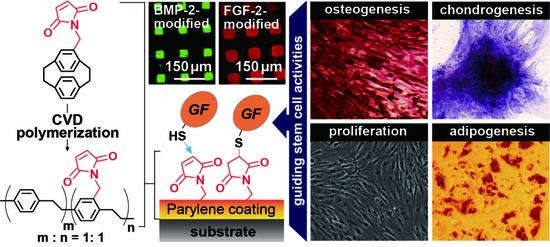
Guiding Stem Cell Differentiation and Proliferation Activities Based on Nanometer-Thick Functionalized Poly-p-xylylene Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. CVD Surface Modifications
2.2. Growth Factor Immobilization
2.3. Characterizations
2.4. µCP and Immunofluorescence
2.5. Induced Cellular Activities
2.6. Osteogenesis
2.7. Chondrogenesis
2.8. Adipogenesis
2.9. Proliferation Activities
2.10. Gene Expression Profiles
2.11. Statistical Analysis
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PPX | Poly-p-xylylene |
| BMP-2 | Bone Morphogenetic Protein 2 |
| FGF-2 | Fibroblast Growth Factor 2 |
| hADSCs | Human Adipose-Derived Stem Cells |
| CVD | Chemical Vapor Deposition |
| Maleimide-PPX | Poly[(4-N-maleimidomethyl-p-xylylene)-co(p-xylylene)] |
| TGF-β | Transforming Growth Factor Beta |
| MSCs | Mesenchymal Stem Cells |
| FT-IR | Fourier Transform Infrared |
| XPS | X-ray Photoelectron Spectroscopy |
| DTT | Dithiothreitol |
| QCM | Quartz Crystal Microbalance |
| µCP | Microcontact Printing |
| PDMS | Poly(dimethylsiloxane) |
| TCPS | Tissue Culture Polystyrene |
| ALP | Alkaline Phosphatase |
| ARS | Alizarin Red S |
| sGAG | Sulfated Glycosaminoglycan |
| DMMB | 1,9-Dimethylmethylene Blue |
| FABP-4 | Fatty Acid-Binding Protein 4 |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide |
| qPCR | Quantitative Real-Time Polymerase Chain Reaction |
References
- Ito, Y.; Tada, S. Bio-orthogonal and combinatorial approaches for the design of binding growth factors. Biomaterials 2013, 34, 7565–7574. [Google Scholar] [CrossRef]
- Fu, R.H.; Wang, Y.C.; Liu, S.P.; Huang, C.M.; Kang, Y.H.; Tsai, C.H.; Shyu, W.C.; Lin, S.Z. Differentiation of stem cells: Strategies for modifying surface biomaterials. Cell Transplant. 2011, 20, 37–47. [Google Scholar] [CrossRef]
- Masters, K.S. Covalent growth factor immobilization strategies for tissue repair and regeneration. Macromol. Biosci. 2011, 11, 1149–1163. [Google Scholar] [CrossRef]
- Kusamori, K.; Takayama, Y.; Nishikawa, M. Stable Surface Modification of Mesenchymal Stem Cells Using the Avidin-Biotin Complex Technique. Curr. Protoc. Stem Cell Biol. 2018, 47, e66. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Prasad, B.L.V. Surface Modification of Polymers for Tissue Engineering Applications: Arginine Acts as a Sticky Protein Equivalent for Viable Cell Accommodation. ACS Omega 2018, 3, 4242–4251. [Google Scholar] [CrossRef] [PubMed]
- Mahla, R.S. Stem Cells Applications in Regenerative Medicine and Disease Therapeutics. Int. J. Cell Biol. 2016, 2016, 6940283. [Google Scholar] [CrossRef] [PubMed]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef] [PubMed]
- Dellatore, S.M.; Garcia, A.S.; Miller, W.M. Mimicking Stem Cell Niches to Increase Stem Cell Expansion. Curr. Opin. Biotechnol. 2008, 19, 534–540. [Google Scholar] [CrossRef]
- Lu, Z.; Roohani-Esfahani, S.I.; Wang, G.; Zreiqat, H. Bone Biomimetic Microenvironment Induces Osteogenic Differentiation of Adipose Tissue-derived Mesenchymal Stem Cells. Nanomedicine 2012, 8, 507–515. [Google Scholar] [CrossRef]
- Donnelly, H.; Salmeron-Sanchez, M.; Dalby, M.J. Designing Stem Cell Niches for Differentiation and Self-renewal. J. R. Soc. Interface 2018, 15, 20180388. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Lahann, J. Designable Biointerfaces Using Vapor-Based Reactive Polymers. Langmuir 2010, 27, 34–48. [Google Scholar] [CrossRef]
