Preparation and Characterization of a Silane Sealed PEO Coating on Aluminum Alloy
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of PEO/Silane Composite Coating
2.2. Characterization Analysis
2.3. Electrochemical Measurements
3. Results and Discussion
3.1. Morphologies of the Coatings
3.2. Formation Mechanism of Silane Coating
3.3. Electrochemical Corrosion Behaviour
3.3.1. Potentiodynamic Polarization Analysis
3.3.2. EIS Analysis
3.4. Corrosion Morphology
4. Conclusions
- In the long-term immersion test, the corrosion evolution of the PEO/silane samples could be divided into three stages: (i) in the initial stage of immersion, the value of ng was close to one, the silane coating formed a capacitive equivalent circuit, and the corrosion rate was controlled by CPEg-T; (ii) in the second stage of immersion, the corrosive media penetrated to the PEO coating through the defects of the outer layer, and the charge transfer in the PEO coating became the main factor controlling the corrosion rate; and (iii) in the third stage of immersion, the value of ng was decreased to close 0, Rg and Rdiff increased rapidly, suggesting that the silane coating formed a resistor equivalent circuit and the corrosion products (e.g., Al(OH)2Cl, Al(OH)Cl2, AlCl3 et al.) diffused in the coating and controlled the corrosion process.
- EIS was used to analyse the corrosion evolution of the PEO/silane composite coating on UNS A97075 alloy. The resistance of PEO-S1 remained at 106 Ω·cm2 and with small changes compared to that of PEO-S2, and the PEO film under the silane layer remained dense and continuous. It could be concluded that the thickness of the silane layer delayed the corrosion process, but the corrosion resistance mainly depended on the densification of the silane coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wernick, S.; Pinner, R.; Sheasby, P.G. The Surface Treatment and Finishing of Aluminum and Its Adlloys, 5th ed.; Finishing Publications Ltd: Warrington, UK, 1987. [Google Scholar]
