Impacts of MAPbBr3 Additive on Crystallization Kinetics of FAPbI3 Perovskite for High Performance Solar Cells
Abstract
:1. Introduction
2. Materials and Experimental
2.1. Materials
2.2. Solution Preparation
2.3. Device Fabrication
2.4. In Situ GIWAXS Experiments
2.5. Device Characterization
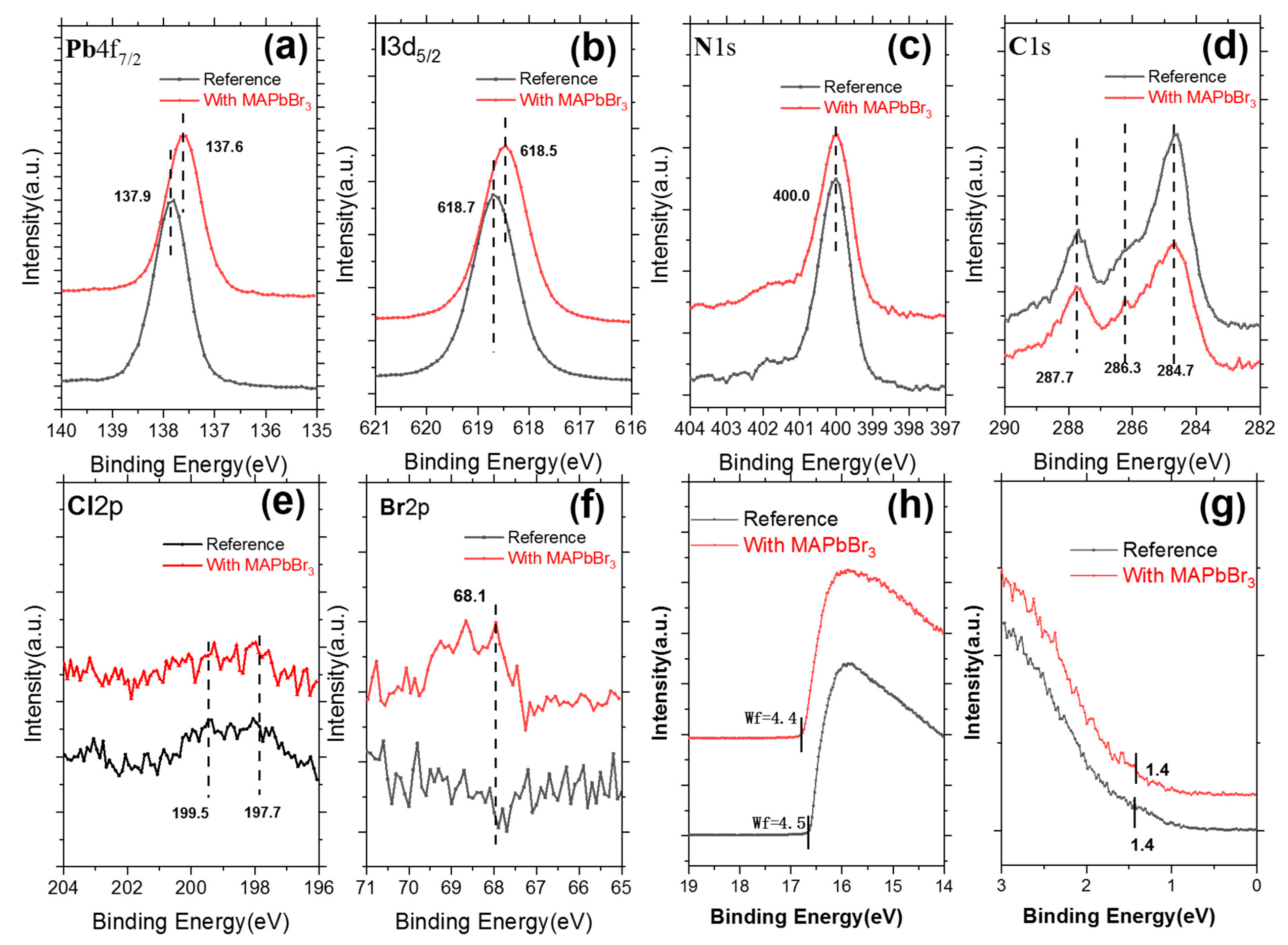
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Ho-Baillie, A.W. Solar cell efficiency tables (Version 55). Prog. Photovolt. 2020, 28, 3–15. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J. Low-temperature solution-processed tin oxide as an alternative electron transporting layer for efficient perovskite solar cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, L.; Wang, H.; Yang, X.; Meng, J.; Liu, H.; Yin, Z.; Wu, J.; Zhang, X.; You, J. Enhanced electron extraction using SnO2 for high-efficiency planar-structure HC (NH2)2PbI3-based perovskite solar cells. Nat. Energy 2016, 2, 1–7. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Turren-Cruz, S.-H.; Hagfeldt, A.; Saliba, M. Methylammonium-free, high-performance, and stable perovskite solar cells on a planar architecture. Science 2018, 362, 449–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saliba, M.; Matsui, T.; Seo, J.-Y.; Domanski, K.; Correa-Baena, J.-P.; Nazeeruddin, M.K.; Zakeeruddin, S.M.; Tress, W.; Abate, A.; Hagfeldt, A. Cesium-containing triple cation perovskite solar cells: Improved stability, reproducibility and high efficiency. Energy Environ. Sci. 2016, 9, 1989–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Zhao, Y.; Fu, R.; Zhou, W.; Zhao, Y.; Liu, X.; Yu, D.; Zhao, Q. Efficient Perovskite Solar Cells Fabricated Through CsCl-Enhanced PbI2 Precursor via Sequential Deposition. Adv. Mater. 2018, 30, 1803095. [Google Scholar] [CrossRef]
- Ye, F.; Ma, J.; Chen, C.; Wang, H.; Xu, Y.; Zhang, S.; Wang, T.; Tao, C.; Fang, G. Roles of MACl in Sequentially Deposited Bromine-Free Perovskite Absorbers for Efficient Solar Cells. Adv. Mater. 2021, 33, 2007126. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal halide perovskites as visible-light sensitizers for photovoltaic cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, H.; Hu, J.; Huang, B.; Sun, M.; Dong, B.; Zheng, G.; Huang, Y.; Chen, Y.; Li, L. A Eu3+-Eu2+ ion redox shuttle imparts operational durability to Pb-I perovskite solar cells. Science 2019, 363, 265–270. [Google Scholar] [CrossRef]
- Luo, D.; Yang, W.; Wang, Z.; Sadhanala, A.; Hu, Q.; Su, R.; Shivanna, R.; Trindade, G.F.; Watts, J.F.; Xu, Z. Enhanced photovoltage for inverted planar heterojunction perovskite solar cells. Science 2018, 360, 1442–1446. [Google Scholar] [CrossRef] [Green Version]
