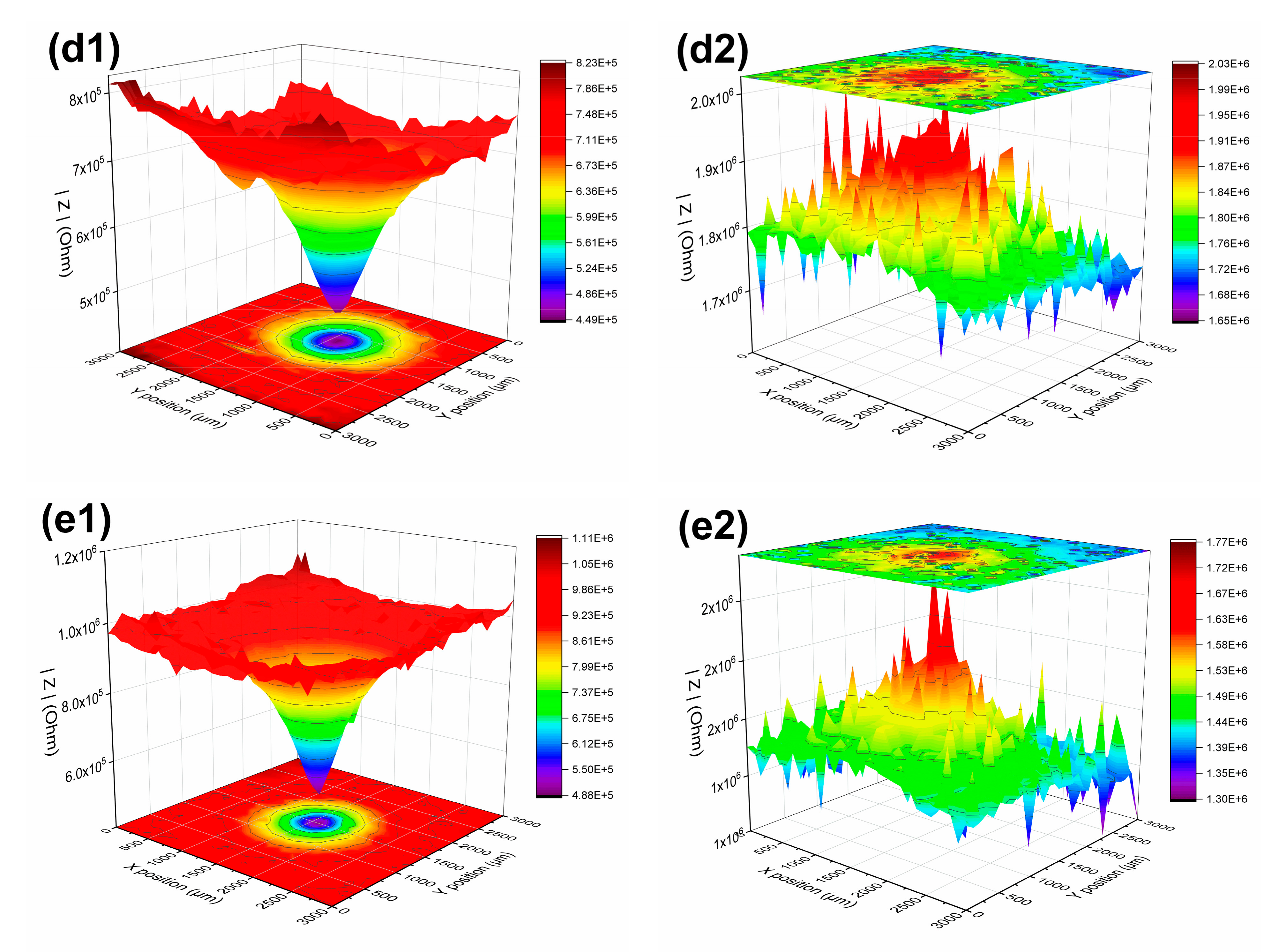
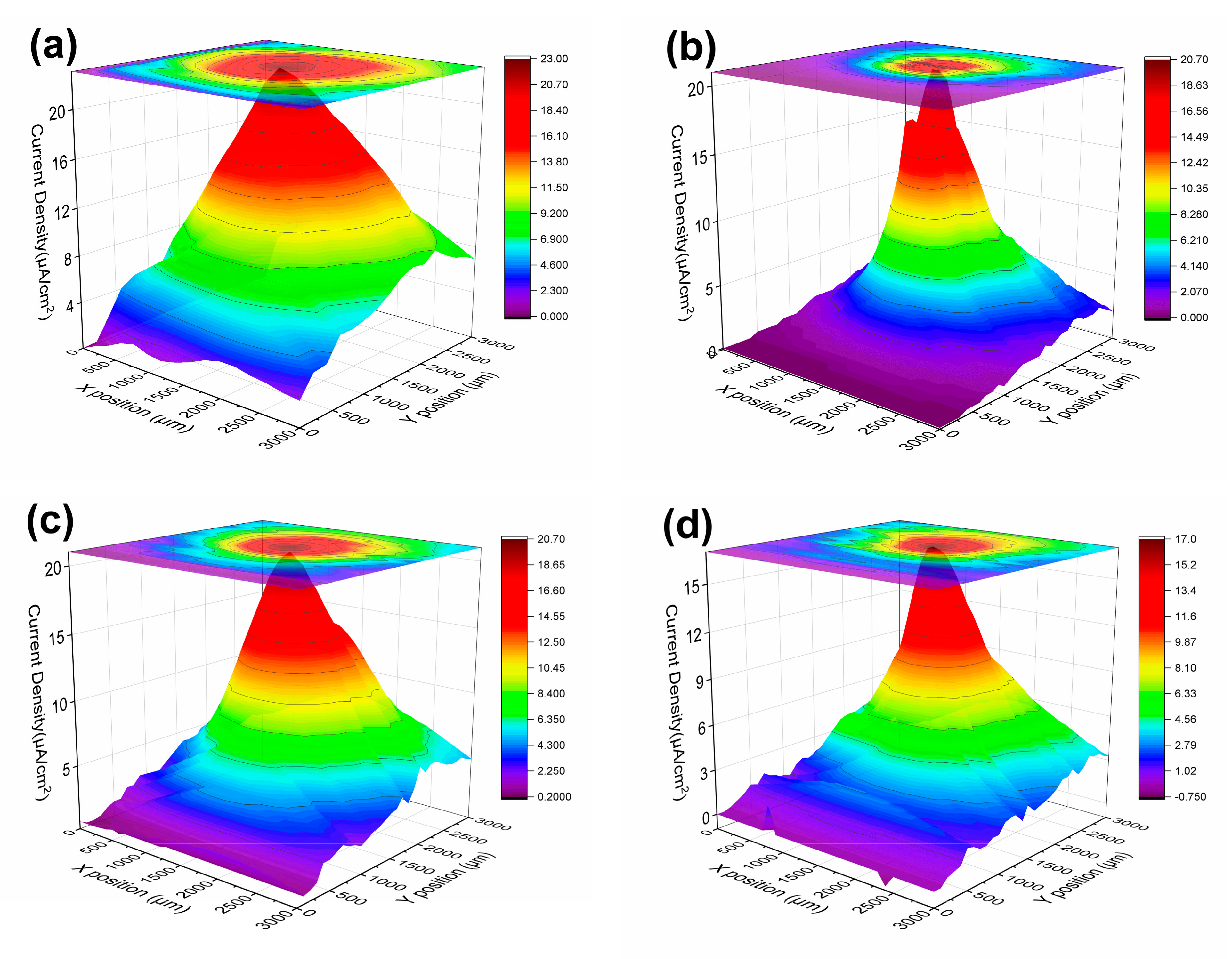
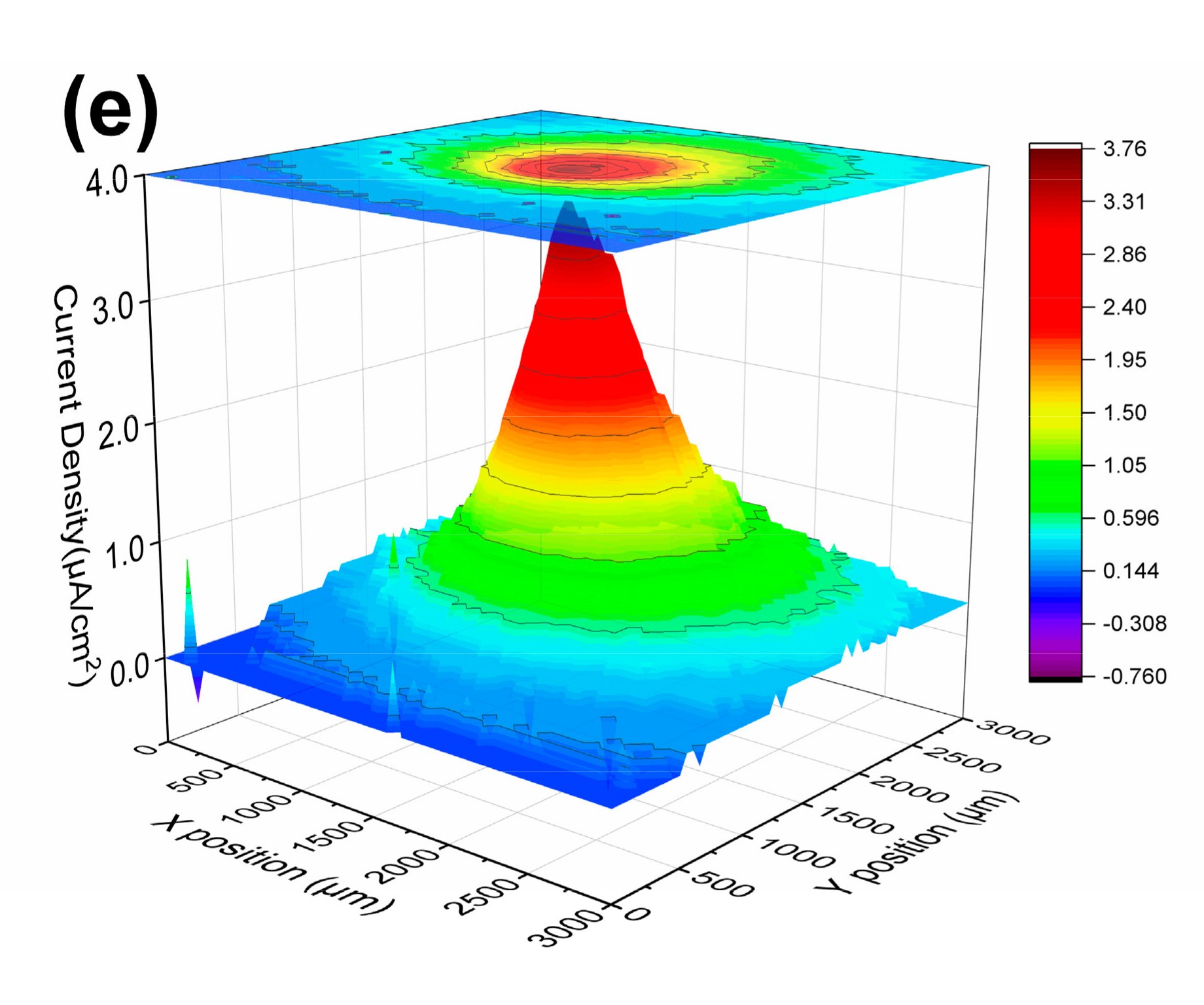
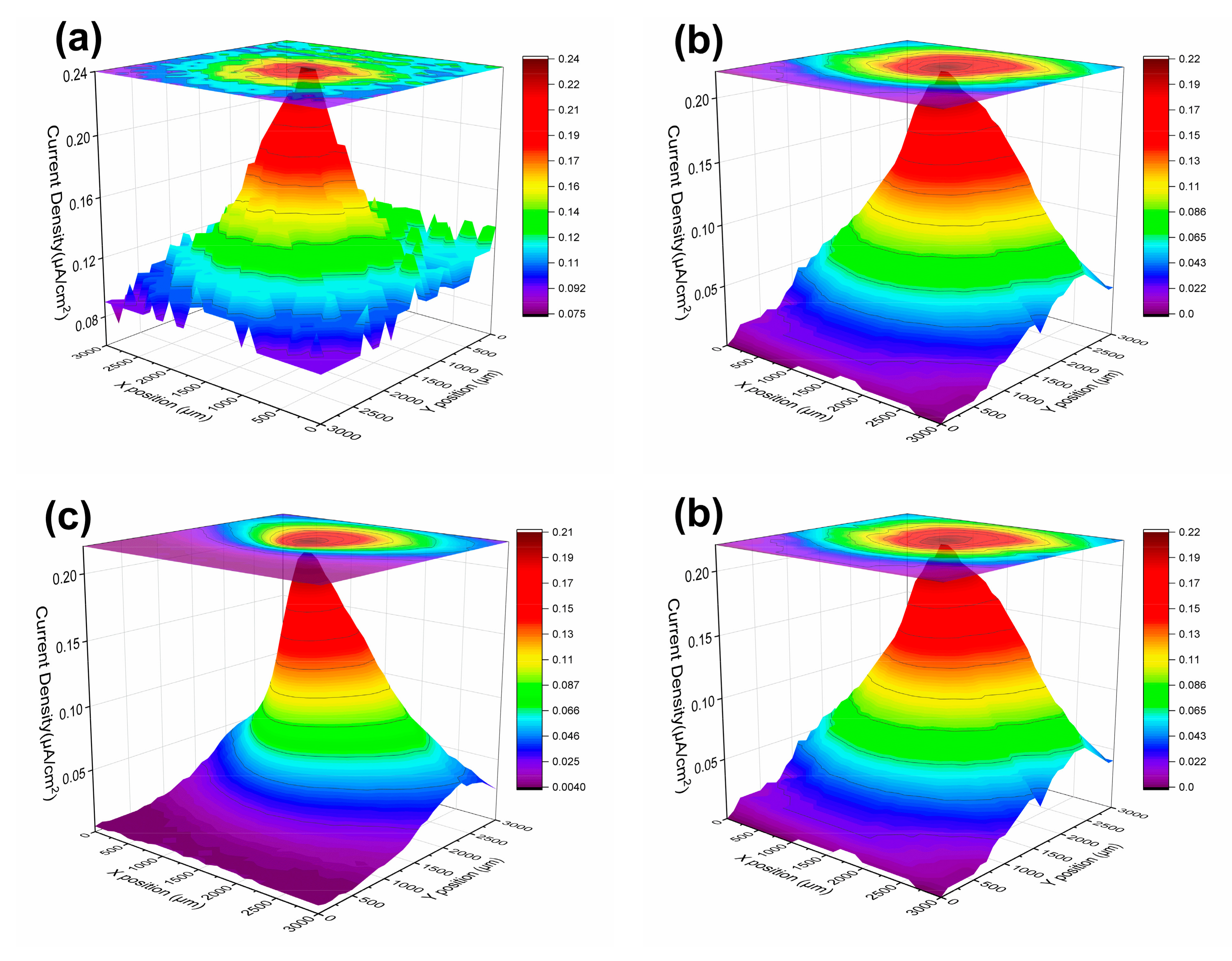
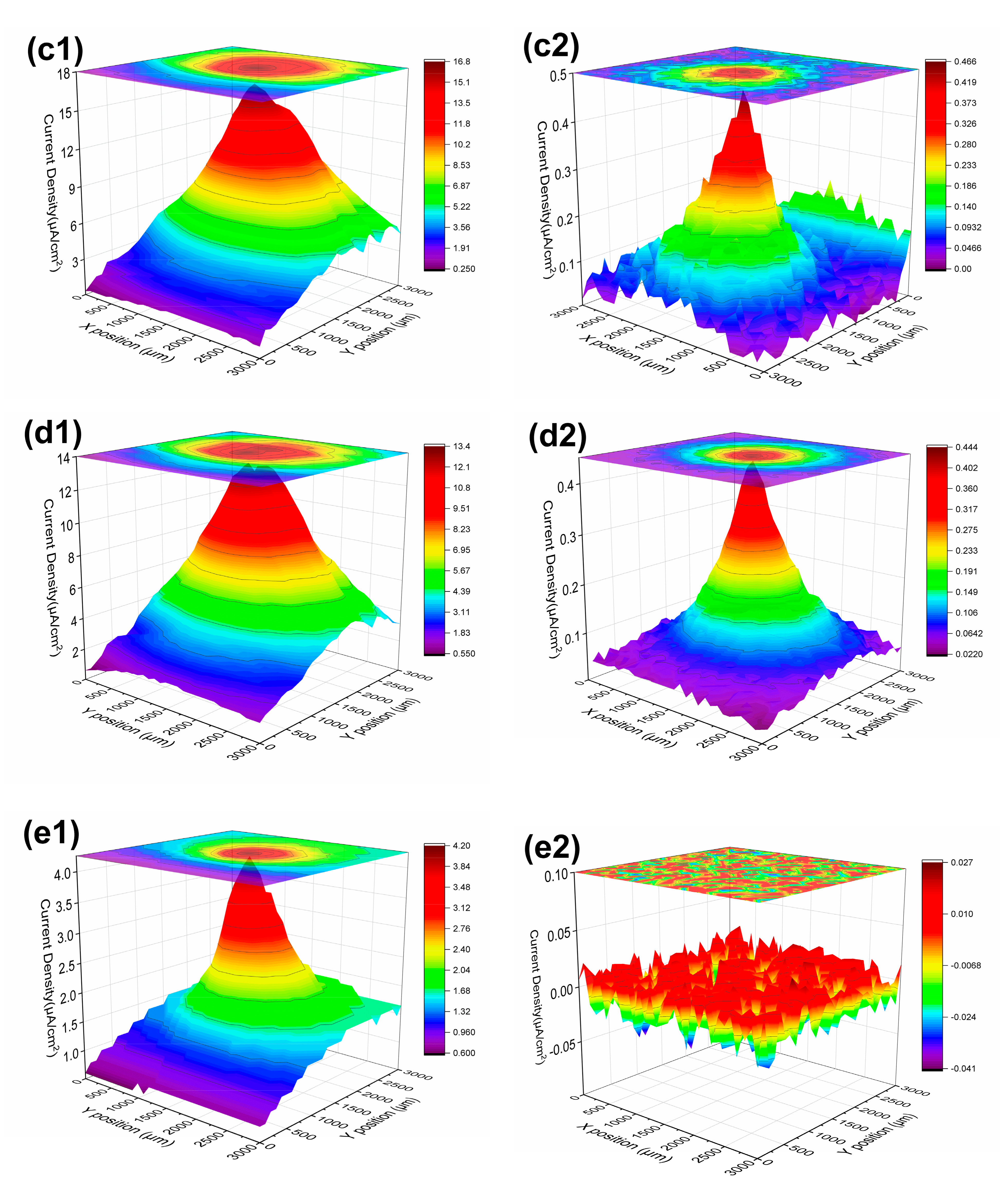
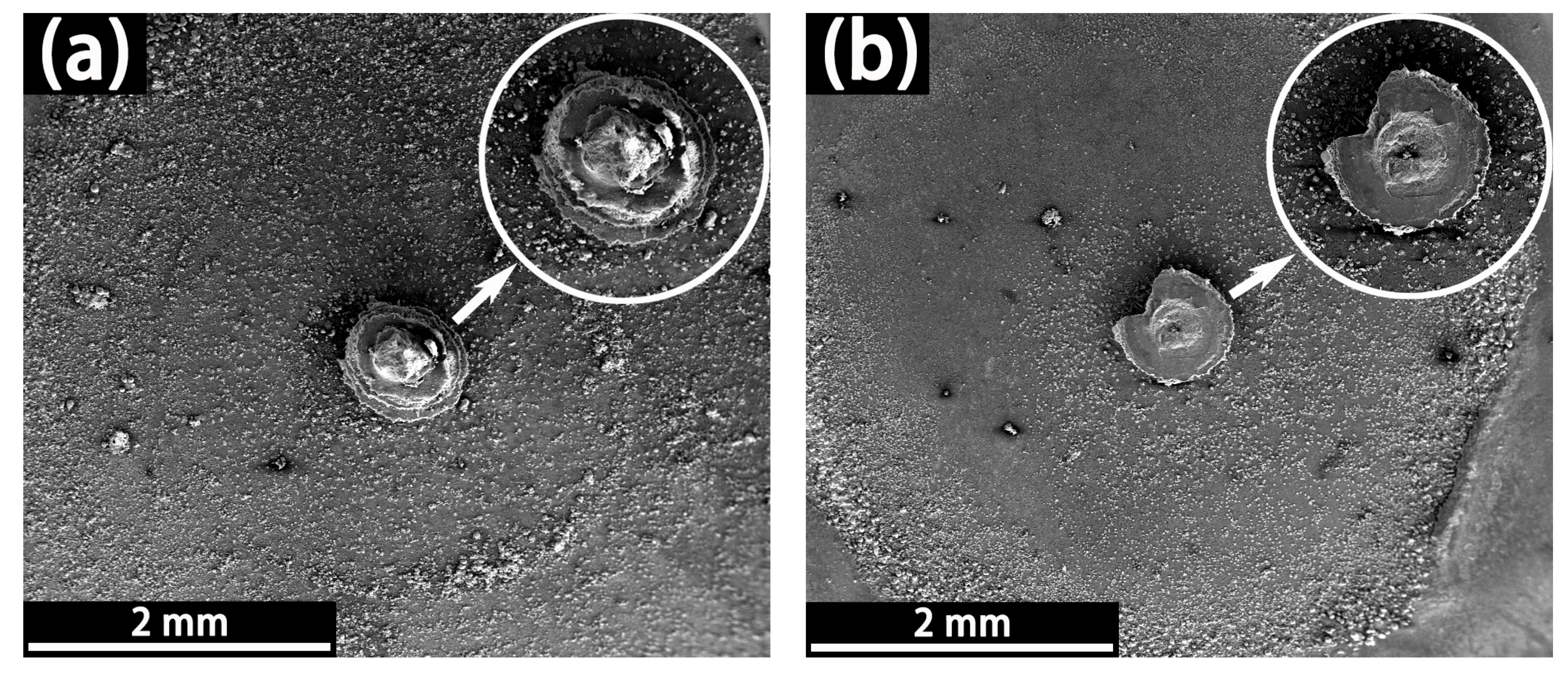
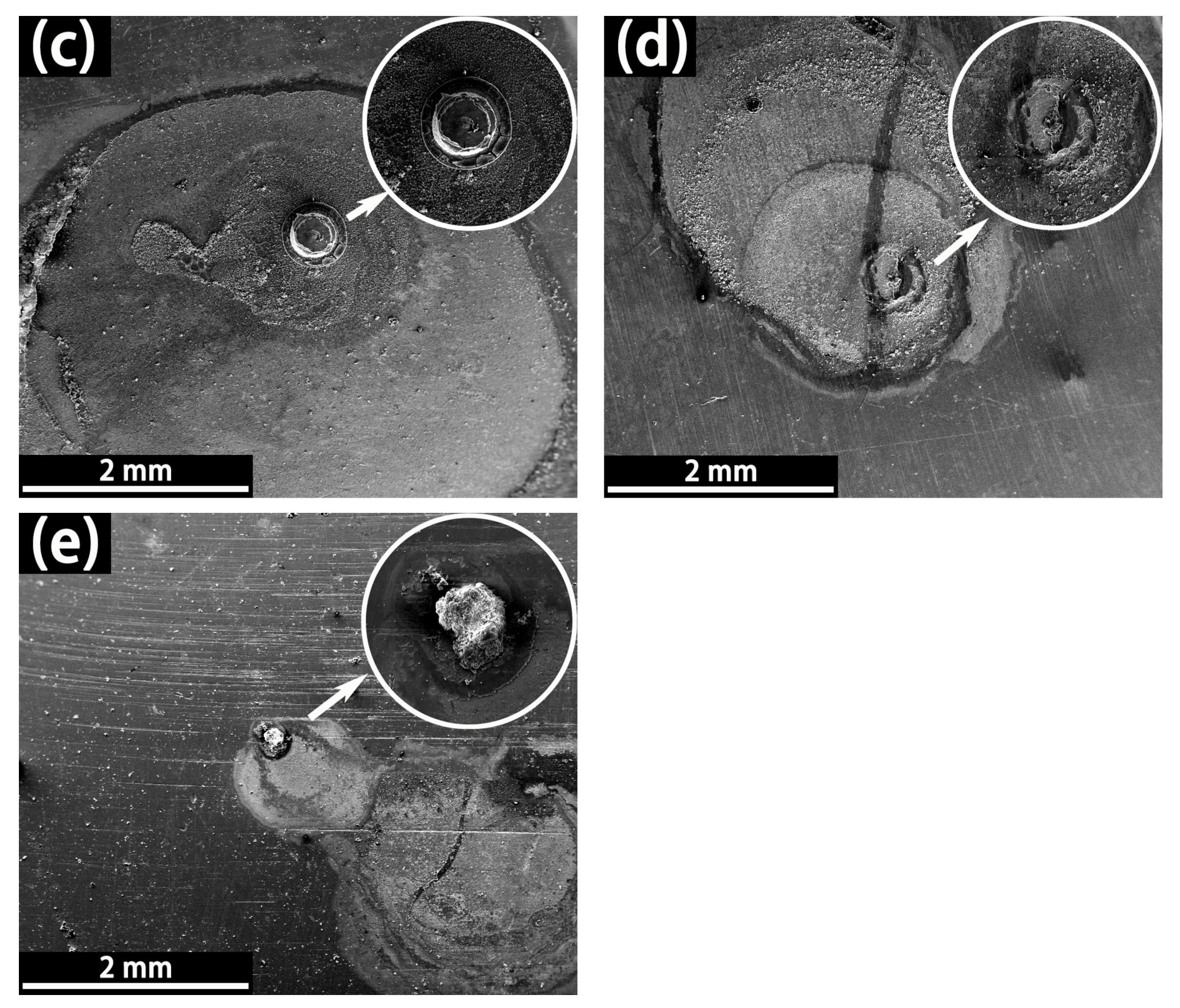