- Henley, V.F. Anodic Oxidation of Aluminium & Its Alloys; Pergamon Press: Oxford, UK, 1982. [Google Scholar]
- Yerokhin, A.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Javidi, M.; Fadaee, H. Plasma electrolytic oxidation of 2024-T3 aluminum alloy and investigation on microstructure and wear behavior. Appl. Surf. Sci. 2013, 286, 212–219. [Google Scholar] [CrossRef]
- Arrabal, R.; Mohedano, M.; Matykina, E.; Pardo, A.; Mingo, B.; Merino, M. Characterization and wear behaviour of PEO coatings on 6082-T6 aluminium alloy with incorporated α-Al2O3 particles. Surf. Coat. Technol. 2015, 269, 64–73. [Google Scholar] [CrossRef]
- Chen, M.-A.; Ou, Y.-C.; Fu, Y.-H.; Li, Z.-H.; Li, J.-M.; Liu, S.-D. Effect of friction stirred Al-Fe-Si particles in 6061 aluminum alloy on structure and corrosion performance of MAO coating. Surf. Coat. Technol. 2016, 304, 85–97. [Google Scholar] [CrossRef]
- Dehnavi, V.; Shoesmith, D.W.; Luan, B.L.; Yari, M.; Liu, X.Y.; Rohani, S. Corrosion properties of plasma electrolytic oxidation coatings on an aluminium alloy—The effect of the PEO process stage. Mater. Chem. Phys. 2015, 161, 49–58. [Google Scholar] [CrossRef]
- Fadaee, H.; Javidi, M. Investigation on the corrosion behaviour and microstructure of 2024-T3 Al alloy treated via plasma electrolytic oxidation. J. Alloy Compd. 2014, 604, 36–42. [Google Scholar] [CrossRef]
- Liu, C.; He, D.; Yan, Q.; Huang, Z.; Liu, P.; Li, D.; Jiang, G.; Ma, H.; Nash, P.; Shen, D. An investigation of the coating/substrate interface of plasma electrolytic oxidation coated aluminum. Surf. Coat. Technol. 2015, 280, 86–91. [Google Scholar] [CrossRef]
