Formulation and Optimization of Nano Lipid Based Oral Delivery Systems for Arthritis
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
2.2.1. HPLC Method
2.2.2. Optimization
2.2.3. Method of Preparation
2.2.4. Particle Characterization
2.2.5. Entrapment Efficiency
2.2.6. Mucoadhesion Study
2.2.7. Release Study
2.2.8. Permeation Study
2.2.9. Antioxidant Study
2.2.10. Anti-Arthritic Activity
Experimental Design
Biochemical Estimation
Paw Edema
Writhing Study
2.2.11. Statistical Analysis
3. Results and Discussion
3.1. Optimization
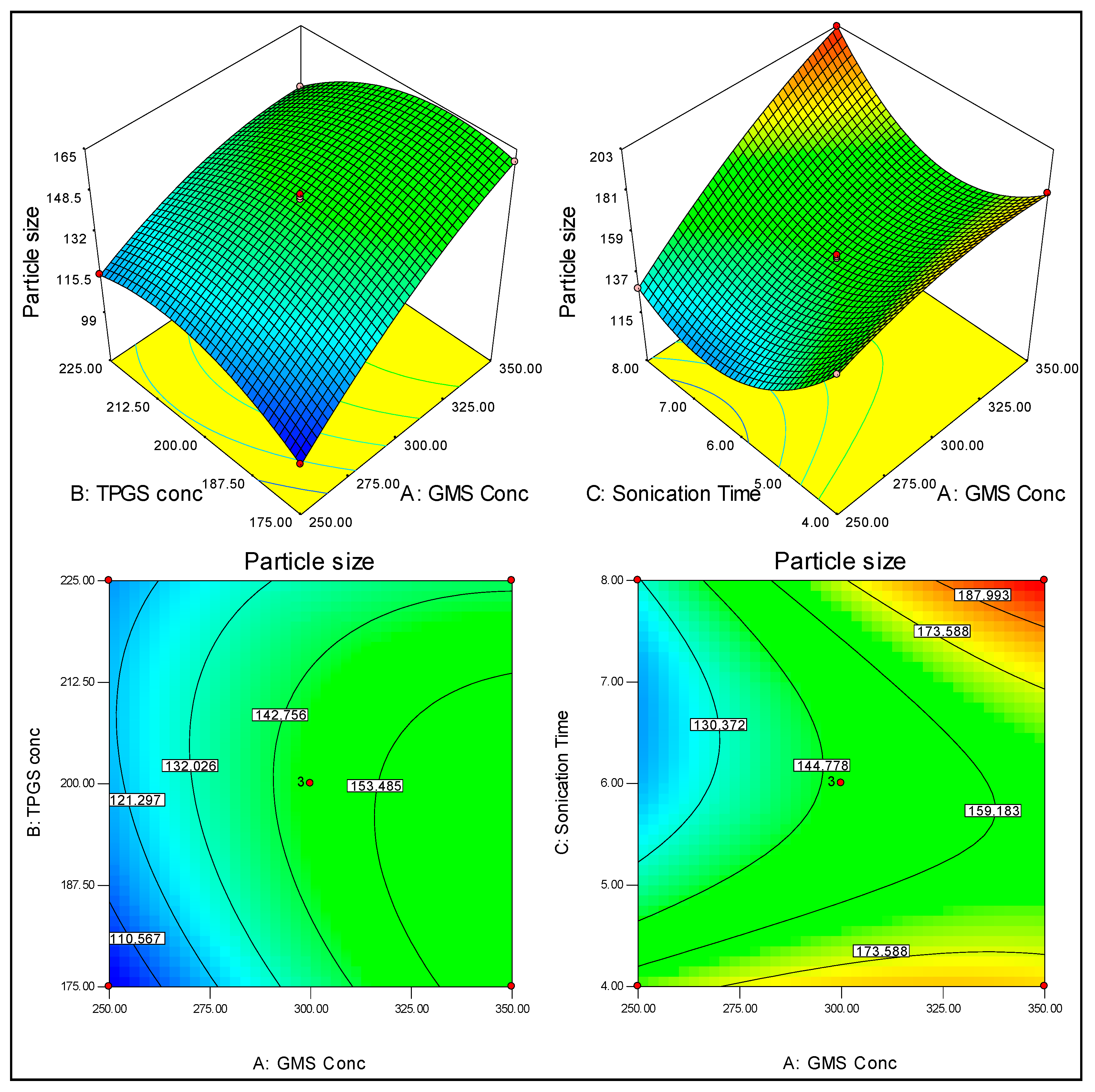
3.2. Effect of GMS, TPGS and ST on Particle Size (PS)
3.3. Effect of GMS, TPGS and ST on the Encapsulation Efficiency (EE)
3.4. Point Prediction
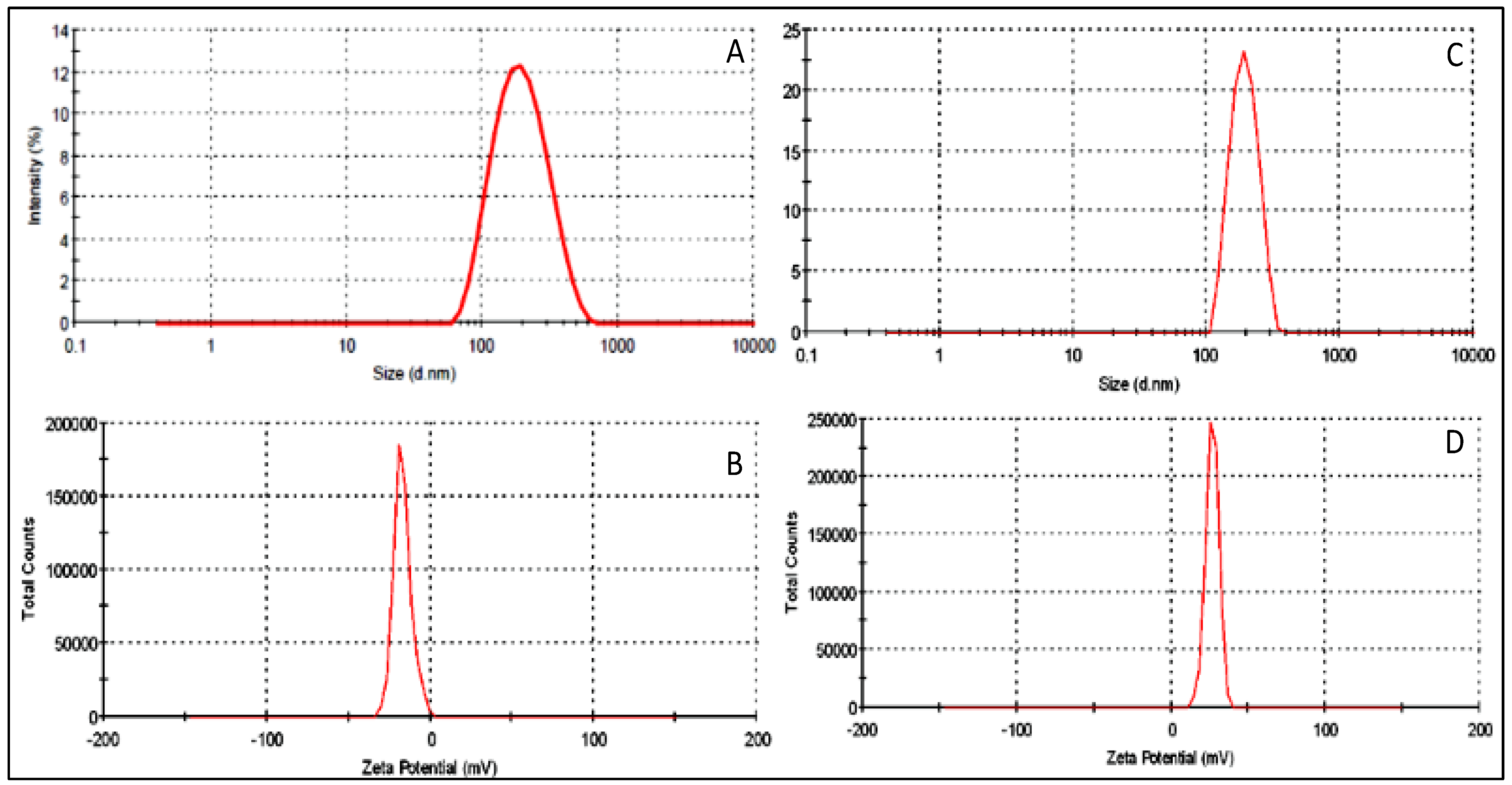
3.5. Particle Characterization
3.6. Release Study
3.7. Permeation Study
3.8. Antioxidant Study
3.9. Mucoadhesive Efficiency
3.10. Anti-Arthritic Activity
Biochemical Estimation
3.11. Paw Volume
3.12. Paw Edema Study
3.13. Writhing Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cui, P.; Qu, F.; Sreeharsha, N.; Sharma, S.; Mishra, A.; Gubbiyappa, S.K. Antiarthritic effect of chitosan nanoparticle loaded with embelin against adjuvant-induced arthritis in Wistar rats. IUBMB Life 2020, 72, 1054–1064. [Google Scholar] [CrossRef]
- Dolati, S.; Sadreddini, S.; Rostamzadeh, D.; Ahmadi, M.; Jadidi-Niaragh, F.; Yousefi, M. Utilization of nanoparticle technology in rheumatoid arthritis treatment. Biomed. Pharmacother. 2016, 80, 30–41. [Google Scholar] [CrossRef]
- Mitragotri, S.; Yoo, J.-W. Designing micro- and nano-particles for treating rheumatoid arthritis. Arch. Pharmacal Res. 2011, 34, 1887–1897. [Google Scholar] [CrossRef]
- Marzo, N.S.; Perez-Sanchez, A.; Ruiz-Torres, V.; Tebar, A.M.; Castillo, J.; López, M.H.; Catalán, E.B. Antioxidant and Photoprotective Activity of Apigenin and Its Potassium Salt Derivative in Human Keratinocytes and Absorption in Caco-2 Cell Monolayers. Int. J. Mol. Sci. 2019, 20, 2148. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Wang, F.; Zhou, R.; Song, X.; Xie, M. Apigenin: A current review on its beneficial biological activities. J. Food Biochem. 2017, 41, e12376. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.K.; Shin, H.D.; Lee, H.J.; Jo, H.S.; Jeong, J.S.; Choi, Y.L.; Lee, C.J.; Hwang, S.C. Apigenin Regulates Inter-leukin-1β-Induced Production of Matrix Metalloproteinase Both in the Knee Joint of Rat and in Primary Cultured Articular Chondrocytes. Biomol Ther 2016, 24, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Bandyopadhyay, S.; Lion, J.M. Attenuation of osteoclastogenesis and osteoclast function by apigenin. Biochem. Pharmacol. 2006, 330, 184–197. [Google Scholar] [CrossRef]
