Anhydride-Cured Epoxy Powder Coatings from Natural-Origin Resins, Hardeners, and Fillers
Abstract
:1. Introduction
- “Ecology” (resulting from the lack of volatile organic compounds dangerous to health and the environment);
- “Excellence of finish” (excellent properties of the coatings, six times less complaints about painting than in the case of liquid paints);
- “Economy” (economy, up to 50% less production costs of powders in relation to other types of paints);
- “Energy” (energy efficiency of the production process of powder paints and coatings is lower by approx. 30%);
- “Efficiency” (high use of paint, reduction of waste by up to 90%).
- Solid state in ambient conditions with melting temperatures in a range of ca. 100–140 °C, which should be lower than curing temperatures of powder coatings (usually 140–220 °C);
- Flexible chains in molecules enabling non-brittle curing of coatings;
- The cross-linking mechanism should be as mild as possible to avoid shrinkage and cracking; this requirement is met only by the anhydride mechanism; it is noteworthy that due to considerable toxicity, petroleum-based anhydride-curing powder paints are not developed nowadays, however rosin-based anhydrides are claimed as non-toxic [30].
2. Materials and Methods
2.1. Materials
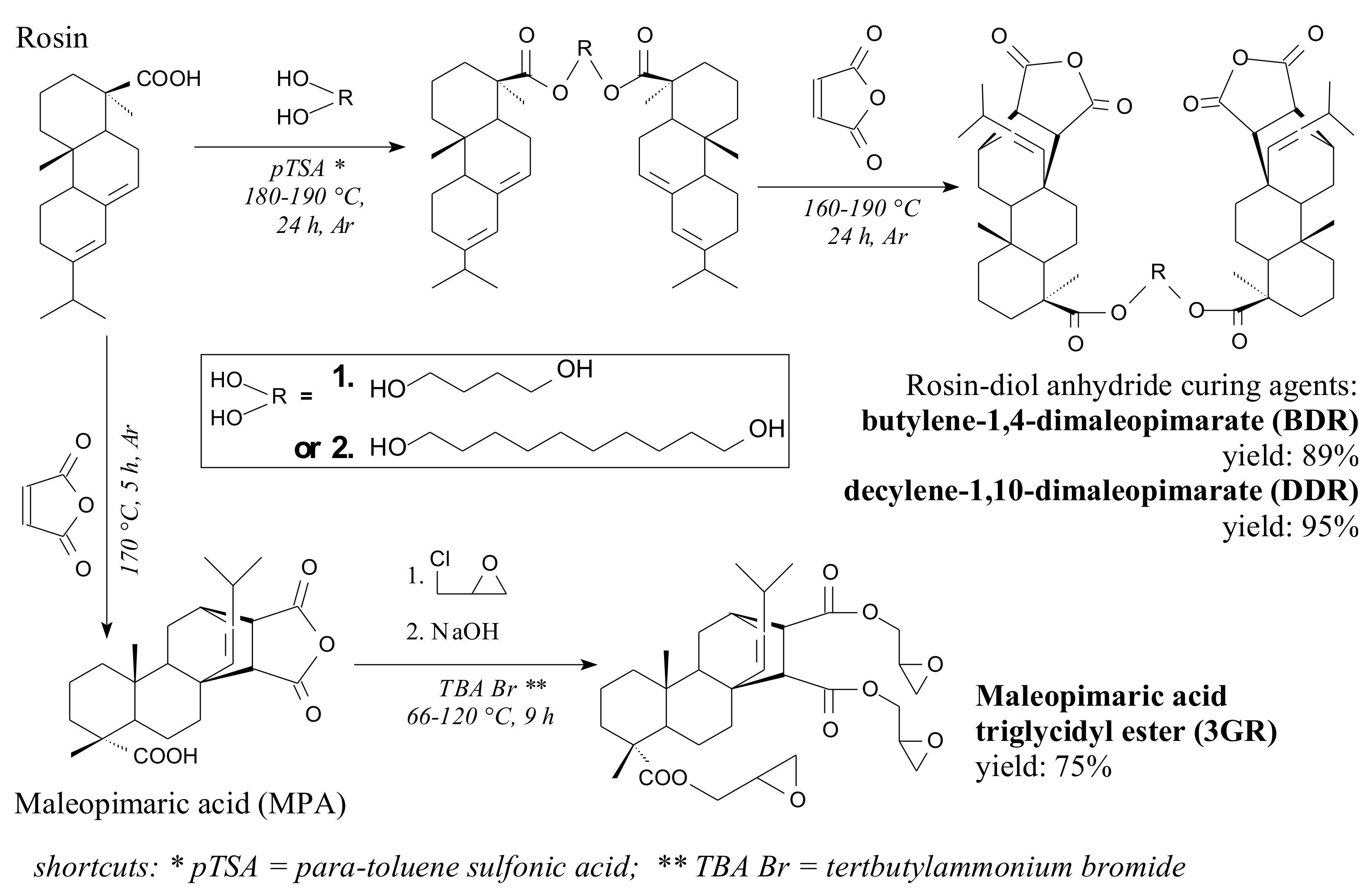
2.2. Synthesis of Rosin-Derived Dianhydrides
2.3. Preparation of Natural-Sourced Anti-Corrosive Filler
2.4. Preparation of Natural-Sourced Powder Coatings
2.5. Evaluation Methods of Coating Components
2.6. Evaluation Methods of Coating Compositions and Coatings
3. Results and Discussion
3.1. Properties of Rosin-Derived Dianhydride Hardeners
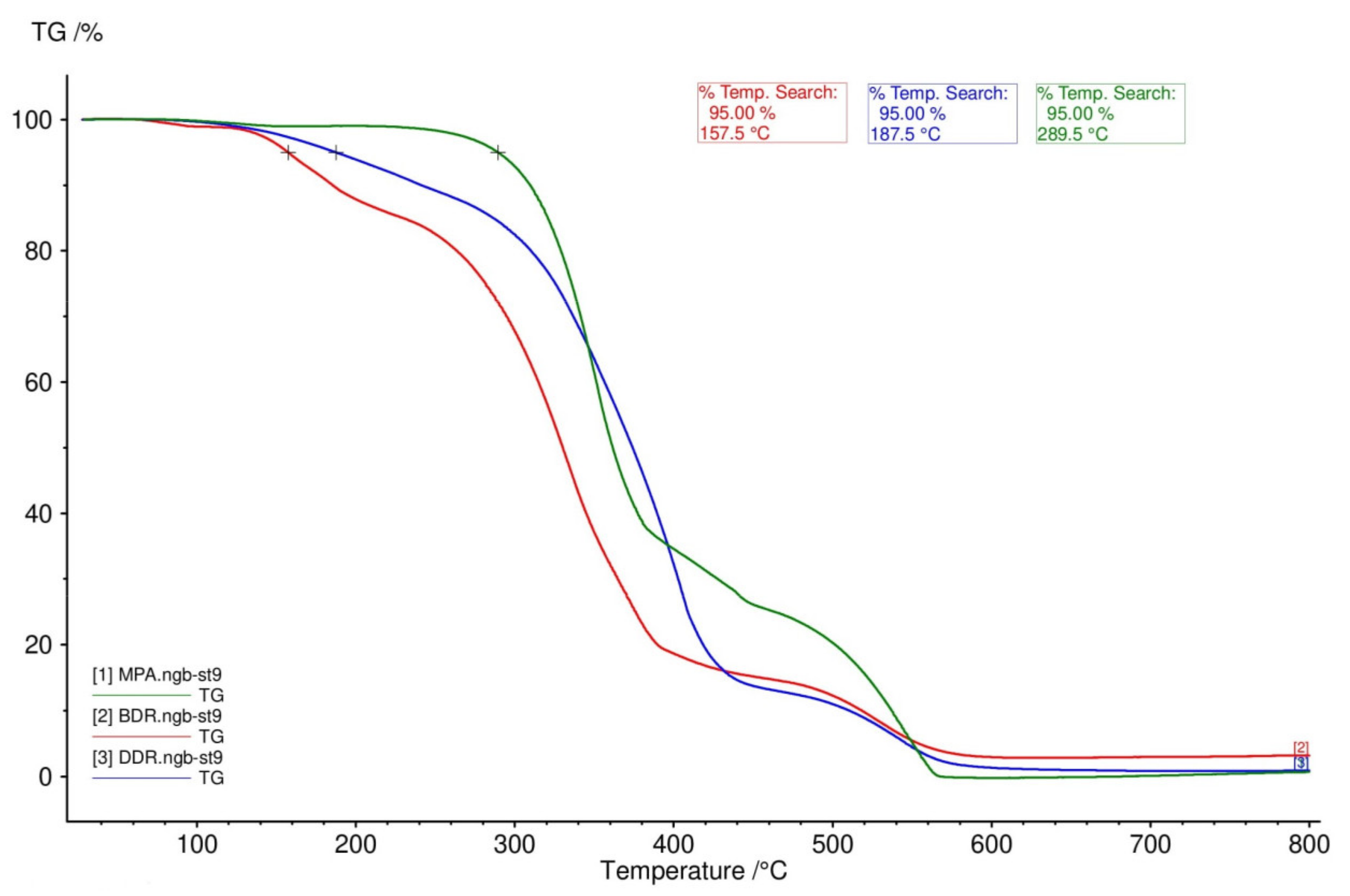
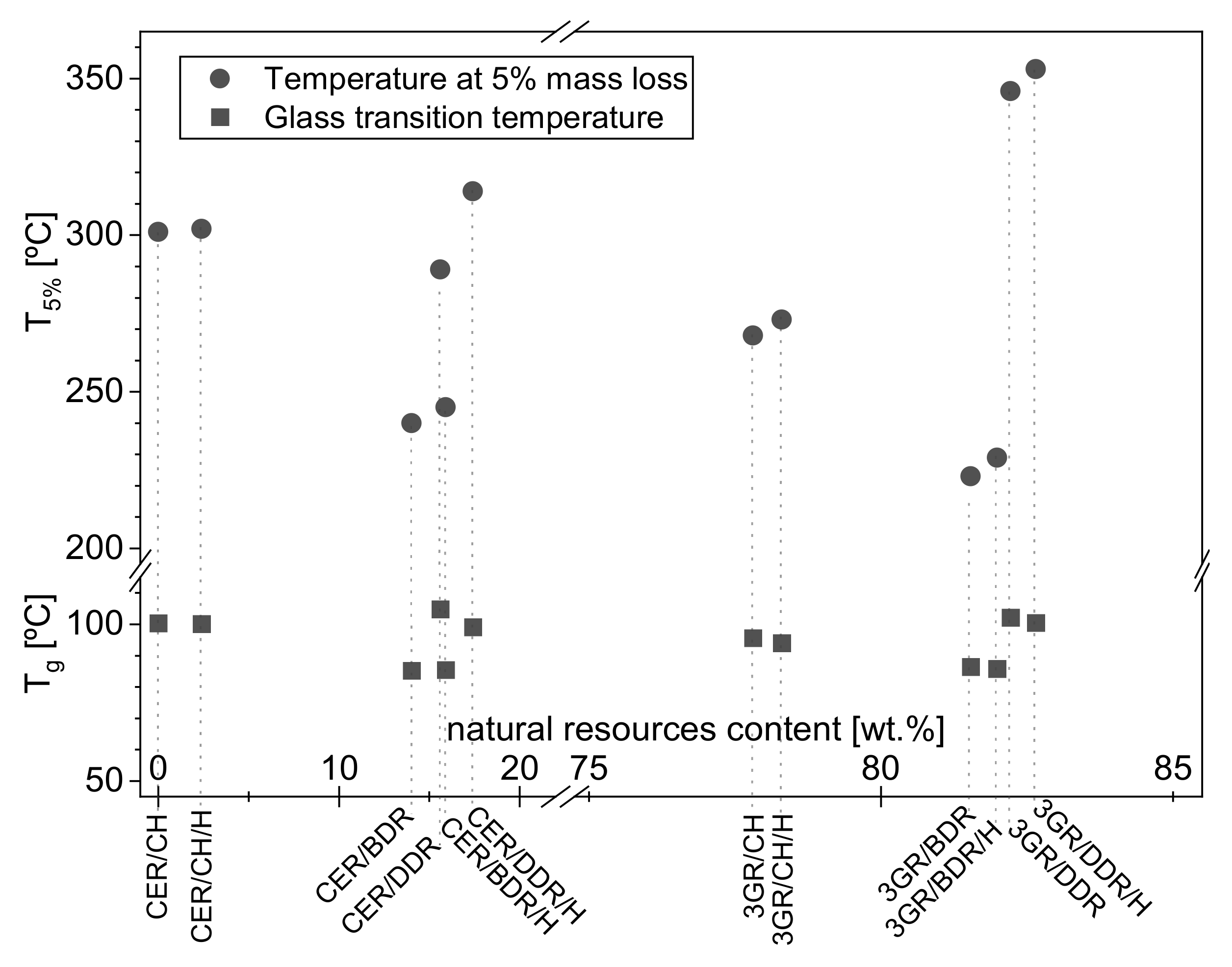