3.1.3. LEIS and SVET Test Results
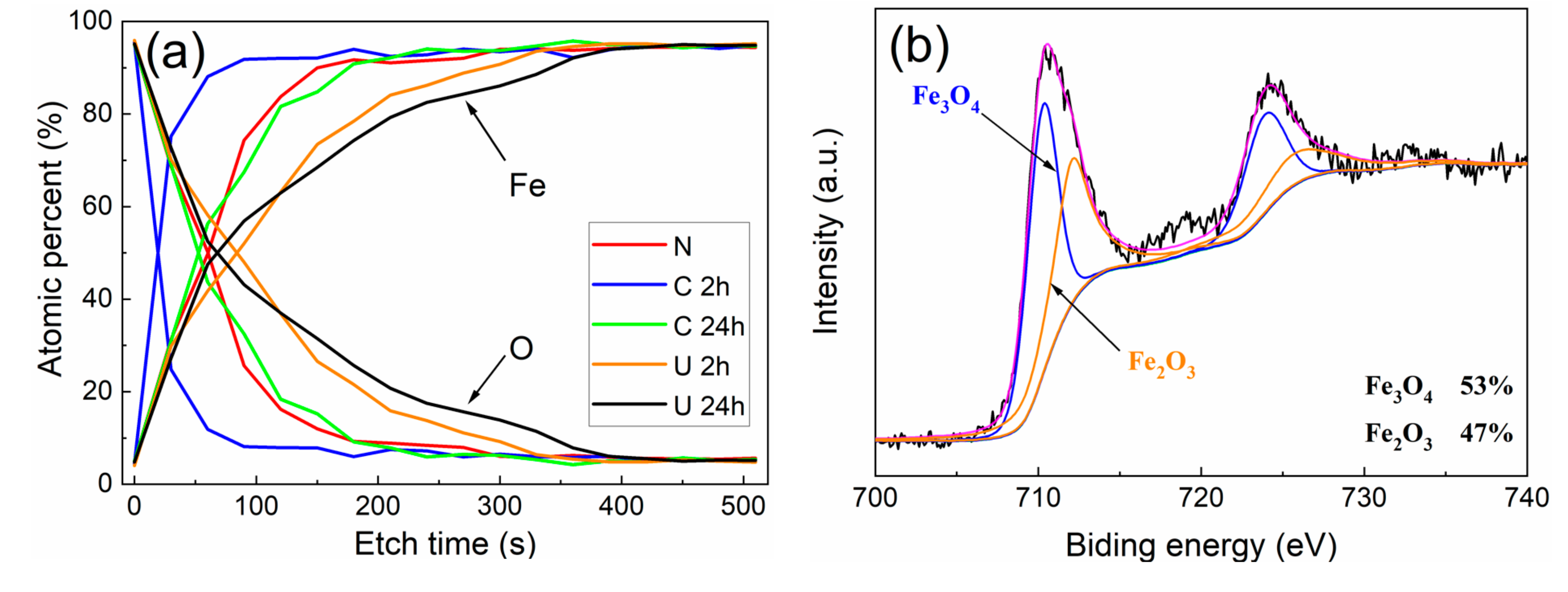
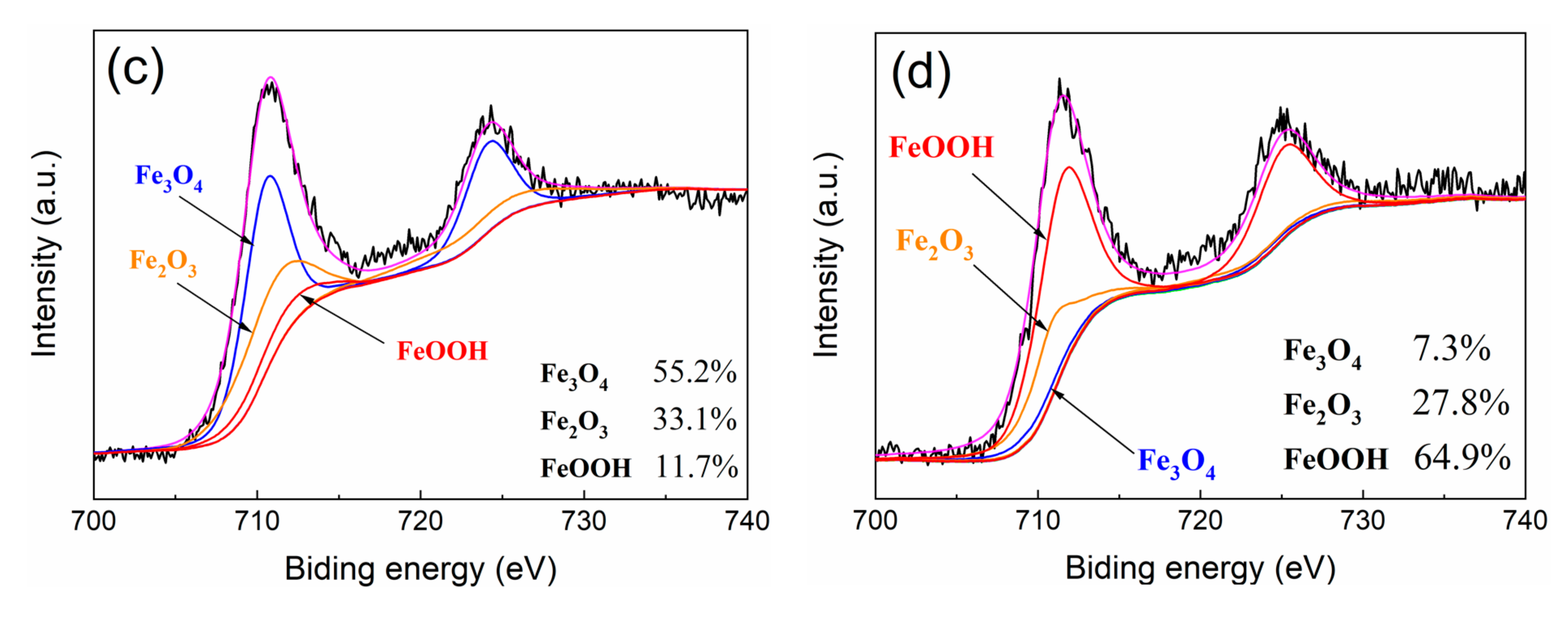
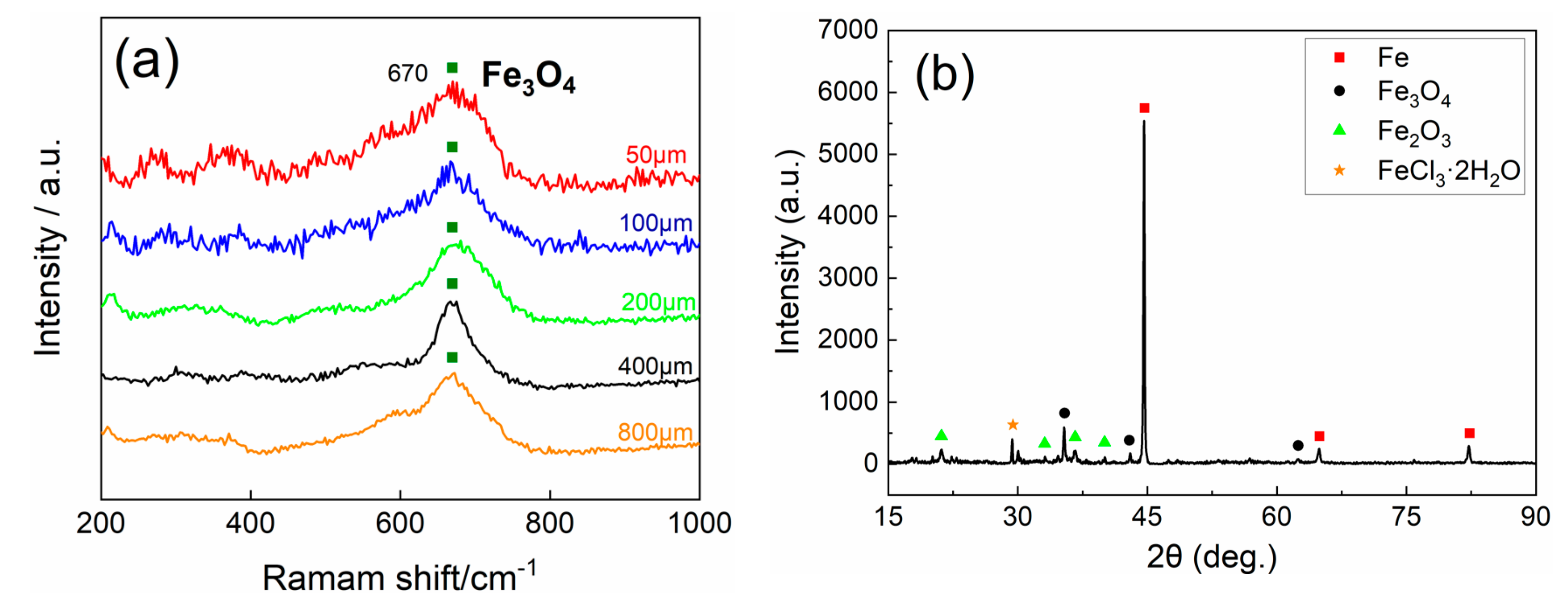
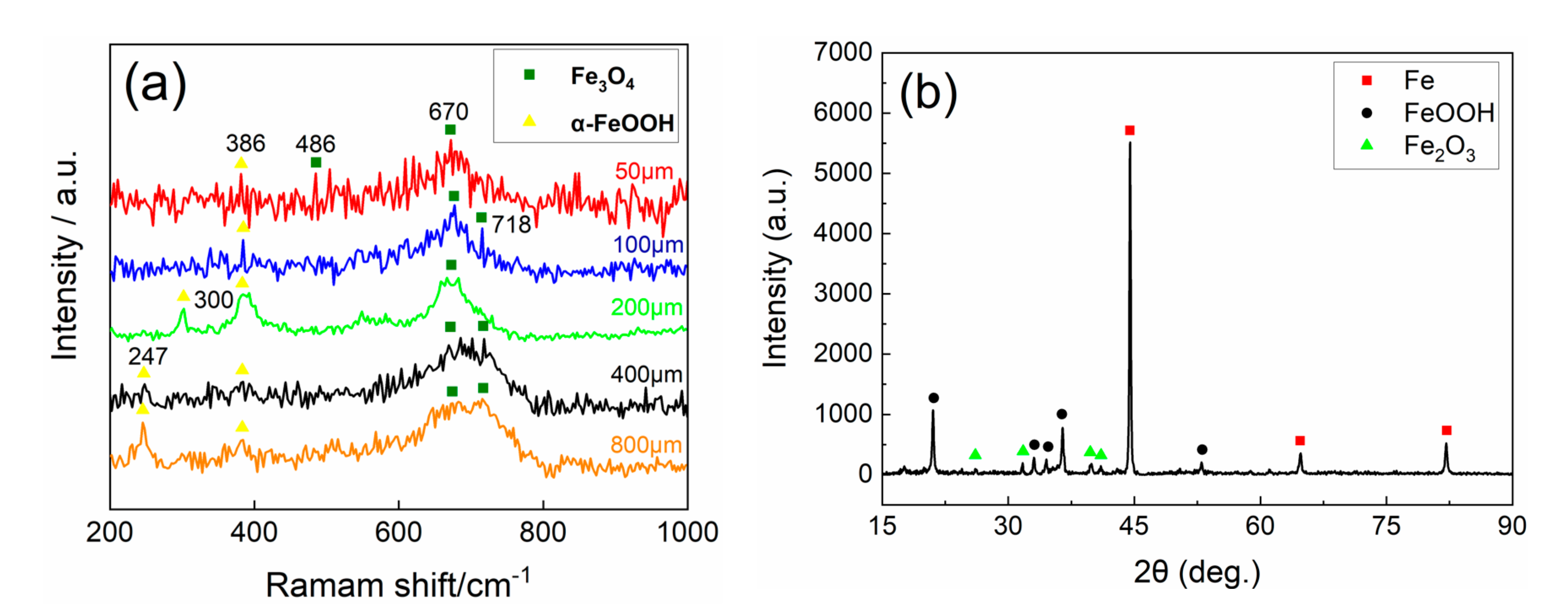
3.2. Corrosion Products Analysis
4. Conclusions
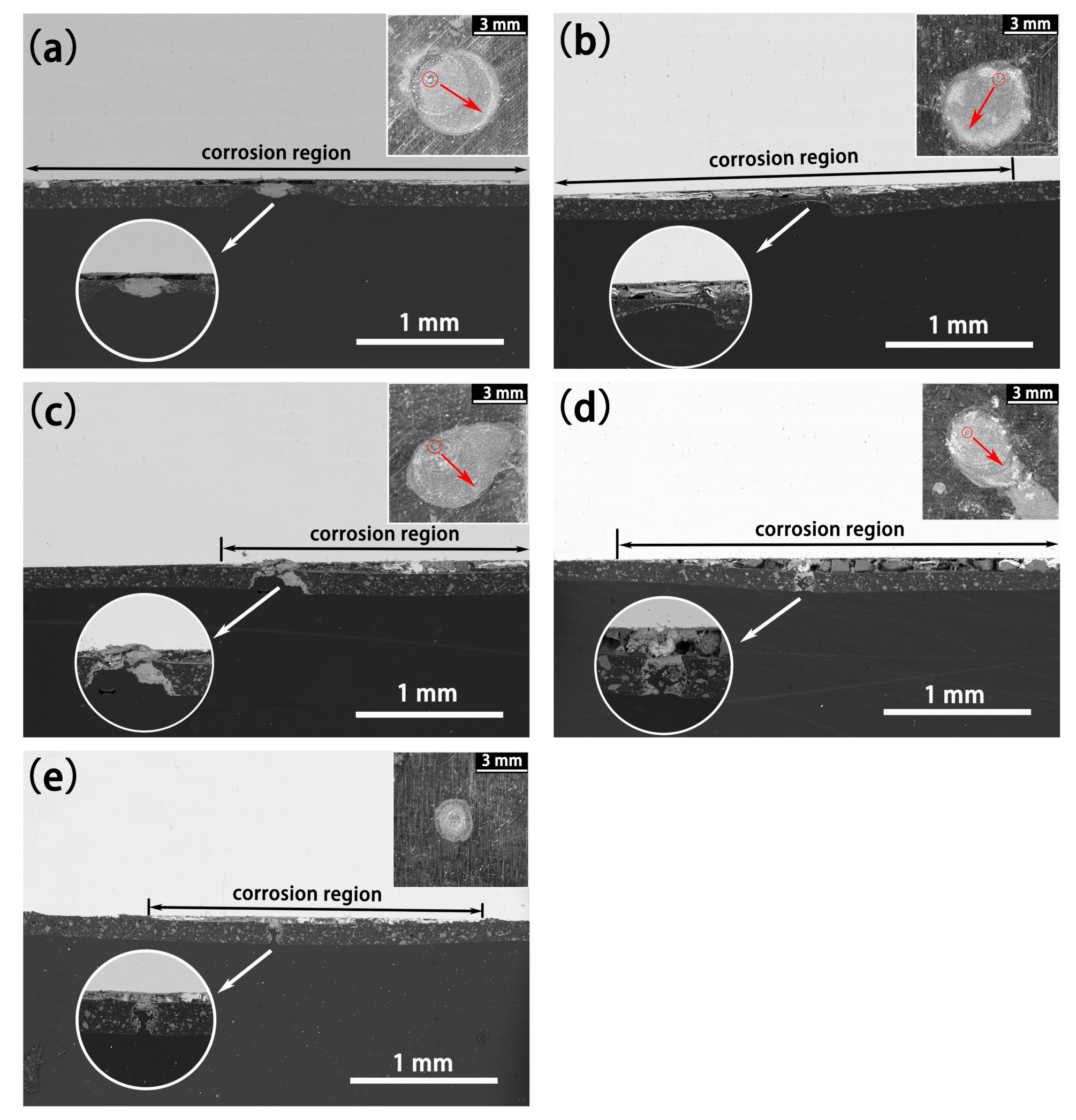