- Karim, R.; Palazzo, C.; Laloy, J.; Delvigne, A.-S.; Vanslambrouck, S.; Jerome, C.; Lepeltier, E.; Orange, F.; Dogne, J.-M.; Evrard, B.; et al. Development and evaluation of injectable nanosized drug delivery systems for apigenin. Int. J. Pharm. 2017, 532, 757–768. [Google Scholar] [CrossRef] [Green Version]
- Ali, H.; Singh, S.K. Biological voyage of solid lipid nanoparticles: A proficient carrier in nanomedicine. Ther. Deliv. 2016, 7, 691–709. [Google Scholar] [CrossRef]
- Singh, A.; Yadagiri, G.; Parvez, S.; Singh, O.P.; Verma, A.; Sundar, S.; Mudavath, S.L. Formulation, characterization and in vitro anti-leishmanial evaluation of amphotericin B loaded solid lipid nanoparticles coated with vitamin B12-stearic acid conjugate. Mater. Sci. Eng. C 2020, 117, 111279. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Wang, P.-W.; Alalaiwe, A.; Lin, Z.-C.; Fang, J.-Y. Use of Lipid Nanocarriers to Improve Oral Delivery of Vitamins. Nutrients 2019, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, P.; Ramalingam, P.; Karthivashan, G.; Ko, Y.T.; Choi, D.-K. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int. J. Nanomed. 2018, 13, 1569–1583. [Google Scholar] [CrossRef] [Green Version]
- Hassan, H.; Bello, R.O.; Adam, S.K.; Alias, E.; Affandi, M.M.R.M.M.; Shamsuddin, A.F.; Basir, R. Acyclovir-Loaded solid lipid nanoparticles: Optimization, characterization and evaluation of its pharmacokinetic profile. Nanomaterials 2020, 10, 1785. [Google Scholar] [CrossRef]
- Arora, R.; Kuhad, A.; Kaur, I.; Chopra, K. Curcumin loaded solid lipid nanoparticles ameliorate adjuvant-induced arthritis in rats. Eur. J. Pain 2015, 19, 940–952. [Google Scholar] [CrossRef]
- Thakkar, H.; Sharma, R.K.; Murthy, R.S.R. Enhanced Retention of Celecoxib-Loaded Solid Lipid??Nanoparticles after Intra-Articular Administration. Drugs R&D 2007, 8, 275–285. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Z.; He, X.; Li, Z.; Shi, B.; Cai, F. β-Sitosterol-loaded solid lipid nanoparticles ameliorate complete Freund’s adjuvant-induced arthritis in rats: Involvement of NF-κB and HO-1/Nrf-2 pathway. Drug Deliv. 2020, 27, 1329–1341. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Zhang, D.; Jiang, S.; Shi, K.; Huang, Y.; Li, R.; Xu, Q. Methazolamide-loaded solid lipid nanoparticles modified with low-molecular weight chitosan for the treatment of glaucoma: Vitro and vivo study. J. Drug Target. 2014, 22, 849–858. [Google Scholar] [CrossRef]