- Hussein, R.; Nie, X.; Northwood, D. Production of high quality coatings on light alloys using plasma electrolytic oxidation (PEO). WIT Trans. Built Environ. 2016, 166, 439–454. [Google Scholar]
- Zhu, L.; Guo, Z.; Zhang, Y.; Li, Z.; Sui, M. A mechanism for the growth of a plasma electrolytic oxide coating on Al. Electrochim. Acta 2016, 208, 296–303. [Google Scholar] [CrossRef]
- Hu, N.; Dong, X.; He, X. Effect of sealing on the morphology of anodized aluminum oxide. Corros. Sci. 2015, 97, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Shulman, G.P.; Bauman, A.J. Organic acid sealants for anodized aluminum-a new method for corrosion protection. Met. Finish. 1995, 93, 16–19. [Google Scholar] [CrossRef]
- Shahzad, M.; Chaussumier, M.; Chieragatti, R.; Mabru, C.; Rezai-Aria, F. Effect of sealed anodic film on fatigue performance of 2214-T6 aluminum alloy. Surf. Coat. Technol. 2012, 206, 2733–2739. [Google Scholar] [CrossRef] [Green Version]
- Shang, W.; Chen, B.; Shi, X.; Chen, Y.; Xiao, X. Electrochemical corrosion behavior of composite MAO/sol–gel coatings on magnesium alloy AZ91D using combined micro-arc oxidation and sol–gel technique. J. Alloy Compd. 2009, 474, 541–545. [Google Scholar] [CrossRef]
- Van Phuong, N.; Fazal, B.R.; Moon, S. Cerium- and phosphate-based sealing treatments of PEO coated AZ31 Mg alloy. Surf. Coat. Technol. 2017, 309, 86–95. [Google Scholar] [CrossRef]
- Yu, X.W.; Yan, C.W.; Cao, C.N. Study on the rare earth sealing procedure of the porous film of anodized 2024 aluminum alloy. Mater. Sci. Technol. 2003, 19, 51–53. [Google Scholar]
- Wang, S.X.; Zhao, Q.; Du, N.; Shao, Z.S.; Shu, W.F.; Chen, Q.L. Sealing effect of cerium salt on boric-sulfuric acid anodic film of aluminum alloy. Chin. J. Nonferrous Met. 2012, 22, 1132–1141. [Google Scholar]