- The defect size of epoxy-coated rebar is a crucial influence on the corrosion behavior. In the same conditions, the corrosion of S800~S100 was much more severe than that of S50. This indicates that the defect with a diameter of 50 μm might be a threshold. The deterioration of substrate and coatings caused by the defect smaller than 50 μm in diameter is much less than that of larger defects.
- The corrosion mechanism in both SPS is crevice corrosion rather than pitting in this experiment, though there are microdefects on the coatings. The corrosion process is autocatalysis and self-sustaining in CSPS.
- Specimens in USPS acquire a better corrosion resistance than those in CSPS, which means the high alkalinity of concrete can effectively alleviate the erosion of Cl−.
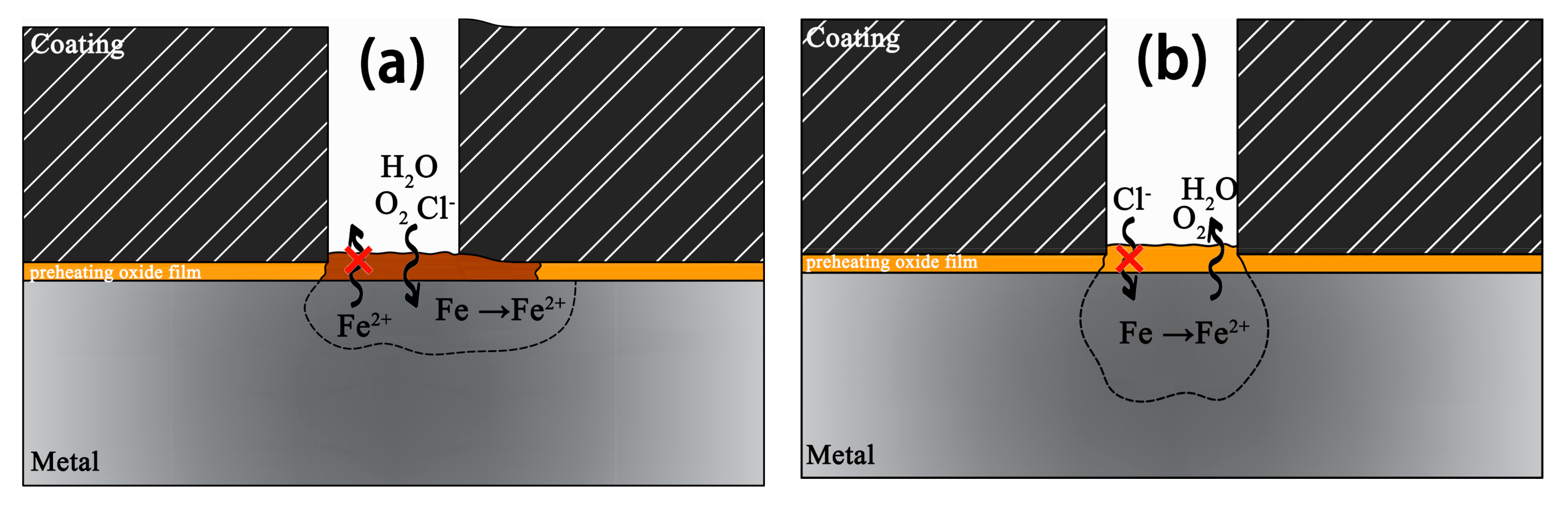
- The corrosion products do have a prominent influence on corrosion behavior. Experiments indicate that α-FeOOH and β-FeOOH exhibit the anion ion-selective property in the corrosion process. The existence of FeOOH facilitates the penetration of Cl− to the substrate and hinders the diffusion of Fe2+ to the rust–solution interface. The enrichment of iron ions and chloridion under the rust induce the crevice corrosion.