- Ahmadifard, Z.; Ahmed, A.; Rasekhian, M.; Moradi, S.; Arkan, E. Chitosan-coated magnetic solid lipid nanoparticles for controlled release of letrozole. J. Drug Deliv. Sci. Tech. 2020, 57, 101621. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.; Junginger, H. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001, 50, S91–S101. [Google Scholar] [CrossRef]
- Alalaiwe, A.; Carpinone, P.; Alshahrani, S.; Alsulays, B.; Ansari, M.; Anwer, M.; Alshehri, S.; Alshetaili, A. Influence of chi-tosan coating on the oral bioavailability of gold nanoparticles in rats. Saudi Pharm. J. 2019, 27, 171–175. [Google Scholar] [CrossRef]
- Kockisch, S.; Rees, G.D.; Young, S.A.; Tsibouklis, J.; Smart, J.D. Polymeric Microspheres for Drug Delivery to the Oral Cavity: An In Vitro Evaluation of Mucoadhesive Potential. J. Pharm. Sci. 2003, 92, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Ahad, A.; Aqil, M.; Akhtar, M.; Sultana, Y.; Ali, A. Formulation by design based risperidone nano soft lipid vesicle as a new strategy for enhanced transdermal drug delivery: In-vitro characterization, and in-vivo appraisal. Mater. Sci. Eng. C 2017, 75, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.; Alayoubi, A.; El-Malah, Y.; Nazzal, S. Modeling the effect of sonication parameters on size and dispersion temperature of solid lipid nanoparticles (SLNs) by response surface methodology (RSM). Pharm. Dev. Technol. 2013, 19, 342–346. [Google Scholar] [CrossRef] [PubMed]
- Shahab, M.S.; Rizwanullah; Alshehri, S.; Imam, S.S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404. [Google Scholar] [CrossRef]
- Imam, S.S.; Aqil, M.; Akhtar, M.; Sultana, Y.; Ali, A. Formulation by design-based proniosome for accentuated transdermal delivery of risperidone: In vitro characterization and in vivo pharmacokinetic study. Drug Deliv. 2015, 22, 1059–1070. [Google Scholar] [CrossRef]
- Dhiman, S.; Mishra, N.; Sharma, S. Development of PEGylated solid lipid nanoparticles of pentoxifylline for their beneficial pharmacological potential in pathological cardiac hypertrophy. Artif. Cells Nanomedicine, Biotechnol. 2015, 44, 1901–1908. [Google Scholar] [CrossRef] [Green Version]