3.2. Natural Resources Content and Thermal Properties of Coatings
3.3. Mechanical Properties of Coatings
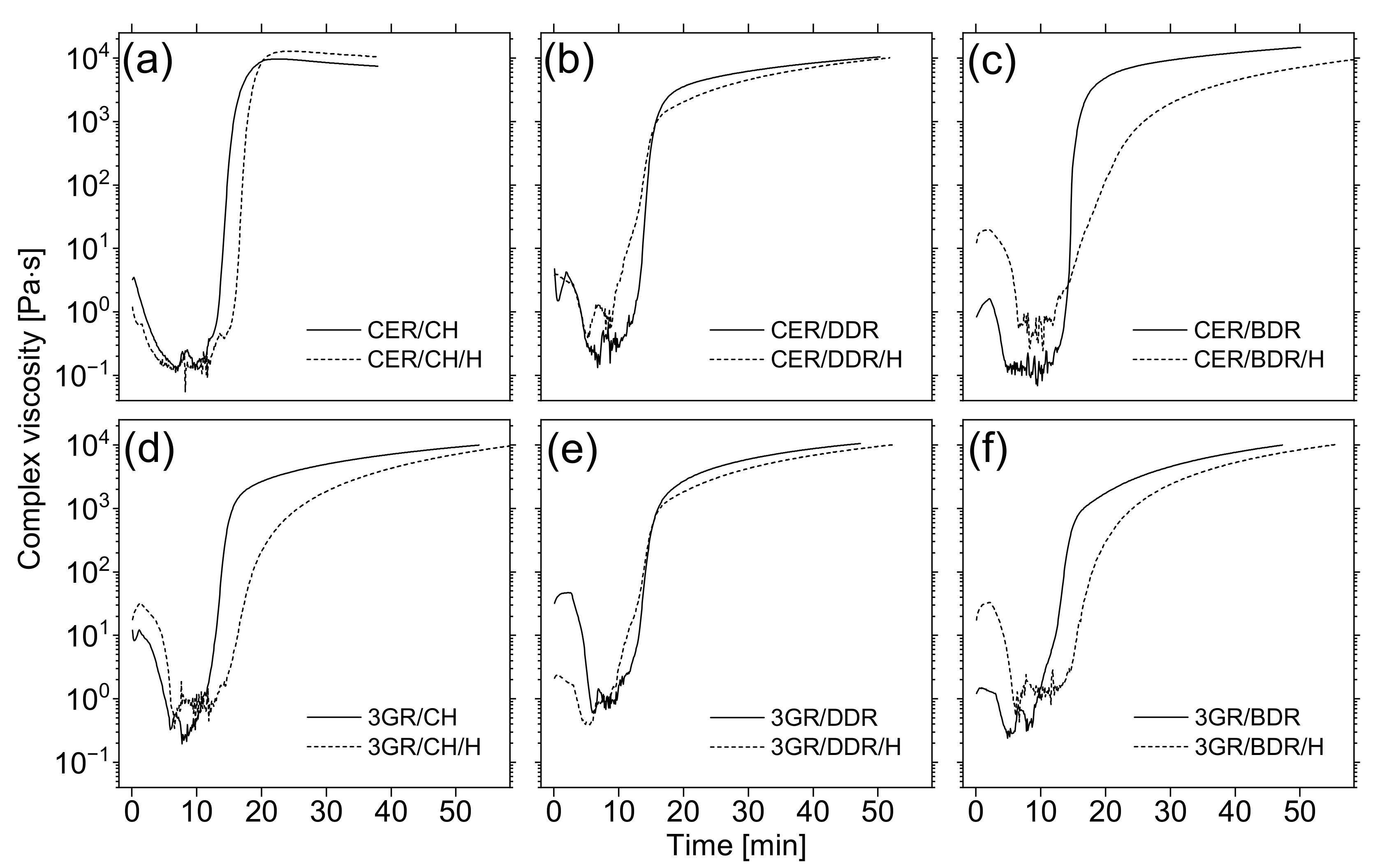
3.4. Functional Properties of Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spyrou, E. Powder Coatings Chemistry and Technology, 3rd rev. ed.; Vincentz Network: Hanover, Germany, 2014; ISBN 978-3-7486-0236-1. [Google Scholar]
- Powder Coatings Market Size, Share, Industry Report, 2027. Available online: https://www.grandviewresearch.com/industry-analysis/powder-coatings-market-analysis (accessed on 1 February 2021).
