Influence of Rust Inhibitor on the Corrosion Resistance of Reinforcement in Cement Paste with Chloride
Abstract
:1. Introduction
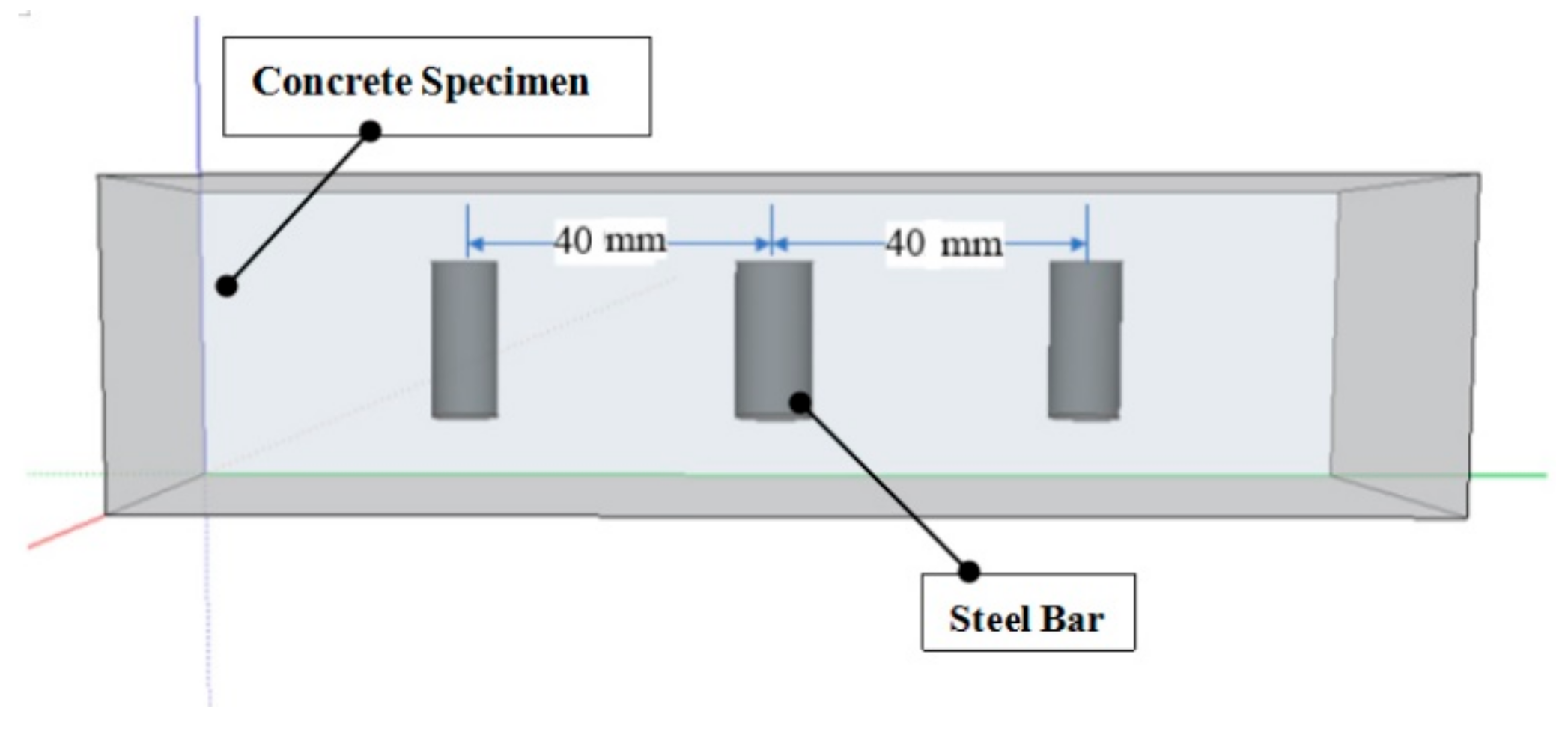
2. Experimental
Raw Materials
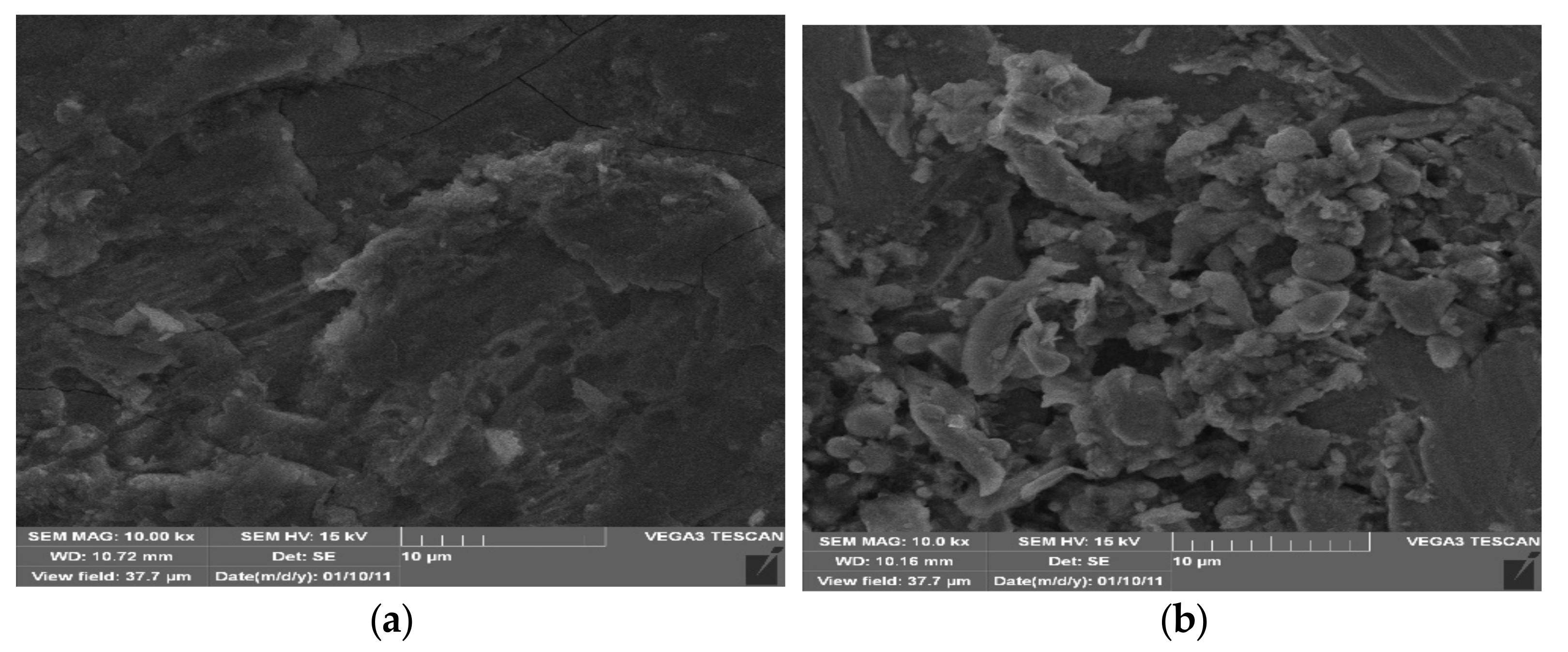