- Campos, J.; Varas-Godoy, M.; Haidar, Z.S. Physicochemical characterization of chitosan-hyaluronan-coated solid lipid nanoparticles for the targeted delivery of paclitaxel: A proof-of-concept study in breast cancer cells. Nanomedicine 2017, 12, 473–490. [Google Scholar] [CrossRef]
- Zhai, Y.; Guo, S.; Liu, C.; Yang, C.; Dou, J.; Li, L.; Zhai, G. Preparation and in vitro evaluation of apigenin-loaded polymeric micelles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 429, 24–30. [Google Scholar] [CrossRef]
- Gilani, S.; Bin-Jumah, M.; Rizwanullah; Imam, S.; Imtiyaz, K.; AlShehri, S.; Rizvi, M. Chitosan Coated Luteolin Nanostructured Lipid Carriers: Optimization, In Vitro-Ex Vivo Assessments and Cytotoxicity Study in Breast Cancer Cells. Coatings 2021, 11, 158. [Google Scholar] [CrossRef]
- Roy, S.; Mallick, S.; Chakraborty, T.; Ghosh, N.; Singh, A.K.; Manna, S.; Majumdar, S. Synthesis, characterisation and antioxidant activity of luteolin–vanadium(II) complex. Food Chem. 2015, 173, 1172–1178. [Google Scholar] [CrossRef]
- Funakoshi-Tago, M.; Nakamura, K.; Tago, K.; Mashino, T.; Kasahara, T. Anti-inflammatory activity of structurally related flavonoids, Apigenin, Luteolin and Fisetin. Int. Immunopharmacol. 2011, 11, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi-Tago, M.; Nakamura, K.; Tsuruya, R.; Hatanaka, M.; Mashino, T.; Sonoda, Y.; Kasahara, T. The fixed structure of Licochalcone A by alpha, beta-unsaturated ketone is necessary for anti-inflammatory activity through the inhibition of NF-kappa B activation. Int. Immunopharmacol. 2010, 10, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Vendramini-Costa, D.B.; Spindola, H.M.; de Mello, G.C.; Antunes, E.; Pilli, R.A.; de Carvalho, J.E. Anti-inflammatory and antinociceptive effects of racemic goniothalamin, a styryl lactone. Life Sci. 2015, 139, 83–90. [Google Scholar] [CrossRef]
- Padhye, S.G.; Nagarsenker, M.S. Simvastatin Solid Lipid Nanoparticles for Oral Delivery: Formulation Development and In vivo Evaluation. Indian J. Pharm. Sci. 2013, 75, 591–598. [Google Scholar] [PubMed]
- Zirak, M.B.; Pezeshki, A. Effect of Surfactant Concentration on the Particle Size, Stability and Potential Zeta of Beta carotene Nano Lipid Carrier. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 924–932. [Google Scholar]