- Vuori, L.; Ali-Löytty, H.; Lahtonen, K.; Hannula, M.; Lehtonen, E.; Niu, Y.; Valden, M. Improved corrosion properties of hot dip galvanized steel by nanomolecular silane layers as hybrid interface between zinc and top coatings. Corrosion 2017, 73, 169–180. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Peikari, M.; Zaarei, D.; Danaee, I.; Fakhraei, J.M.; Mohammadi, M. Using silane films to produce an alternative for chromate conversion coatings. Corrosion 2013, 69, 372–387. [Google Scholar] [CrossRef]
- Wojciechowski, J.; Szubert, K.; Peipmann, R. Anti-corrosive properties of silane coatings deposited on anodised aluminium. Electrochim. Acta 2016, 220, 1–10. [Google Scholar] [CrossRef]
- Whelan, M.; Cassidy, J.; Duffy, B. Sol–gel sealing characteristics for corrosion resistance of anodised aluminium. Surf. Coat. Technol. 2013, 235, 86–96. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, Z.; Bai, S.; Wang, J. Influence of sealing treatment on the corrosion resistance of PEO coated Al-Zn-Mg-Cu alloy in various environments. Coatings 2019, 9, 867. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Du, K.; Guo, Q.; Ge, H.; Wang, Y.; Wang, R.; Wang, F. Water uptake of as andwich-structured composite film coated on a Mg-Gd-Y alloy: Experimentsand model validation. J. Electrochem. Soc. 2014, 161, 188–194. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, Z.; Bai, S.; Zhao, J.; Wang, J. Corrosion resistance of bis-silane-modified epoxy coatings on an Al-Zn-Mg-Cu alloy. J. Mater. Eng. Perform. 2020, 29, 5282–5290. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.M.; Zhou, Y.; Ouyang, J.H.; Guo, L.; Jia, D. Corrosion evaluation of microarc oxidation coatings formed on 2024 aluminum alloy. Corros. Sci. 2010, 52, 2687–2696. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Sinebryukhov, S.L.; Egorkin, V.S. Wettability and electrochemical properties of the highly hydrophobic coatings on PEO-pretreated aluminum alloy. Surf. Coat. Technol. 2016, 307, 1241–1248. [Google Scholar] [CrossRef]