- It is speculated that Fe3O4 has the cation ion-selective property, hindering the invasion of Cl− and promoting the penetration of iron ions to the rust–solution interface. Iron ions react with O2, and the new Fe3O4 layer generates on the rust, which protects the substrate ulteriorly.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Li, Y.; Zhao, X.; Singh, R.R.; Al-Saadi, S. Experimental study on seawater and sea sand concrete filled GFRP and stainless steel tubular stub columns. Thin-Walled Struct. 2016, 106, 390–406. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.-L.; Xian, G.; Wu, G.; Raman, R.S.; Al-Saadi, S.; Haque, A. Long-term durability of basalt- and glass-fibre reinforced polymer (BFRP/GFRP) bars in seawater and sea sand concrete environment. Constr. Build. Mater. 2017, 139, 467–489. [Google Scholar] [CrossRef]
- Ann, K.Y.; Song, H.-W. Chloride threshold level for corrosion of steel in concrete. Corros. Sci. 2007, 49, 4113–4133. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S.; Fedel, M. Organic coatings degradation: Comparison between natural and artificial weathering. Corros. Sci. 2008, 50, 2360–2366. [Google Scholar] [CrossRef]
- Xia, D.H.; Wang, J.; Wu, Z.; Qin, Z.; Xu, L.; Hu, W.; Behnamian, Y.; Luo, J.L. Sensing corrosion within an artificial defect in or-ganic coating using SECM. Sens. Actuators B Chem. 2019, 280, 235–242. [Google Scholar] [CrossRef]
- Rosales, B.M.; Sarli, A.R.D.; Rincón, O.d.; Rincón, A.; Elsner, C.I.; Marchisio, B. An evaluation of coil coating formulations in marine environments. Prog. Org. Coat. 2004, 50, 105–114. [Google Scholar] [CrossRef]
- Zayed, A.M.; Sagues, A.A. Corrosion at surface damage on an epoxy-coated reinforcing steel. Corros. Sci. 1990, 30, 1025–1044. [Google Scholar] [CrossRef]
- Fernández-Álvarez, M.; Velasco, F.; Bautista, A.; Gonzalez-Garcia, Y.; Galiana, B. Corrosion Protection in Chloride Environments of Nanosilica Containing Epoxy Powder Coatings with Defects. J. Electrochem. Soc. 2020, 167, 161507. [Google Scholar] [CrossRef]
- Funke, W. Blistering of paint films and filiform corrosion. Prog. Org. Coat. 1981, 9, 29–46. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Zheng, Y. Electrochemical techniques for determining corrosion rate of rusted steel in seawater. Corros. Sci. 2011, 53, 208–216. [Google Scholar] [CrossRef]
- Leng, A.; Streckel, H.; Stratmann, M. The delamination of polymeric coatings from steel. Part 1: Calibration of the Kelvinprobe and basic delamination mechanism. Corros. Sci. 1998, 41, 547–578. [Google Scholar] [CrossRef]
- Deflorian, F.; Rossi, S. An EIS study of ion diffusion through organic coatings. Electrochim. Acta 2006, 51, 1736–1744. [Google Scholar] [CrossRef]
- Leidheiser, H.L., Jr.; Wang, W.; Igetoft, L. The mechanism for the cathodic delamination of organic coatings from a metal surface. Prog. Org. Coat. 1983, 11, 19–40. [Google Scholar] [CrossRef]
- Mao, Y.-Z.; Wei, Y.-H.; Zhao, H.-T.; Lv, C.-X.; Cao, H.-J.; Li, J. Corrosion Behavior of Epoxy-Coated Rebar with Pinhole Defect in Seawater Concrete. Acta Met. Sin. Engl. Lett. 2018, 31, 1171–1182. [Google Scholar] [CrossRef]
- Jorcin, J.-B.; Aragon, E.; Merlatti, C.; Pébère, N. Delaminated areas beneath organic coating: A local electrochemical impedance approach. Corros. Sci. 2006, 48, 1779–1790. [Google Scholar] [CrossRef]
- Burstein, G.; Ilevbare, G. The effect of specimen size on the measured pitting potential of stainless steel. Corros. Sci. 1996, 38, 2257–2265. [Google Scholar] [CrossRef]
- Li, L.; Sagüés, A.A. Chloride Corrosion Threshold of Reinforcing Steel in Alkaline Solutions—Effect of Specimen Size. Corrosion 2004, 60, 195–202. [Google Scholar] [CrossRef]
- Angst, U.; Rønnquist, A.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Probabilistic considerations on the effect of specimen size on the critical chloride content in reinforced concrete. Corros. Sci. 2011, 53, 177–187. [Google Scholar] [CrossRef]
- Zhong, C.; Tang, X.; Cheng, Y. Corrosion of steel under the defected coating studied by localized electrochemical impedance spectroscopy. Electrochim. Acta 2008, 53, 4740–4747. [Google Scholar] [CrossRef]
- De Vooys, A.; van der Weijde, H. Investigating cracks and crazes on coated steel with simultaneous SVET and EIS. Prog. Org. Coat. 2011, 71, 250–255. [Google Scholar] [CrossRef]
- Kessler, S.; Angst, U.; Zintel, M.; Gehlen, C. Defects in epoxy-coated reinforcement and their impact on the service life of a concrete structure A study of critical chloride content and macro-cell corrosion A study of critical chloride content and mac-ro-cell corrosion. Struct. Concr. 2015, 16, 398–405. [Google Scholar] [CrossRef]
- Nishimura, T.; Katayama, H.; Noda, K.; Kodama, T. Electrochemical behavior of rust formed on carbon steel in a wet/dry en-vironment containing chloride ions. Corrosion 2000, 56, 935–941. [Google Scholar] [CrossRef]
- Musić, S.; Gotić, M.; Popović, S. X-ray diffraction and Fourier transform-infrared analysis of the rust formed by corrosion of steel in aqueous solutions. J. Mater. Sci. 1993, 28, 5744–5752. [Google Scholar] [CrossRef]
- Refait, P.; Génin, J.-M. The oxidation of ferrous hydroxide in chloride-containing aqueous media and pourbaix diagrams of green rust one. Corros. Sci. 1993, 34, 797–819. [Google Scholar] [CrossRef]
- King, A.D.; Birbilis, N.; Scully, J.R. Accurate Electrochemical Measurement of Magnesium Corrosion Rates; a Combined Im-pedance, Mass-Loss and Hydrogen Collection Study. Electrochim. Acta 2014, 121, 394–406. [Google Scholar] [CrossRef]