- Pradhan, S.; Hedberg, J.; Blomberg, E.; Wold, S.; Wallinder, I.O. Effect of sonication on particle dispersion, administered dose and metal release of non-functionalized, non-inert metal nanoparticles. J. Nanoparticle Res. 2016, 18, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telange, D.R.; Patil, A.T.; Pethe, A.M.; Fegade, H.; Anand, S.; Dave, V.S. Formulation and characterization of an apigenin-phospholipid phytosome (APLC) for improved solubility, in vivo bioavailability, and antioxidant potential. Eur. J. Pharm. Sci. 2017, 108, 36–49. [Google Scholar] [CrossRef] [Green Version]
- Al Shaal, L.; Shegokar, R.; Müller, R.H. Production and characterization of antioxidant apigenin nanocrystals as a novel UV skin protective formulation. Int. J. Pharm. 2011, 420, 133–140. [Google Scholar] [CrossRef]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharm 2017, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, S.; Yoo, H.S. The Application of Mucoadhesive Chitosan Nanoparticles in Nasal Drug Delivery. Mar. Drugs 2020, 18, 605. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Zheng, Z.; Sun, Y.; Liu, Z.; Zhang, M.; Li, C. The effect of curcumin and its nanoformulation on adjuvant-induced arthritis in rats. Drug Des. Dev. Ther. 2015, 9, 4931–4942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeom, M.; Hahm, D.H.; Sur, B.J.; Han, J.J.; Lee, H.J.; Yang, H.I.; Kim, K.S. Phosphatidylserine inhibits inflammatory responses in interleukin-1b-stimulated fibroblast-like synoviocytes and alleviates carrageenan-induced arthritis in rat. Nutr. Res. 2013, 33, 242–250. [Google Scholar] [CrossRef]
- Gautam, R.; Singh, M.; Gautam, S.; Rawat, J.K.; Saraf, S.A.; Kaithwas, G. Rutin attenuates intestinal toxicity induced by Methotrexate linked with anti-oxidative and anti-inflammatory effects. BMC Complement. Altern. Med. 2016, 16, 99. [Google Scholar] [CrossRef] [Green Version]
- Frank, L.A.; Onzi, G.R.; Morawski, A.S.; Pohlmann, A.R.; Guterres, S.S.; Contri, R.V. Chitosan as a coating material for nanoparticles intended for biomedical Applications. React. Funct. Polym. 2020, 147, 104459. [Google Scholar] [CrossRef]
- Li, Y.; Kakkar, R.; Wang, J. In vivo and in vitro Approach to Anti-arthritic and Anti-inflammatory Effect of Crocetin by Al-teration of Nuclear Factor-E2-Related Factor 2/hem Oxygenase (HO)-1 and NF-κB Expression. Front. Pharmacol. 2018, 9, 1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rayhana, B.; Sheliya, M.A.; Pillai, K.K.; Aeri, V.; Sharma, M. Evaluation of anti-inflammatory effect of Careya arborea in CFA induced chronic inflammation. Int. J. Pharm. Sci. Rev. Res. 2014, 26, 292–298. [Google Scholar]