- Barik, R.C.; Wharton, J.A.; Wood, R.J.K.; Stokes, K.R.; Jones, R.L. Corrosion, erosion and erosion–corrosion performance of plasma electrolytic oxidation (PEO) deposited Al2O3 coatings. Surf. Coat. Technol. 2005, 199, 158–167. [Google Scholar] [CrossRef]
- ChruscielJerzy, J.J.; Lesniak, E. Modification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Williams, K.G.; Gido, S.P.; Coughlin, E.B. Polymers and Copolymers Containing Covalently Bonded Polyhedral Oligomeric Silsesquioxanes Moieties. In Applications of Polyhedral Oligomeric Silsesquioxanes; Springer Netherlands: Dordrecht, The Netherlands, 2011. [Google Scholar]
- Vejar, N.D.; Azocar, M.I.; Tamayo, L.A.; Gonzalez, E.; Pavez, J.; Gulppi, M.; Zagal, J.H.; Zhou, X.; Santibanez, F.; Thompson, G.E.; et al. Anti biofouling properties of sol-gel type polymers for aluminium alloys: Biocorrosion protection against pseudomonas aeruginosa. Int. J. Electrochem. Sci. 2013, 8, 12062–12077. [Google Scholar]
- Hu, J.M.; Liu, L.; Zhang, J.Q.; Cao, C.N. Electro deposition of silane films on aluminum alloys for corrosion protection. Prog. Org. Coat. 2007, 58, 265–271. [Google Scholar] [CrossRef]
- Jothi, K.J.; Palanivelu, K. Synergistic effect of silane modified nano-composites for active corrosion protection. Ceram. Int. 2013, 39, 7619–7625. [Google Scholar] [CrossRef]
- Rubio, F.; Rubio, J.; Oteo, L. A FT-IR study of the hydrolysis of tetraethylorthosilicate (TEOS). Spectrosc. Lett. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- Pena-Alonso, R.; Rubio, F.; Rubio, J.; Oteo, J.L. Study of the hydrolysis and condensation of γ-Aminopropyltriethoxysilane by FT-IR spectroscopy. J. Mater. Sci. 2006, 42, 595–603. [Google Scholar] [CrossRef]
- Jiang, H.; Zheng, Z.; Wang, X. Kinetic study of methyltriethoxysilane (MTES) hydrolysis by FTIR spectroscopy under different temperatures and solvents. Vib. Spectrosc. 2008, 46, 1–7. [Google Scholar] [CrossRef]