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and characterization of petroleum and biobased epoxy resins: A review. Polym. Int. 2018, 67, 815–839. [Google Scholar] [CrossRef]
- Ma, S.; Li, T.; Liu, X.; Zhu, J. Research Progress on Bio-based thermosetting resins. Polym. Int. 2016, 65, 164–173. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent development of biobased epoxy resins: a review. Polym.-Plast Technol. 2018, 57, 133–155. [Google Scholar] [CrossRef]
- Ding, C.; Matharu, A.S. Recent developments on biobased curing agents: a review of their preparation and use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F. Recent advances in bio-based epoxy resins and bio-based epoxy curing agents. J. Appl. Polym. Sci. 2016, 133, 44103. [Google Scholar] [CrossRef] [Green Version]
- Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K.; Wierzbicka, E. Advances in rosin-based chemicals: The latest recipes, applications and future trends. Molecules 2019, 24, 1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Fina, A.; Malucelli, G. Thermal shielding performances of nano-structured intumescent coatings containing organo-modified layered double hydroxides. Prog. Org. Coat. 2015, 78, 504–510. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Zhang, G.; Qian, P.Y. Environmentally friendly antifouling coatings based on biodegradable polymer and natural antifoulant. ACS Sustain. Chem. Eng. 2017, 5, 6304–6309. [Google Scholar] [CrossRef]
- Dong, Y.; Yan, Y.; Wang, K.; Li, J.; Zhang, S.; Xia, C.; Shi, S.Q.; Cai, L. Improvement of water resistance, dimensional stability, and mechanical properties of poplar wood by rosin impregnation. Eur. J. Wood Prod. 2016, 74, 177–184. [Google Scholar] [CrossRef]
- Pinori, E.; Berglin, M.; Brive, L.M.; Hulander, M.; Dahlström, M.; Elwing, H. Multi-seasonal barnacle (balanus improvisus) protection achieved by trace amounts of a macrocyclic lactone (ivermectin) included in rosin-based coatings. Biofouling 2011, 27, 941–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellotti, N.; del Amo, B.; Romagnoli, R. Quaternary ammonium “tannate” for antifouling coatings. Ind. Eng. Chem. Res. 2012, 51, 16626–16632. [Google Scholar] [CrossRef] [Green Version]
- Yousaf, B.; Liu, G.; Abbas, Q.; Wang, R.; Ullah, H.; Mian, M.M.; Amina; Rashid, A. Enhanced removal of hexavalent chromium from aqueous media using a highly stable and magnetically separable rosin-biochar-coated TiO2@c nanocomposite. RSC Adv. 2018, 8, 25983–25996. [Google Scholar] [CrossRef] [Green Version]
- Makhutov, N.A.; Ushakov, B.N.; Vasil’ev, I.E. Strength assessment and defect detection in welded pipeline seams by means of brittle tensosensitive coatings. Russ. Eng. Res. 2011, 31, 123–127. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, B.-R.; Oh, E.-S. Application of a Bio-Derivative, Rosin, as a Binder additive for lithium titanium oxide electrodes in lithium-ion batteries. J. Power Sources 2015, 273, 608–612. [Google Scholar] [CrossRef]
- Liu, X.Q.; Huang, W.; Jiang, Y.H.; Zhu, J.; Zhang, C.Z. Preparation of a bio-based epoxy with comparable properties to those of petroleum-based counterparts. Express Polym. Lett. 2012, 6, 293–298. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J. High-performance biobased epoxy derived from rosin. Polym. Int. 2010, 59, 607–609. [Google Scholar] [CrossRef]