- Kaithwas, G.; Gautam, R.; Jachak, S.M.; Saklani, A. Antiarthritic effects of Ajuga bracteosa Wall ex Benth. in acute and chronic models of arthritis in albino rats. Asian Pac. J. Trop. Biomed. 2012, 2, 185–188. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.-J.; Chen, J.; Xiong, X.-G.; Wu, D.; Zhu, H.; Liang, Q.-H. Effect of Bizhongxiao decoction and its dismantled formulae on IL-1 and TNF levels in collagen-induced arthritis in rat synovial joints. Theor. Biol. Med. Model. 2012, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; He, H.; Zhu, L.; Gao, J.; Wei, T.; Ma, Z.; Yan, T. Protective effect of apigenin on Freund’s complete adjuvant-induced arthritis in rats via inhibiting P2X7/NF-κB pathway. Chem. Biol. Interact. 2015, 236, 41–46. [Google Scholar] [CrossRef]
- Spindola, H.M.; Vendramini-Costa, D.B.; Rodrigues, M.T., Jr.; Foglio, M.A.; Pilli, R.A.; Carvalho, J.E. The antinociceptive activity of harmicine on chemical-induced neurogenic and inflammatory pain models in mice. Pharmacol. Biochem. Behav. 2012, 102, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Boonyarikpunchai, W.; Sukrong, S.; Towiwat, P. Antinociceptive and anti-inflammatory effects of rosmarinic acid isolated from Thunbergia laurifolia Lindl. Pharmacol. Biochem. Behav. 2014, 124, 67–73. [Google Scholar] [CrossRef] [PubMed]







| Independent Variables | Level | Dependent Variables | Target | ||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| GMS (mg) | 250 | 300 | 350 | Particle size (nm) | 100–250 nm |
| TPGS (mg) | 175 | 200 | 225 | Encapsulation efficiency (%) | 50–80% |
| Sonication time (min) | 4 | 6 | 8 | - | - |
| Formulations | GMS | TPGS | Sonication Time | Particle Size (nm) | Encapsulation Efficiency (%) | ||
|---|---|---|---|---|---|---|---|
| mg | mg | min | Actual | Predicted | Actual | Predicted | |
| 1 | 250 | 175 | 6 | 100.13 | 103.84 | 48.27 | 52.28 |
| 2 | 350 | 175 | 6 | 160.31 | 164.58 | 67.32 | 69.26 |
| 3 | 250 | 225 | 6 | 115.69 | 111.42 | 71.18 | 75.24 |
| 4 | 350 | 225 | 6 | 140.88 | 137.16 | 66.83 | 68.82 |
| 5 | 250 | 200 | 4 | 166.59 | 160.60 | 76.49 | 72.54 |
| 6 | 350 | 200 | 4 | 180.90 | 187.35 | 80.66 | 83.77 |
| 7 | 250 | 200 | 8 | 129.10 | 122.65 | 66.94 | 61.83 |