- Zhang, J.-T.; Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Studies of water transport behavior and impedance models of epoxy-coated metals in NaCl solution by EIS. Prog. Org. Coat. 2004, 51, 145–151. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Gao, R.; Wang, J.; Yang, W.; Liu, L. One-step method for the fabrication of superhydrophobic surface on magnesium alloy and its corrosion protection, antifouling performance. Corros. Sci. 2014, 80, 177–183. [Google Scholar] [CrossRef]
- Liu, Q.; Kang, Z.X. One-step electrodeposition process to fabricate superhydrophobic surface with improved anticorrosion property on magnesium alloy. Mater. Lett. 2014, 137, 210–213. [Google Scholar] [CrossRef]
- Han, M.; Go, S.; Ahn, Y. Fabrication of super-hydrophobic surface on magnesium substrate by chemical etching. Bull. Korean Chem. Soc. 2012, 33, 1363–1366. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.M.; Zhang, J.Q.; Cao, C.N. Determination of water uptake and diffusion of Cl− ion in epoxy primer on aluminum alloys in NaCl solution by electrochemical impedance spectroscopy. Prog. Org. Coat. 2003, 46, 273–279. [Google Scholar] [CrossRef]
- Zhang, J.T.; Hu, J.M.; Zhang, J.Q.; Cao, C.N. Studies of impedance models and water transport behaviors of polypropylene coated metals in NaCl solution. Prog. Org. Coat. 2004, 49, 293–301. [Google Scholar] [CrossRef]
- Shen, D.; He, D.; Liu, F.; Guo, C.; Cai, J.; Li, G.; Ma, H. Effects of ultrasound on the evolution of plasma electrolytic oxidation process on 6061Al alloy. Ultrasonics 2014, 54, 1065–1070. [Google Scholar] [CrossRef]
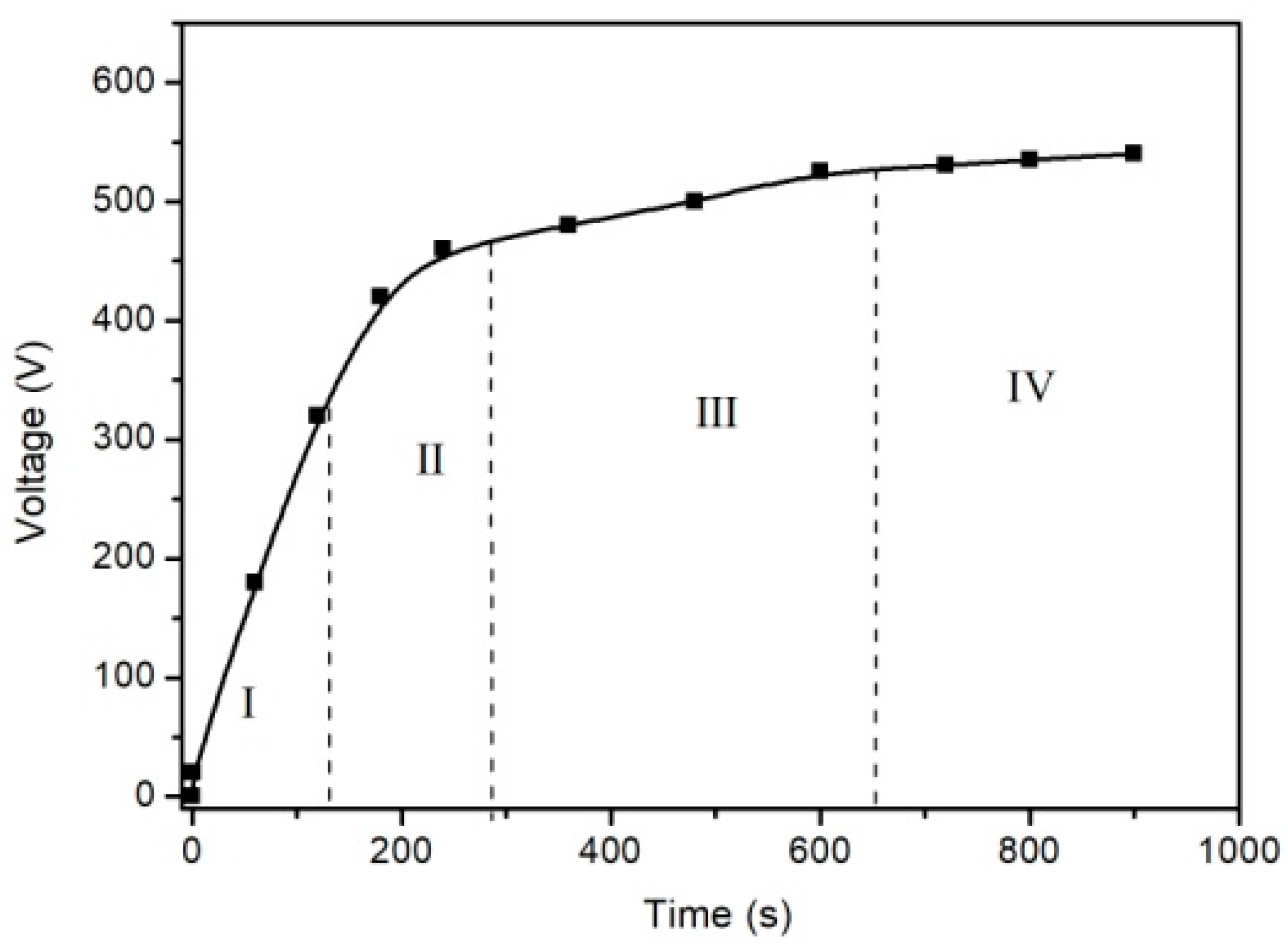

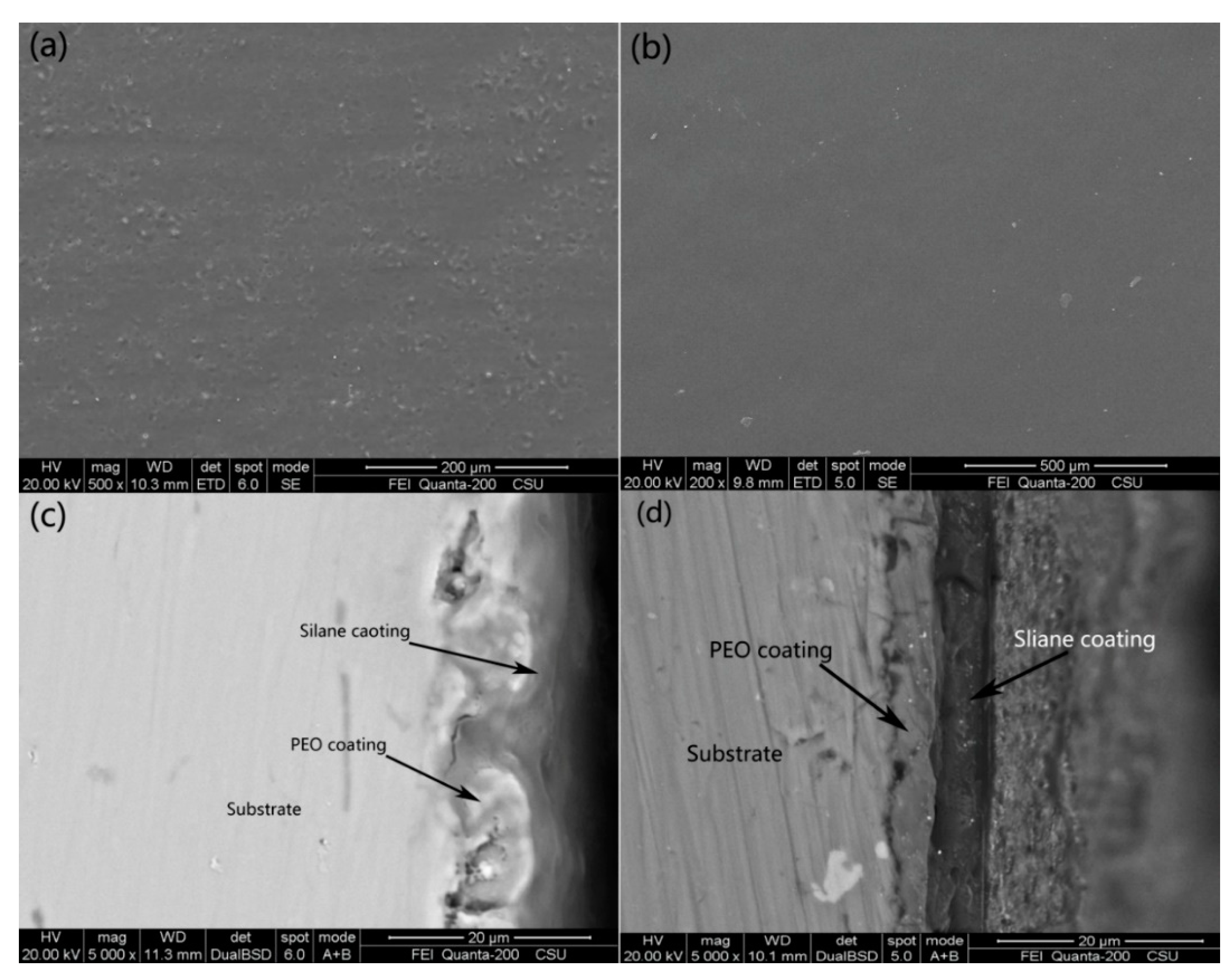
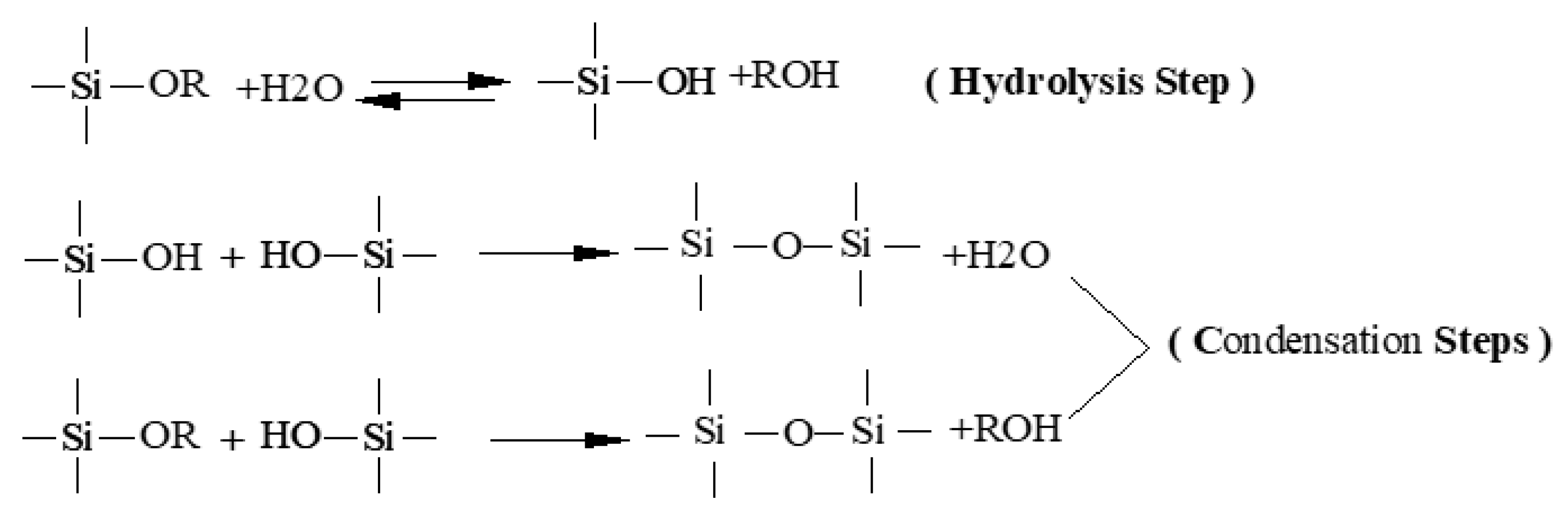
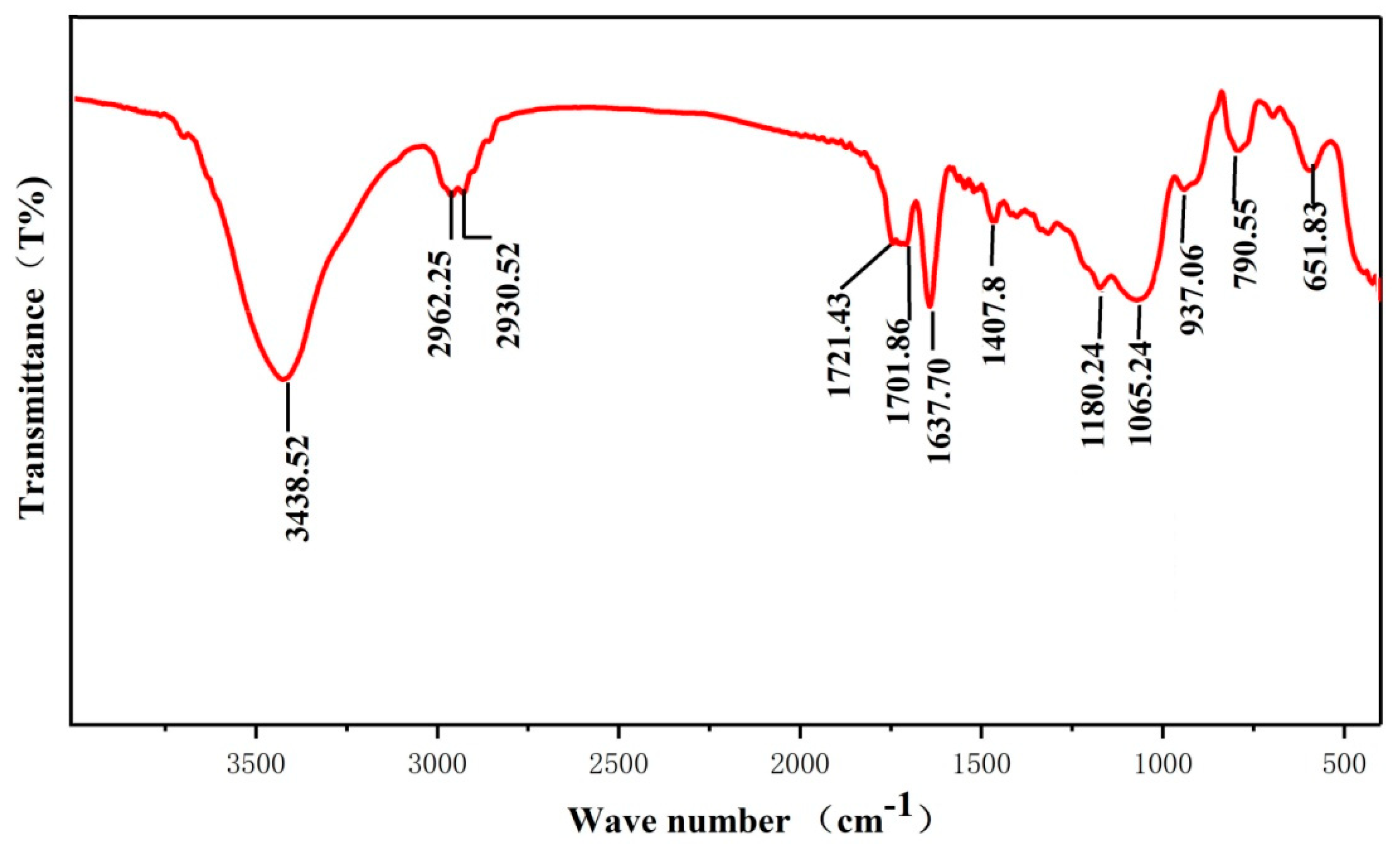
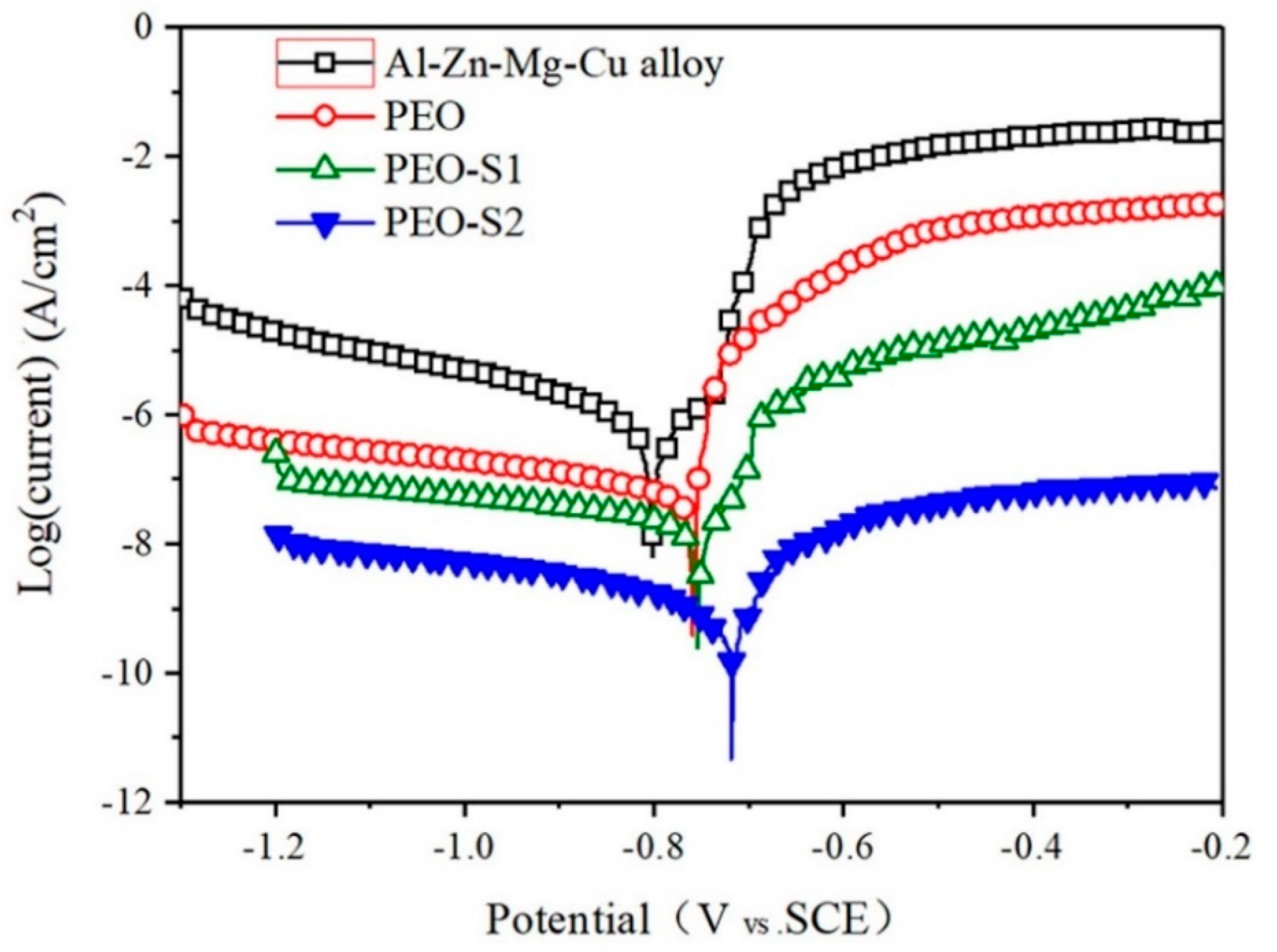