- Sun, H.-Y.; Fang, C.-Y.; Lin, T.-J.; Chen, Y.-C.; Lin, C.-Y.; Ho, H.-Y.; Chen, M.H.C.; Yu, J.; Lee, D.-J.; Chang, C.-H.; et al. Thiol-Reactive Parylenes as a Robust Coating for Biomedical Materials. Adv. Mater. Interfaces 2014, 1. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Wu, C.-Y.; Guan, Z.-Y.; Sun, H.-Y.; Cheng, N.-C.; Yeh, S.-Y.; Chen, H.-Y. Topologically Controlled Cell Differentiation Based on Vapor-Deposited Polymer Coatings. Langmuir 2017, 33, 8943–8949. [Google Scholar] [CrossRef] [PubMed]
- Lahann, J.; Langer, R. Novel Poly(p-xylylenes): Thin Films with Tailored Chemical and Optical Properties. Macromolecules 2002, 35, 4380–4386. [Google Scholar] [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen zur Wägung dünner Schichten und zur Mikrowägung. Z. Phys. 1959, 155, 206–222. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Liu, H.-Y.; Huang, C.-W.; Yeh, S.-Y.; Cheng, N.-C.; Ding, S.-T.; Chen, H.-Y. Synergistically Controlled Stemness and Multilineage Differentiation Capacity of Stem Cells on Multifunctional Biointerfaces. Adv. Mater. Interfaces 2017, 4, 1700243. [Google Scholar] [CrossRef]
- Zheng, C.H.; Levenston, M.E. Fact versus artifact: Avoiding erroneous estimates of sulfated glycosaminoglycan content using the dimethylmethylene blue colorimetric assay for tissue-engineered constructs. Eur. Cells Mater. 2015, 29, 224–236. [Google Scholar] [CrossRef]
- Kraus, N.A.; Ehebauer, F.; Zapp, B.; Rudolphi, B.; Kraus, B.J.; Kraus, D. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte 2016, 5, 351–358. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hendrick, M.H. Human Adipose Tissue is a Source of Multipotent Stem Cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tsai, M.-Y.; Lin, C.-Y.; Huang, C.-H.; Gu, J.-A.; Huang, S.-T.; Yu, J.; Chen, H.-Y. Vapor-based synthesis of maleimide-functionalized coating for biointerface engineering. Chem. Commun. 2012, 48, 10969–10971. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol-ene click chemistry. Angew. Chem. Int. Ed. Engl. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Brewer, C.F.; Riehm, J.P. Evidence for possible nonspecific reactions between N-ethylmaleimide and proteins. Anal. Biochem. 1967, 18, 248–255. [Google Scholar] [CrossRef]
- Chen, Y.C.; Sun, T.P.; Su, C.T.; Wu, J.T.; Lin, C.Y.; Yu, J.; Huang, C.W.; Chen, C.J.; Chen, H.Y. Sustained immobilization of growth factor proteins based on functionalized parylenes. ACS Appl. Mater. Interfaces 2014, 6, 21906–21910. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-T.; Sun, T.-P.; Huang, C.-W.; Su, C.-T.; Wu, C.-Y.; Yeh, S.-Y.; Yang, D.-K.; Chen, L.-C.; Ding, S.-T.; Chen, H.-Y. Tunable coverage of immobilized biomolecules for biofunctional interface design. Biomater. Sci. 2015, 3, 1266–1269. [Google Scholar] [CrossRef]
- Della Ventura, B.; Sakac, N.; Funari, R.; Velotta, R. Flexible immunosensor for the detection of salivary alpha-amylase in body fluids. Talanta 2017, 174, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.P.; Tai, C.H.; Wu, J.T.; Wu, C.Y.; Liang, W.C.; Chen, H.Y. Multifaceted and route-controlled click reactions based on vapor-deposited coatings. Biomater. Sci. 2016, 4, 265–271. [Google Scholar] [CrossRef]