- Jaswal, S.; Gaur, B. Structure-property correlation study of bio-based multifunctional vinyl ester resin in presence of methacrylated lignin model compounds. Polym. Sci. Ser. B 2015, 57, 417–433. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Jiang, Y.; Ma, S.; Zhu, J. Bio-based shape memory epoxy resin synthesized from rosin acid. Iran. Polym. J. 2016, 25, 957–965. [Google Scholar] [CrossRef]
- Deng, L.; Shen, M.; Yu, J.; Wu, K.; Ha, C. Preparation, characterization, and flame retardancy of novel rosin-based siloxane epoxy resins. Ind. Eng. Chem. Res. 2012, 51, 8178–8184. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Zhu, J.; Zhang, C.; Guo, J. Synthesis, characterization of a rosin-based epoxy monomer and its comparison with a petroleum-based counterpart. J. Macromol. Sci. A 2013, 50, 321–329. [Google Scholar] [CrossRef]
- El-Ghazawy, R.A.; El-Saeed, A.M.; Al-Shafey, H.I.; Abdul-Raheim, A.-M.; El-Sockary, M.A. Rosin based epoxy coating: Synthesis, identification and characterization. Eur. Polym. J. 2015, 69, 403–415. [Google Scholar] [CrossRef]
- Brocas, A.-L.; Llevot, A.; Mantzaridis, C.; Cendejas, G.; Auvergne, R.; Caillol, S.; Carlotti, S.; Cramail, H. Epoxidized rosin acids as co-precursors for epoxy resins. Des. Monomers Polym. 2014, 17, 301–310. [Google Scholar] [CrossRef] [Green Version]
- Mantzaridis, C.; Brocas, A.-L.; Llevot, A.; Cendejas, G.; Auvergne, R.; Caillol, S.; Carlotti, S.; Cramail, H. Rosin acid oligomers as precursors of dgeba-free epoxy resins. Green Chem. 2013, 15, 3091–3098. [Google Scholar] [CrossRef]
- Liu, X.; Xin, W.; Zhang, J. Rosin-based acid anhydrides as alternatives to petrochemical curing agents. Green Chem. 2009, 11, 1018–1025. [Google Scholar] [CrossRef]
- Liu, X.; Xin, W.; Zhang, J. Rosin-derived imide-diacids as epoxy curing agents for enhanced performance. Bioresour. Technol. 2010, 101, 2520–2524. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Liu, B.; Zhang, J.; Xian, M. Synthesis of rosin-based flexible anhydride-type curing agents and properties of the cured epoxy. Polym. Int. 2009, 58, 1435–1441. [Google Scholar] [CrossRef]
- Mustata, F.R.; Tudorachi, N. Epoxy resins cross-linked with rosin adduct derivatives. cross-linking and thermal behaviors. Ind. Eng. Chem. Res. 2010, 49, 12414–12422. [Google Scholar] [CrossRef]
- Karlberg, A.-T.; Gäfveri, E.; Hagelthorn, G.; Nilsson, J.L.G. Maleopimaric acid—a potent sensitizer in modified rosin. Contact Dermat. 1990, 22, 193–201. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K. Modifier of epoxy coating compositions properties. preparation and characterization. Mater. Eng. 2020, 5. [Google Scholar] [CrossRef]
- ISO 3001:1999 Plastics — Epoxy compounds — Determination of epoxy equivalent; ISO: Geneva, Switzerland, 1999; Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/02/96/29610.html (accessed on 27 April 2021).
- ISO 2813:2014 Paints and Varnishes — Determination of Gloss Value at 20°, 60° and 85°; ISO: Geneva, Switzerland, 2014; Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/05/68/56807.html (accessed on 27 April 2021).
- ISO 2409:2020 Paints and Varnishes — Cross-Cut Test; ISO: Geneva, Switzerland, 2020; Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/60/76041.html (accessed on 27 April 2021).
- ISO 1520:2006 Paints and Varnishes — Cupping Test; ISO: Geneva, Switzerland, 2006; Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/09/40923.html (accessed on 27 April 2021).
- PN-EN 13523-11:2020-01 Metale Powlekane Metodą Ciągłą – Metody Badań – Część 11: Odporność na Rozpuszczalniki (Próba Pocierania); Polish Committee for Standardization: Warsaw, Poland, 2020; Available online: https://sklep.pkn.pl/pn-en-13523-11-2020-01e.html (accessed on 27 April 2021).