- Hou, Y.; Du, X.; Scheiner, S.; McMeekin, D.P.; Wang, Z.; Li, N.; Killian, M.S.; Chen, H.; Richter, M.; Levchuk, I. A generic interface to reduce the efficiency-stability-cost gap of perovskite solar cells. Science 2017, 358, 1192–1197. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Chen, B.; Dai, J.; Fang, Y.; Bai, Y.; Lin, Y.; Wei, H.; Zeng, X.C.; Huang, J. Defect passivation in hybrid perovskite solar cells using quaternary ammonium halide anions and cations. Nat. Energy 2017, 2, 1–9. [Google Scholar] [CrossRef]
- Kim, M.; Kim, G.-H.; Lee, T.K.; Choi, I.W.; Choi, H.W.; Jo, Y.; Yoon, Y.J.; Kim, J.W.; Lee, J.; Huh, D. Methylammonium chloride induces intermediate phase stabilization for efficient perovskite solar cells. Joule 2019, 3, 2179–2192. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional engineering of perovskite materials for high-performance solar cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, H.; Li, G.; Fang, G. Stabilizer-assisted growth of formamdinium-based perovskites for highly efficient and stable planar solar cells with over 22% efficiency. Nano Energy 2019, 63, 103835. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Li, W.; Lin, N.; Wang, L.; Qiao, J. Stable α/δ phase junction of formamidinium lead iodide perovskites for enhanced near-infrared emission. Chem. Sci. 2017, 8, 800–805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, S.; Qian, F.; Yang, S.; Cai, Y.; Wang, Q.; Sun, J.; Liu, Z.; Liu, S. NbF5: A Novel α-Phase Stabilizer for FA-Based Perovskite Solar Cells with High Efficiency. Adv. Funct. Mater. 2019, 29, 1807850. [Google Scholar] [CrossRef]
- Shi, B.; Yao, X.; Hou, F.; Guo, S.; Li, Y.; Wei, C.; Ding, Y.; Li, Y.; Zhao, Y.; Zhang, X. Unraveling the passivation process of PbI2 to enhance the efficiency of planar perovskite solar cells. J. Phys. Chem. C 2018, 122, 21269–21276. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Yang, Z.; Xu, Y.; Ding, Y.; Tan, L.; Yi, C.; Zhang, Z.; Meng, K.; Chen, G. Ligand-Modulated Excess PbI2 Nanosheets for Highly Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2020, 32, 2000865. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zhao, B.; Song, F.; Zheng, G.; Zhang, X.; Shen, K.; Yang, Y.; Chen, S.; Gao, X. The energy level alignment at the CH3NH3PbI3/pentacene interface. Appl. Surf. Sci. 2017, 393, 417–421. [Google Scholar] [CrossRef]
- Jacobsson, T.J.; Correa-Baena, J.-P.; Halvani Anaraki, E.; Philippe, B.; Stranks, S.D.; Bouduban, M.E.; Tress, W.; Schenk, K.; Teuscher, J.L.; Moser, J.-E. Unreacted PbI2 as a double-edged sword for enhancing the performance of perovskite solar cells. J. Am. Chem. Soc. 2016, 138, 10331–10343. [Google Scholar] [CrossRef] [Green Version]