- Tang, Y.M.; Dun, Y.C.; Miao, Y.F.; Zhao, X.H.; Zuo, Y. Influence of the C-S-H Amount on [Cl−]/[OH−] Ratio of Simulated Con-crete SPS and the Corrosion Susceptibility of Steel. J. Wuhan Univ. Technol. 2017, 32, 430–436. [Google Scholar] [CrossRef]
- Macedo, M.C.S.S.; Margarit-Mattos, I.C.P.; Fragata, F.L.; Jorcin, J.B.; Pébère, N.; Mattos, O.R. Contribution to a better under-standing of different behaviour patterns observed with organic coatings evaluated by electrochemical impedance spectros-copy. Corros. Sci. 2009, 51, 1322–1327. [Google Scholar] [CrossRef]
- Zou, F.; Thierry, D. Localized electrochemical impedance spectroscopy for studying the degradation of organic coatings. Electrochim. Acta 1997, 42, 3293–3301. [Google Scholar] [CrossRef]
- Mi, W.B.; Jiang, E.Y.; Bai, H.L. Fe3+/Fe2+ ratio controlled magnetic and electrical transport properties of polycrystalline Fe3(1-delta)O4 films. J. Phys. D Appl. Phys. 2009, 42, 7. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situXPS analysis of various iron oxide films grown byNO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef]
- Aronniemi, M.; Sainio, J.; Lahtinen, J. Chemical state quantification of iron and chromium oxides using XPS: The effect of the background subtraction method. Surf. Sci. 2005, 578, 108–123. [Google Scholar] [CrossRef]
- Crist, B.V. Handbook of Monochromatic XPS Spectra. IEEE Electr. Insul. Mag. 1999, 19, 47. [Google Scholar]
- Asami, K.; Kikuchi, M. In-depth distribution of rusts on a plain carbon steel and weathering steels exposed to coastal–industrial atmosphere for 17 years. Corros. Sci. 2003, 45, 2671–2688. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides: Structure, Properties, Reactions, Occurrences and Uses, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Zhang, Q.C.; Wu, J.S.; Wang, J.J.; Zheng, W.L.; Chen, J.G.; Li, A.B. Corrosion behavior of weathering steel in marine atmos-phere. Mater. Chem. Phys. 2003, 77, 603–608. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.Y.; Ke, W. A study of the evolution of rust on weathering steel submitted to the Qinghai salt lake atmos-pheric corrosion. Mater. Chem. Phys. 2013, 139, 225–232. [Google Scholar] [CrossRef]






| Element | C | Si | Mn | P | S | Cr | Ni | Cu | V | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 0.22 | 0.43 | 1.25 | 0.017 | 0.02 | 0.03 | 0.02 | 0.02 | 0.035 | Bal |
| Diameter of Defect (μm) | 800 | 400 | 200 | 100 | 50 |
| Name | S800 | S400 | S200 | S100 | S50 |
| Solution | Concentration (mol/L) | pH | ||||
|---|---|---|---|---|---|---|
| NaOH | Ca(OH)2 | NaHCO3 | Na2CO3 | NaCl | ||
| USPS | 0.1 | saturated | 0 | 0 | 0.6 | 13.2 |
| CSPS | 0 | 0 | 0.06 | 0.04 | 0.6 | 9.8 |
| Solution | Fitting Data | Specimen | ||||
|---|---|---|---|---|---|---|
| 800 | 400 | 200 | 100 | 50 | ||
| USPS | Ecorr (V) | −0.368 | −0.261 | −0.183 | −0.161 | −0.149 |
| Icorr (A/cm2) | 7.74 × 10−10 | 3.22 × 10−10 | 9.546 × 10−11 | 1.07 × 10−10 | 9.31× 10−11 | |
| CSPS | Ecorr (V) | −0.488 | −0.437 | −0.379 | −0.349 | −0.169 |
| Icorr (A/cm2) | 1.06× 10−7 | 9.39 × 10−8 | 3.24 × 10−9 | 3.89 × 10−10 | 1.17 × 10−10 | |
| S800 | S400 | S200 | S100 | S50 | |||
|---|---|---|---|---|---|---|---|
| Impedance (Ohm) | |||||||
| USPS | 0 h | defect | 1.05 × 105 | 0.90 × 105 | 0.99 × 105 | 1.17 × 105 | 0.96 × 105 |
| periphery | 1.14 × 105 | 1.32 × 105 | 1.53 × 105 | 1.31 × 105 | 1.04 × 105 | ||
| 24 h | defect | 1.00 × 106 | 0.74 × 106 | 1.60 × 106 | 3.10 × 106 | 4.40 × 106 | |
| periphery | 8.56 × 106 | 5.40 × 106 | 1.07 × 107 | 2.83 × 107 | 2.57 × 107 | ||
| 84 days | defect | 1.21 × 106 | 1.08 × 106 | 1.24 × 106 | 1.21 × 106 | 1.22 × 106 | |
| periphery | 1.64 × 106 | 1.33 × 106 | 1.63 × 106 | 1.54 × 106 | 1.40 × 106 | ||
| CSPS | 0 h | defect | 1.05 × 105 | 0.90 × 105 | 0.99 × 105 | 1.17 × 105 | 0.96 × 105 |
| periphery | 1.14 × 105 | 1.32 × 105 | 1.53 × 105 | 1.31 × 105 | 1.04 × 105 | ||
| 21 days | defect | 3.21 × 105 | 5.34 × 105 | 3.28 × 105 | 4.49 × 105 | 4.88 × 105 | |
| periphery | 4.04 × 105 | 6.97 × 105 | 4.43 × 105 | 8.23 × 105 | 1.11 × 106 | ||
| 63 days | defect | 1.47 × 106 | 1.30 × 106 | 1.38 × 106 | 1.65 × 106 | 1.30 × 106 | |
| periphery | 1.81 × 106 | 1.73 × 106 | 1.78 × 106 | 2.03 × 106 | 1.77 × 106 | ||
| S800 | S400 | S200 | S100 | S50 | ||
|---|---|---|---|---|---|---|
| Current Density (μA/cm2) | ||||||
| USPS | 0 h | 23.0 | 20.7 | 20.7 | 17.0 | 3.76 |
| 84 d | 0.24 | 0.22 | 0.21 | 0.090 | −0.015~0.045 | |
| CSPS | 0 h | 23.0 | 20.7 | 20.7 | 17.0 | 3.76 |
| 21 d | 14.9 | 12.4 | 16.8 | 13.4 | 4.20 | |
| 63 d | 0.676 | 0.530 | 0.466 | 0.444 | −0.041~0.027 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, L.; Wei, Y.; Zhao, H.; Cao, H.; Li, J. Influence of Microdefect Size on Corrosion Behavior of Epoxy-Coated Rebar for Application in Seawater-Mixed Concrete. Coatings 2021, 11, 439. https://doi.org/10.3390/coatings11040439
Wan L, Wei Y, Zhao H, Cao H, Li J. Influence of Microdefect Size on Corrosion Behavior of Epoxy-Coated Rebar for Application in Seawater-Mixed Concrete. Coatings. 2021; 11(4):439. https://doi.org/10.3390/coatings11040439
Chicago/Turabian StyleWan, Li, Yinghua Wei, Hongtao Zhao, Haijiao Cao, and Jing Li. 2021. "Influence of Microdefect Size on Corrosion Behavior of Epoxy-Coated Rebar for Application in Seawater-Mixed Concrete" Coatings 11, no. 4: 439. https://doi.org/10.3390/coatings11040439
APA StyleWan, L., Wei, Y., Zhao, H., Cao, H., & Li, J. (2021). Influence of Microdefect Size on Corrosion Behavior of Epoxy-Coated Rebar for Application in Seawater-Mixed Concrete. Coatings, 11(4), 439. https://doi.org/10.3390/coatings11040439