- Pagán, M.; Suazo, D.; Del Toro, N.; Griebenow, K. A Comparative Study of Different Protein Immobilization Methods for the Construction of an Efficient Nano-structured Lactate Oxidase-SWCNT-biosensor. Biosens. Bioelectron. 2015, 64, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.H.; Modo, M. Human neural stem cell-induced endothelial morphogenesis requires autocrine/paracrine and juxtacrine signaling. Sci. Rep. 2016, 6, 29029. [Google Scholar] [CrossRef]
- Shi, Y.; Massagué, J. Mechanisms of TGF-β Signaling from Cell Membrane to the Nucleus. Cell 2003, 113, 685–700. [Google Scholar] [CrossRef]
- Mueller, T.D. Chapter One—Mechanisms of BMP–Receptor Interaction and Activation. In Vitamins & Hormones; Litwack, G., Ed.; Academic Press: Los Angeles, CA, USA, 2015; Volume 99, pp. 1–61. [Google Scholar]
- Shea, C.M.; Edgar, C.M.; Einhorn, T.A.; Gerstenfeld, L.C. BMP Treatment of C3H10T1/2 Mesenchymal Stem Cells Induces Both Chondrogenesis and Osteogenesis. J. Cell. Biochem. 2003, 90, 1112–1127. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, J.H.; Kim, J.Y.; Bae, Y.C.; Suh, K.T.; Jung, J.S. BMP2 increases adipogenic differentiation in the presence of dexamethasone, which is inhibited by the treatment of TNF-alpha in human adipose tissue-derived stromal cells. Cell. Physiol. Biochem. 2014, 34, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Sottile, V.; Seuwen, S. Bone morphogenetic protein-2 stimulates adipogenic di¡erentiation of mesenchymal precursor cells in synergy with BRL 49653 (rosiglitazone). FEBS Lett. 2000, 475, 201–204. [Google Scholar] [CrossRef]
- Vanhatupa, S.; Ojansivu, M.; Autio, R.; Juntunen, M.; Miettinen, S. Bone Morphogenetic Protein-2 Induces Donor-Dependent Osteogenic and Adipogenic Differentiation in Human Adipose Stem Cells. Stem Cells Transl. Med. 2015, 4, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Chiou, M.; Xu, Y.; Longaker, M.T. Mitogenic and Chondrogenic Effects of Fibroblast Growth Factor-2 in Adipose Derived Mesenchymal Cells. Biochem. Biophys. Res. Commun. 2006, 343, 644–652. [Google Scholar] [CrossRef]
- Hankemeier, S.; Keus, M.; Zeichen, J.; Jagodzinski, M.; Barkhausen, T.; Bosch, U.; Krettek, C.; Van Griensven, M. Modulation of Proliferation and Differentiation of Human Bone Marrow Stromal Cells by Fibroblast Growth Factor 2: Potential Implication for Tissue Engineering of Tendons and Ligaments. Tissue Eng. 2005, 11, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Kakudo, N.; Shimotsuma, A.; Kusumoto, K. Fibroblast Growth Factor-2 Stimulates Adipogenic Differentiation of Human Adipose-derived Stem Cell. Biochem. Biophys. Res. Commun. 2007, 395, 239–244. [Google Scholar] [CrossRef]
- Merrell, A.J.; Stanger, B.Z. Adult cell plasticity in vivo: De-differentiation and transdifferentiation are back in style. Nat. Rev. Mol. Cell Biol. 2016, 17, 413–425. [Google Scholar] [CrossRef]
- Chiacchiera, F.; Morey, L.; Mozzetta, C. Editorial: Epigenetic Regulation of Stem Cell Plasticity in Tissue Regeneration and Disease. Front. Cell Dev. Biol. 2020, 8, 82. [Google Scholar] [CrossRef]
- Hernigou, P.; Poignard, A.; Beaujean, F.; Rouard, H. Percutaneous Autologous Bone-Marrow Grafting for Nonunions: Influence of the Number and Concentration of Progenitor Cells. JBJS 2005, 87, 1430–1437. [Google Scholar] [CrossRef]