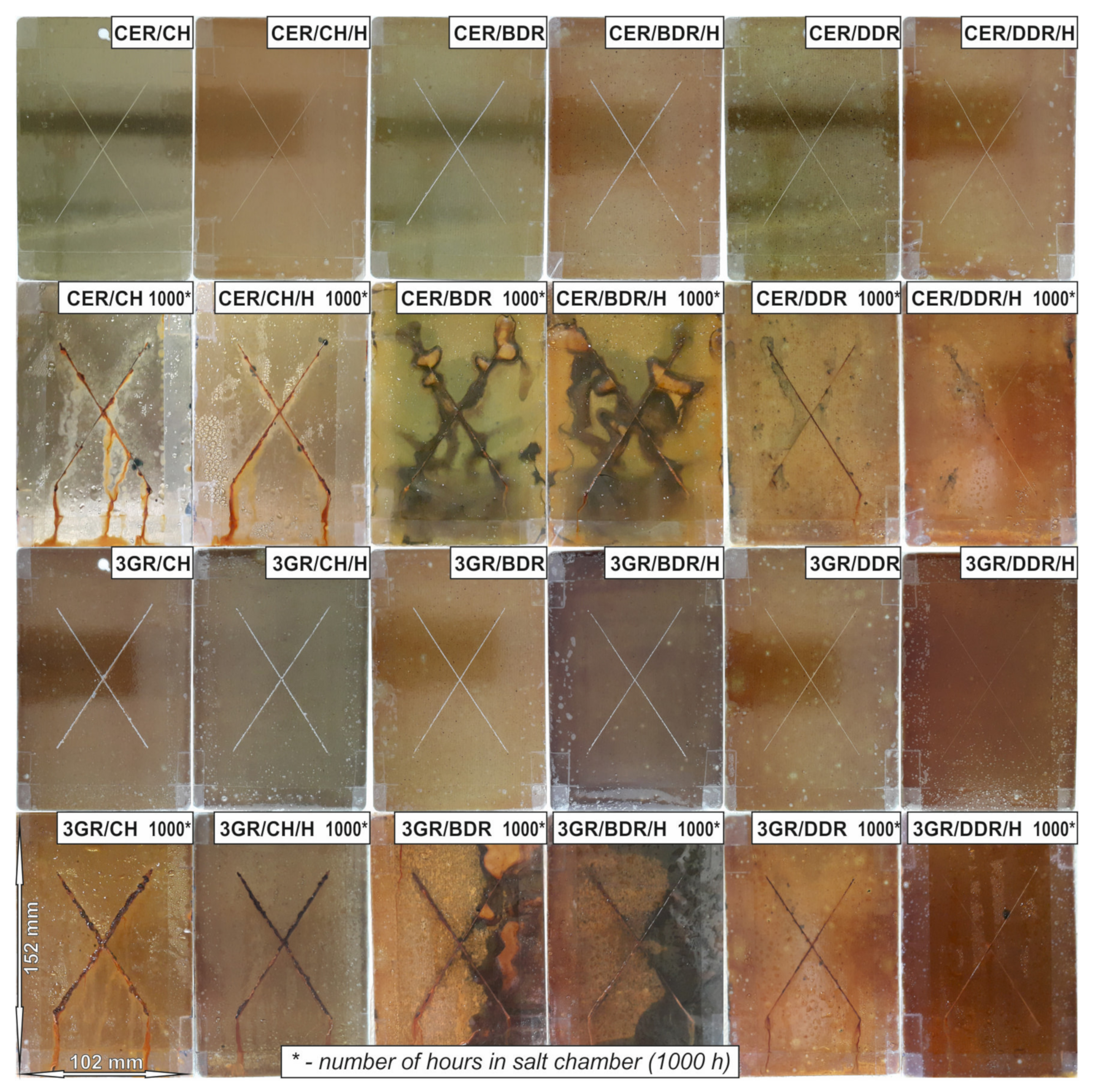
- ISO 9227:2017 Corrosion Tests in Artificial Atmospheres — Salt Spray Tests; ISO: Geneva, Switzerland, 2017; Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/06/35/63543.html (accessed on 27 April 2021).