- Tumen-Ulzii, G.; Qin, C.; Klotz, D.; Leyden, M.R.; Wang, P.; Auffray, M.; Fujihara, T.; Matsushima, T.; Lee, J.W.; Lee, S.J. Detrimental Effect of Unreacted PbI2 on the Long-Term Stability of Perovskite Solar Cells. Adv. Mater. 2020, 32, 1905035. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.; Zheng, G.; Zhao, B.; Song, F.; Zhang, X.; Shen, K.; Yang, Y.; Xiong, Y.; Gao, X.; Cao, L. Interfacial electronic structures revealed at the rubrene/CH3 NH3 PbI3 interface. Phys. Chem. Chem. Phys. 2017, 19, 6546–6553. [Google Scholar] [CrossRef]
- Zhang, X.; Su, Z.; Zhao, B.; Yang, Y.; Xiong, Y.; Gao, X.; Qi, D.-C.; Cao, L. Chemical interaction dictated energy level alignment at the N, N′-dipentyl-3, 4, 9, 10-perylenedicarboximide/CH3NH3PbI3 interface. Appl. Phys. Lett. 2018, 113, 113901. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-López, J.; Cambarau, W.; Cabau, L.; Palomares, E. Charge injection, carriers recombination and HOMO energy level relationship in perovskite solar cells. Sci. Rep. 2017, 7, 1–10. [Google Scholar]
- Jeon, N.J.; Lee, H.G.; Kim, Y.C.; Seo, J.; Noh, J.H.; Lee, J.; Seok, S.I. o-Methoxy substituents in spiro-OMeTAD for efficient inorganic–organic hybrid perovskite solar cells. J. Am. Chem. Soc. 2014, 136, 7837–7840. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Lin, J.; Liu, X.; He, X.; Wang, Y.; Noda, T.; Wu, T.; Yang, X.; Han, L. Highly Stable and Efficient FASnI3-Based Perovskite Solar Cells by Introducing Hydrogen Bonding. Adv. Mater. 2019, 31, 1903721. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 percent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, Y.; Wang, J.; Ma, H.; Sun, Z.; Huang, W. Influence of Cl incorporation in perovskite precursor on the crystal growth and storage stability of perovskite solar cells. ACS Appl. Mater. Interfaces 2019, 11, 6022–6030. [Google Scholar] [CrossRef] [PubMed]






| Element Content | Pb | I | Br | C | N | Cl |
|---|---|---|---|---|---|---|
| Reference | 1.00 | 2.97 | 0 | 1.88 | 2.51 | 0.02 |
| With MAPbBr3 | 1.00 | 2.85 | 0.06 | 1.72 | 2.30 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Z.; Wang, C.; Zheng, G.; Gao, X. Impacts of MAPbBr3 Additive on Crystallization Kinetics of FAPbI3 Perovskite for High Performance Solar Cells. Coatings 2021, 11, 545. https://doi.org/10.3390/coatings11050545
Su Z, Wang C, Zheng G, Gao X. Impacts of MAPbBr3 Additive on Crystallization Kinetics of FAPbI3 Perovskite for High Performance Solar Cells. Coatings. 2021; 11(5):545. https://doi.org/10.3390/coatings11050545
Chicago/Turabian StyleSu, Zhenhuang, Chenyue Wang, Guanhaojie Zheng, and Xingyu Gao. 2021. "Impacts of MAPbBr3 Additive on Crystallization Kinetics of FAPbI3 Perovskite for High Performance Solar Cells" Coatings 11, no. 5: 545. https://doi.org/10.3390/coatings11050545