- Giuliani, N.; Lisignoli, G.; Magnani, M.; Racano, C.; Bolzoni, M.; Dalla Palma, B.; Spolzino, A.; Manferdini, C.; Abati, C.; Toscani, D.; et al. New Insights into Osteogenic and Chondrogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells and Their Potential Clinical Applications for Bone Regeneration in Pediatric Orthopaedics. Stem Cells Int. 2013, 2013, 312501. [Google Scholar] [CrossRef]
- Chen, H.; Wang, H.; Li, B.; Feng, B.; He, X.; Fu, W.; Yuan, H.; Xu, Z. Enhanced chondrogenic differentiation of human mesenchymal stems cells on citric acid-modified chitosan hydrogel for tracheal cartilage regeneration applications. RSC Adv. 2018, 8, 16910–16917. [Google Scholar] [CrossRef]
- Tan, S.S.; Ng, Z.Y.; Zhan, W.; Rozen, W. Role of Adipose-derived Stem Cells in Fat Grafting and Reconstructive Surgery. J. Cutan. Aesthet. Surg. 2016, 9, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Inoue, K.; Suga, H.; Eto, H.; Kato, H.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for facial lipoatrophy: Efficacy of clinical use of adipose-derived stem cells. Dermatol. Surg. 2008, 34, 1178–1185. [Google Scholar] [CrossRef]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast. Surg. 2008, 32, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]






| Gene Name | Primer Sequence (5′-3′) | Product Length (bp) |
|---|---|---|
| Collagen type II | Forward- ACG GCG AGA AGG GAG AAG TTG Reverse- GGG GGT CCA GGG TTG CCA TTG | 352 |
| FABP-4 | Forward- TGG GCC AGG AAT TTG ACG AA Reverse- GAC GCA TTC CAC CAC CAG TT | 158 |
| β-Actin | Forward- CAG GAG ATG GCC ACT GCC GCA Reverse- TCC TTC TGC ATC CTG TCA GCA | 275 |
| Induction b | BMP-2 Modified | FGF-2 Modified | Maleimide-PPX Coating | Unmodified TCPS | Potential Applications |
|---|---|---|---|---|---|
| osteogenesis | 277% | - | 108% | 100% | bone regeneration [41,42] |
| chondrogenesis | 156% | - | 100% | 100% | cartilage regeneration [43] |
| adipogenesis | - | 172% | 94% | 100% | soft tissue reconstruction [44,45,46] |
| proliferation | - | 378% | 94% | 100% | repair damage from wound or cuts [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-Y.; Chiang, Y.-C.; Christy, J.; Huang, A.P.-H.; Chang, N.-Y.; Wenny; Chiu, Y.-C.; Yang, Y.-C.; Chen, P.-C.; Wang, P.-Y.; et al. Guiding Stem Cell Differentiation and Proliferation Activities Based on Nanometer-Thick Functionalized Poly-p-xylylene Coatings. Coatings 2021, 11, 582. https://doi.org/10.3390/coatings11050582
Wu C-Y, Chiang Y-C, Christy J, Huang AP-H, Chang N-Y, Wenny, Chiu Y-C, Yang Y-C, Chen P-C, Wang P-Y, et al. Guiding Stem Cell Differentiation and Proliferation Activities Based on Nanometer-Thick Functionalized Poly-p-xylylene Coatings. Coatings. 2021; 11(5):582. https://doi.org/10.3390/coatings11050582
Chicago/Turabian StyleWu, Chih-Yu, Yu-Chih Chiang, Jane Christy, Abel Po-Hao Huang, Nai-Yun Chang, Wenny, Yu-Chih Chiu, Yen-Ching Yang, Po-Chun Chen, Peng-Yuan Wang, and et al. 2021. "Guiding Stem Cell Differentiation and Proliferation Activities Based on Nanometer-Thick Functionalized Poly-p-xylylene Coatings" Coatings 11, no. 5: 582. https://doi.org/10.3390/coatings11050582
APA StyleWu, C.-Y., Chiang, Y.-C., Christy, J., Huang, A. P.-H., Chang, N.-Y., Wenny, Chiu, Y.-C., Yang, Y.-C., Chen, P.-C., Wang, P.-Y., & Chen, H.-Y. (2021). Guiding Stem Cell Differentiation and Proliferation Activities Based on Nanometer-Thick Functionalized Poly-p-xylylene Coatings. Coatings, 11(5), 582. https://doi.org/10.3390/coatings11050582