| Sample Symbol | The Weight Ratio of Components in Coating Composition [wt. part] | |||||
|---|---|---|---|---|---|---|
| Commercial Epoxy Resin (CER) | Rosin-based Epoxy Resin (3GR) | Commercial Hardener (CH) | BD**/Rosin Hardener(BDR) | DD***/Rosin Hardener (DDR) | Modified Halloysite (H) | |
| CER/CH * | 489 | - | 11 | - | - | - |
| CER/CH/H * | 318 | - | 7 | - | - | 8 |
| CER/BDR | 88 | - | - | 19 | - | - |
| CER/BDR/H | 3592 | - | - | 760 | - | 109 |
| CER/DDR | 88 | - | - | - | 21 | - |
| CER/DDR/H | 3592 | - | - | - | 840 | 111 |
| 3GR/CH | - | 330 | 22 | - | - | - |
| 3GR/CH/H | - | 165 | 11 | - | - | 4 |
| 3GR/BDR | - | 33 | - | 19 | - | - |
| 3GR/BDR/H | - | 330 | - | 190 | - | 13 |
| 3GR/DDR | - | 11 | - | - | 7 | - |
| 3GR/DDR/H | - | 660 | - | - | 420 | 27 |
| Sample Symbol | Hardness [a.u.] a | Cupping Resistance [mm] | Adhesion [°] b | Chemical Resistance [double rub] | Gloss [G.U.] c | Color [RAL] |
|---|---|---|---|---|---|---|
| CER/CH | 200 ± 3 | 9.1 ± 0.1 | 0 | >400 | 68 ± 3 | 7034 (yellow grey) |
| CER/CH/H | 206 ± 3 | 9.5 ± 0.1 | 0 | >400 | 62 ± 4 | 8000 (green brown) |
| CER/BDR | 159 ± 4 | 5.5±0.2 | 1 | 255 ± 6 | 63 ± 2 | 1020 (olive yellow) |
| CER/BDR/H | 165 ± 4 | 5.8±0.3 | 1 | 258 ± 5 | 60 ± 3 | 1035 (pearl beige) |
| CER/DDR | 200 ± 3 | 7.4±0.1 | 0 | >400 | 66 ± 4 | 6013 (red green) |
| CER/DDR/H | 202 ± 4 | 7.5±0.1 | 0 | >400 | 65 ± 2 | 1019 (grey beige) |
| 3GR/CH | 177 ± 4 | 6.1±0.2 | 2 | 247 ± 5 | 61 ± 2 | 1036 (pearl gold) |
| 3GR/CH/H | 183 ± 3 | 6.3±0.2 | 2 | 270 ± 3 | 55 ± 3 | 7002 (olive grey) |
| 3GR/BDR | 145 ± 4 | 4.8±0.2 | 1 | 204 ± 3 | 56 ± 2 | 1011 (brown beige) |
| 3GR/BDR/H | 147 ± 4 | 5.1±0.2 | 1 | 210 ± 3 | 49 ± 4 | 8025 (pale brown) |
| 3GR/DDR | 202 ± 3 | 7.1±0.1 | 0 | >400 | 60 ± 3 | 8003 (clay brown) |
| 3GR/DDR/H | 209 ± 4 | 7.5±0.1 | 0 | >400 | 57 ± 2 | 8007 (fawn brown) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kugler, S.; Ossowicz-Rupniewska, P.; Wierzbicka, E.; Łopiński, J. Anhydride-Cured Epoxy Powder Coatings from Natural-Origin Resins, Hardeners, and Fillers. Coatings 2021, 11, 531. https://doi.org/10.3390/coatings11050531
Kugler S, Ossowicz-Rupniewska P, Wierzbicka E, Łopiński J. Anhydride-Cured Epoxy Powder Coatings from Natural-Origin Resins, Hardeners, and Fillers. Coatings. 2021; 11(5):531. https://doi.org/10.3390/coatings11050531
Chicago/Turabian StyleKugler, Szymon, Paula Ossowicz-Rupniewska, Ewa Wierzbicka, and Jakub Łopiński. 2021. "Anhydride-Cured Epoxy Powder Coatings from Natural-Origin Resins, Hardeners, and Fillers" Coatings 11, no. 5: 531. https://doi.org/10.3390/coatings11050531