| 8 | 350 | 200 | 8 | 202.41 | 211.40 | 77.21 | 75.16 |
| 9 | 300 | 175 | 4 | 156.91 | 164.19 | 69.31 | 66.25 |
| 10 | 300 | 225 | 4 | 180.08 | 171.26 | 85.02 | 81.91 |
| 11 | 300 | 175 | 8 | 175.20 | 179.74 | 66.87 | 69.98 |
| 12 | 300 | 225 | 8 | 148.09 | 147.81 | 73.79 | 70.85 |
| 13 | 300 | 200 | 6 | 146.12 | 152.12 | 68.10 | 65.43 |
| 14 | 300 | 200 | 6 | 147.21 | 151.12 | 68.80 | 65.43 |
| 15 | 300 | 200 | 6 | 148.32 | 145.12 | 68.38 | 64.43 |
| Statistics | PS | EE | Coefficient of Determination | PS | EE |
|---|---|---|---|---|---|
| Std. Dev. | 0.8 | 0.25 | R-Squared | 0.9997 | 0.9997 |
| Mean | 153.13 | 70.34 | Adj R-Squared | 0.9991 | 0.9991 |
| CV % | 0.52 | 1.68 | Pred R-Squared | 0.9975 | 0.9983 |
| PS = + 147.00 + 21.62 × A − 0.96 × B − 3.72 × C − 8.75 × A × B + 14.75 × A × C − 12.50 × B × C −6.50 × A2 − 11.25 × B2 + 29.25 × C2 EE = + 68.43 + 3.64 × A + 5.63 × B − 3.33 × C − 5.85 × A × B + 1.53 × A × C − 2.20 × B × C − 1.72 × A2 − 3.30 × B2 + 8.62 × C2 | |||||
| Groups | TNF-α (pg/mL) | IL-1β (pg/mL) | MDA (nmol/mg Protein) | SOD (U/mg Protein) | CAT (U/mg Protein) |
|---|---|---|---|---|---|
| Normal Control | 55.16 ± 4.27 | 121.72 ± 10.26 | 8.36 ± 0.89 | 38.17 ± 2.17 | 19.05 ± 1.04 |
| Arthritic Control | 138.19 ± 12.19 x | 193.20 ± 16.27 x | 15.27 ± 1.21 x | 12.94 ± 1.82 x | 7.16 ± 0.21 x |
| APG-CH-SLNs | 61.73 ± 6.83 c | 130.04 ± 10.51 c | 10.26 ± 1.04 c | 33.20 ± 2.04 c | 16.20 ± 1.21 b |
| APG | 102.83 ± 8.04 b | 157.26 ± 12.66 a | 12.21 ± 1.20 a | 27.79 ± 1.60 b | 12.05 ± 1.05 a |
| Indomethacin | 65.29 ± 5.37 c | 127.41± 10.05 c | 9.04 ± 0.43 c | 36.26 ± 2.71 c | 18.31 ± 1.61 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilani, S.J.; Bin-Jumah, M.N.; Imam, S.S.; Alshehri, S.; Jahangir, M.A.; Zafar, A. Formulation and Optimization of Nano Lipid Based Oral Delivery Systems for Arthritis. Coatings 2021, 11, 548. https://doi.org/10.3390/coatings11050548
Gilani SJ, Bin-Jumah MN, Imam SS, Alshehri S, Jahangir MA, Zafar A. Formulation and Optimization of Nano Lipid Based Oral Delivery Systems for Arthritis. Coatings. 2021; 11(5):548. https://doi.org/10.3390/coatings11050548
Chicago/Turabian StyleGilani, Sadaf Jamal, May Nasser Bin-Jumah, Syed Sarim Imam, Sultan Alshehri, Mohammed Asadullah Jahangir, and Ameeduzzafar Zafar. 2021. "Formulation and Optimization of Nano Lipid Based Oral Delivery Systems for Arthritis" Coatings 11, no. 5: 548. https://doi.org/10.3390/coatings11050548