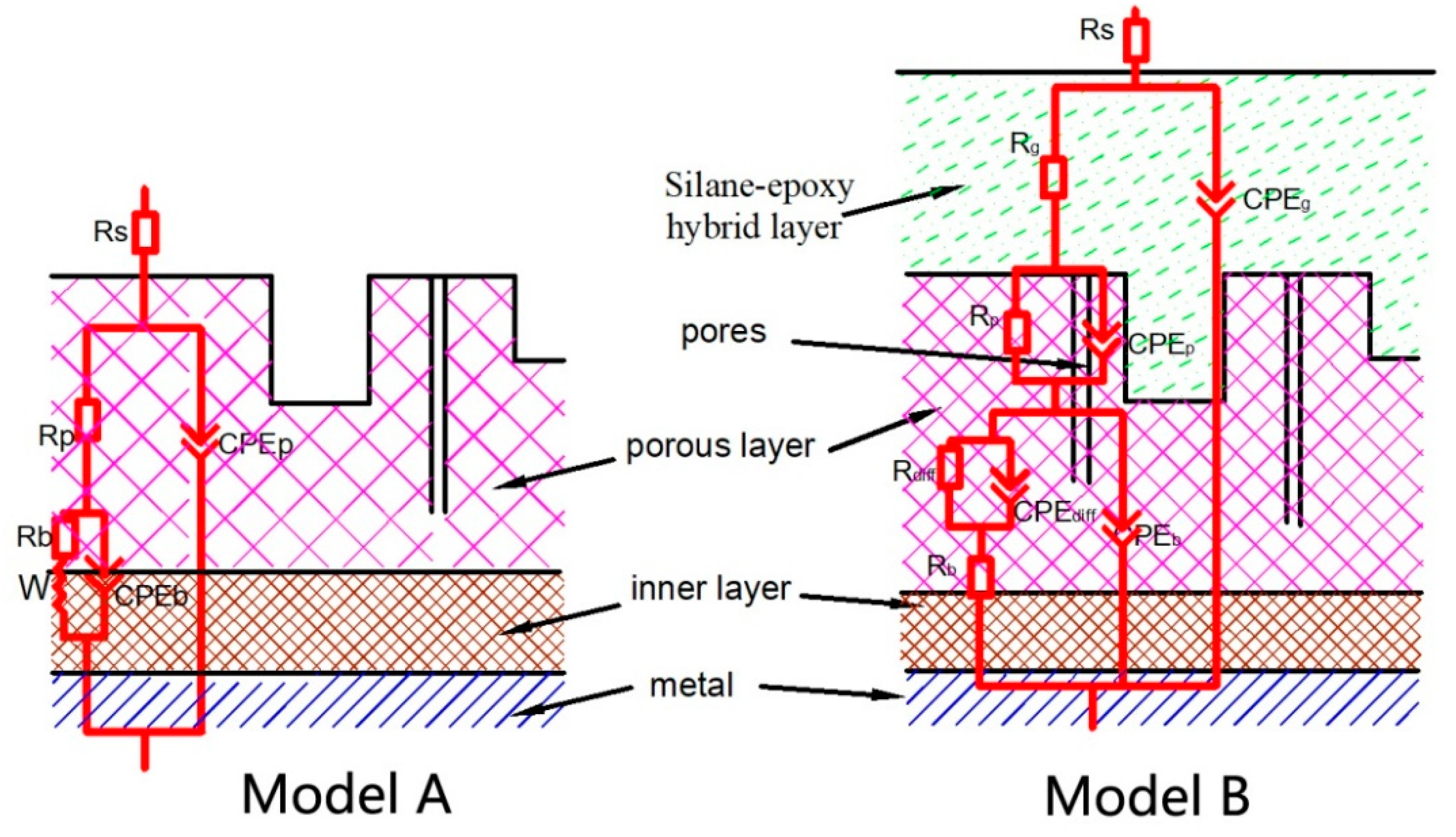

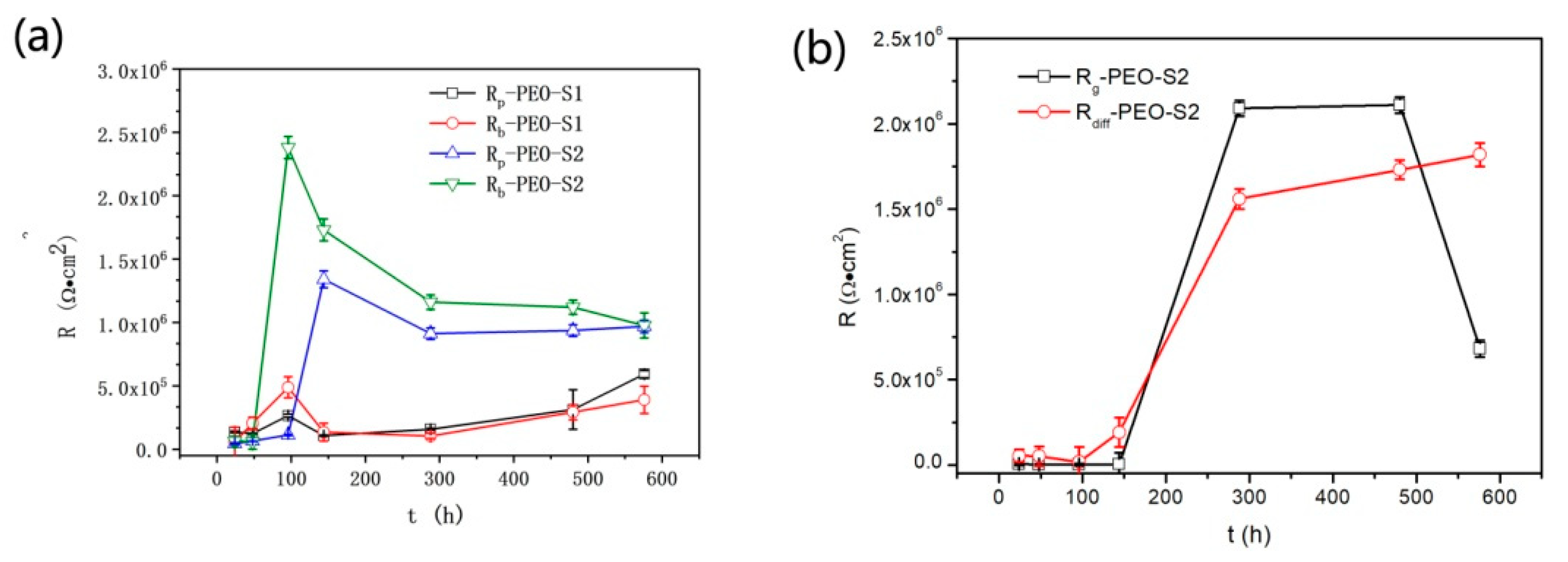
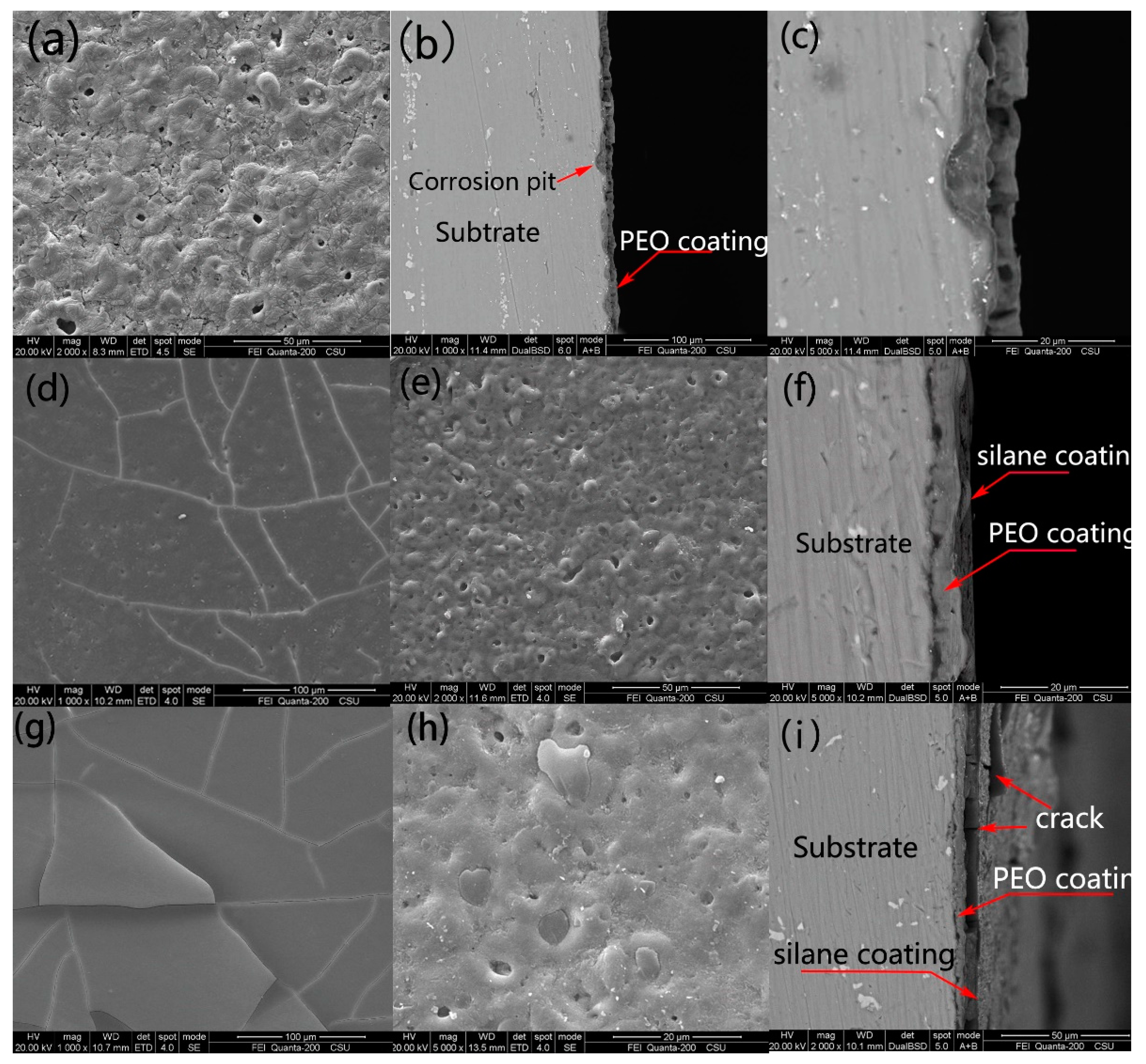
| Samples | Ecorr, (Vvs SCE) | Standard Deviation | icorr (A/cm2) | Standard Deviation |
|---|---|---|---|---|
| Bare | −0.80 | 0.018 | 1.02 × 10−6 | 0.012 × 10−6 |
| PEO PEO-S1 | −0.75 −0.73 | 0.012 0.014 | 1.08 × 10−8 2.24 × 10−9 | 0.06 × 10−6 0.08 × 10−6 |
| PEO-S2 | −0.71 | 0.016 | 3.48 × 10−11 | 0.04 × 10−6 |
| PEO | 24 h | 48 h | 96 h | 144 h | 288 h | 576 h |
|---|---|---|---|---|---|---|
| Rp(Ω·cm2) | 333 | 310 | 67 | 44 | 150 | 1.32×103 |
| CPEp-T (F·cm−2) | 6.6 × 10−7 | 5.72 × 10−8 | 1.84 × 10−8 | 4.42 × 10−8 | 4.21 × 10−6 | 1.34 × 10−5 |
| np | 0.85 | 0.99 | 0.98 | 0.99 | 0.92 | 0.82 |
| Rb(Ω·cm2) | 8.63 × 104 | 7.16 × 104 | 6.62 × 104 | 6.84 × 104 | 6.56 × 104 | 5.10 × 104 |
| CPEb-T(F·cm−2) | 1.10 × 10−6 | 8.21 × 10−6 | 8.64 × 10−5 | 6.11 × 10−6 | 6.12 × 10−5 | 1.03 × 10−5 |
| nb | 0.46 | 0.68 | 0.73 | 0.81 | 0.95 | 0.98 |
| χ2 (Chi-squared) | 0.002 | 0.0006 | 0.003 | 0.002 | 0.001 | 0.0004 |
| PEO-S2 | 24 h | 48 h | 96 h | 144 h | 288 h | 480 h | 576 h |
|---|---|---|---|---|---|---|---|
| Rg (Ω·cm2) | 4230 | 2928 | 3221 | 3907 | 2.09 × 106 | 2.11 × 106 | 6.82 × 105 |
| CPEg-T (F·cm−2) | 3.21 × 10−12 | 1.87 × 10−10 | 4.23 × 10−9 | 1.84 × 10−9 | 3.63 × 10−9 | 5.69 × 10−9 | 2.58 × 10−10 |
| Rp (Ω·cm2) | 6.36 × 104 | 4.36 × 104 | 1.12 × 105 | 2.84 × 106 | 9.12 × 105 | 1.36 × 106 | 9.68 × 105 |
| CPEp-T (F·cm−2) | 3.26 × 10−7 | 3.12 × 10−6 | 1.14 × 10−7 | 7.42 × 10−7 | 7.61 × 10−7 | 3.31 × 10−5 | 7.84 × 10−7 |
| Rb (Ω·cm2) | 6.41 × 104 | 5.86 × 104 | 2.38 × 106 | 1.73 × 106 | 1.16 × 106 | 1.12 × 106 | 9.76 × 105 |
| CPEb-T (F·cm−2) | 3.52 × 10−7 | 3.84 × 10−6 | 1.64 × 10−6 | 2.1 × 10−6 | 9.32 × 10−6 | 8.78 × 10−6 | 6.97 × 10−7 |
| Rdiff (Ω·cm2) | 5.41 × 104 | 5.16 × 104 | 1.81 × 104 | 1.91 × 105 | 1.56 × 106 | 1.73 × 106 | 1.82 × 106 |
| CPEdiff-T (F·cm−2) | 7.41 × 10−5 | 2.41 × 10−7 | 9.01 × 10−7 | 2.82 × 10−6 | 7.39 × 10−7 | 6.49 × 10−7 | 9.82 × 10−6 |
| χ2 (Chi-squared) | 0.003 | 0.002 | 0.0004 | 0.005 | 0.0007 | 0.0008 | 0.003 |
| PEO-S1 | 24 h | 48 h | 96 h | 144 h | 288 h | 480 h | 576 h |
| Rg (Ω·cm2) | 6374 | 3236 | 2657 | 4513 | 4360 | 1317 | 2158 |
| CPEg -T (F·cm−2) | 3.98 × 10−12 | 4.58 × 10−12 | 3.81 × 10−12 | 7.70 × 10−12 | 1.08 × 10−12 | 9.78 × 10−12 | 8.51 × 10−12 |
| Rp (Ω·cm2) | 1.36 × 105 | 1.22 × 105 | 1.16 × 105 | 1.49 × 105 | 4.97 × 105 | 6.41 × 106 | 4.92 × 105 |
| CPEp-T (F·cm−2) | 4.32 × 10−4 | 3.69 × 10−6 | 1.3 × 10−4 | 5.18 × 10−8 | 1.78 × 10−6 | 1.75 × 10−5 | 1.76 × 10−6 |
| Rb (Ω·cm2) | 5.74 × 104 | 2.04 × 105 | 4.88 × 105 | 1.35 × 105 | 1.04 × 105 | 4.85 × 105 | 8.89 × 105 |
| CPEb-T (F·cm−2) | 4.04 × 10−8 | 8.24 × 10−6 | 5.66 × 10−6 | 1.03 × 10−6 | 5.52 × 10−6 | 1.62 × 10−6 | 2.34 × 10−6 |
| Rdiff (Ω·cm2) | 4.57 × 105 | 3.67 × 104 | 6.45 × 105 | 8.26 × 104 | 1.11 × 105 | 4.81 × 105 | 8.84 × 105 |
| CPEdiff-T (F·cm−2) | 7.68 × 10−7 | 3.78 × 10−7 | 7.66 × 10−7 | 8.76 × 10−7 | 5.68 × 10−6 | 2.06 × 10−6 | 1.73 × 10−6 |
| χ2 (Chi-squared) | 0.001 | 0.003 | 0.0006 | 0.004 | 0.002 | 0.004 | 0.0007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, D.; Liu, Z.; Bai, S.; Zhao, J. Preparation and Characterization of a Silane Sealed PEO Coating on Aluminum Alloy. Coatings 2021, 11, 549. https://doi.org/10.3390/coatings11050549
Zeng D, Liu Z, Bai S, Zhao J. Preparation and Characterization of a Silane Sealed PEO Coating on Aluminum Alloy. Coatings. 2021; 11(5):549. https://doi.org/10.3390/coatings11050549
Chicago/Turabian StyleZeng, Diping, Zhiyi Liu, Song Bai, and Juangang Zhao. 2021. "Preparation and Characterization of a Silane Sealed PEO Coating on Aluminum Alloy" Coatings 11, no. 5: 549. https://doi.org/10.3390/coatings11050549