3. Results and Discussion
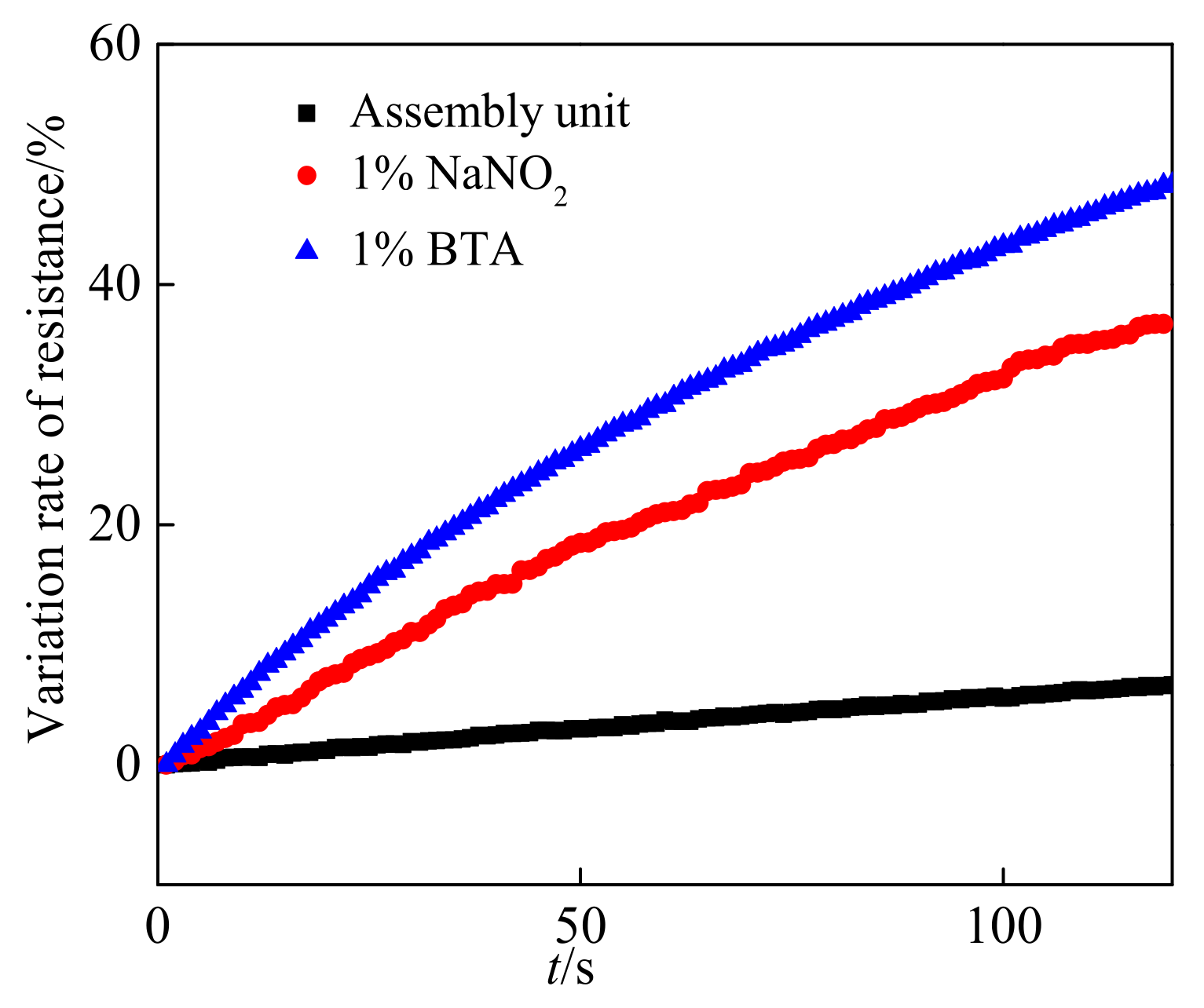
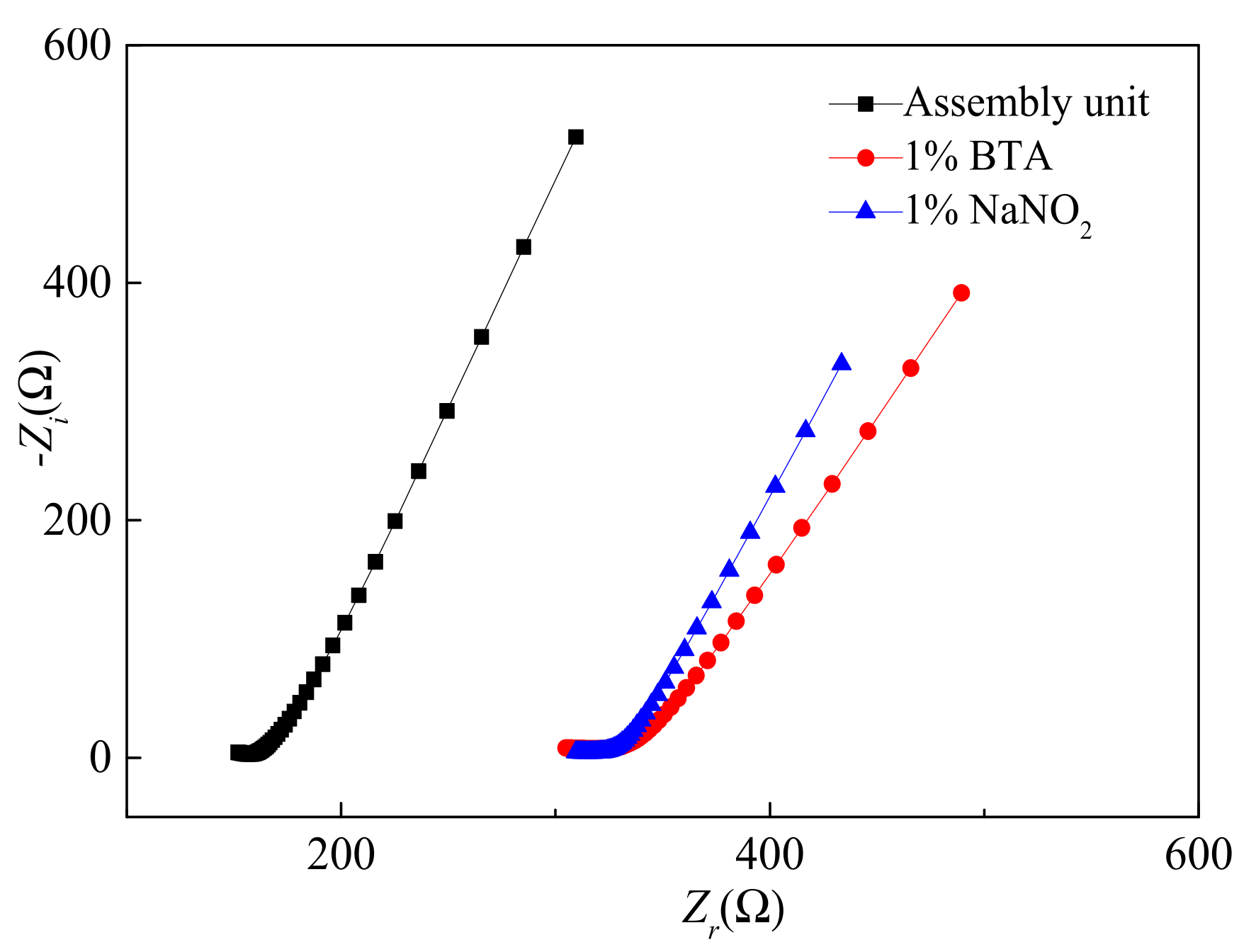
3.1. Electrical Performance
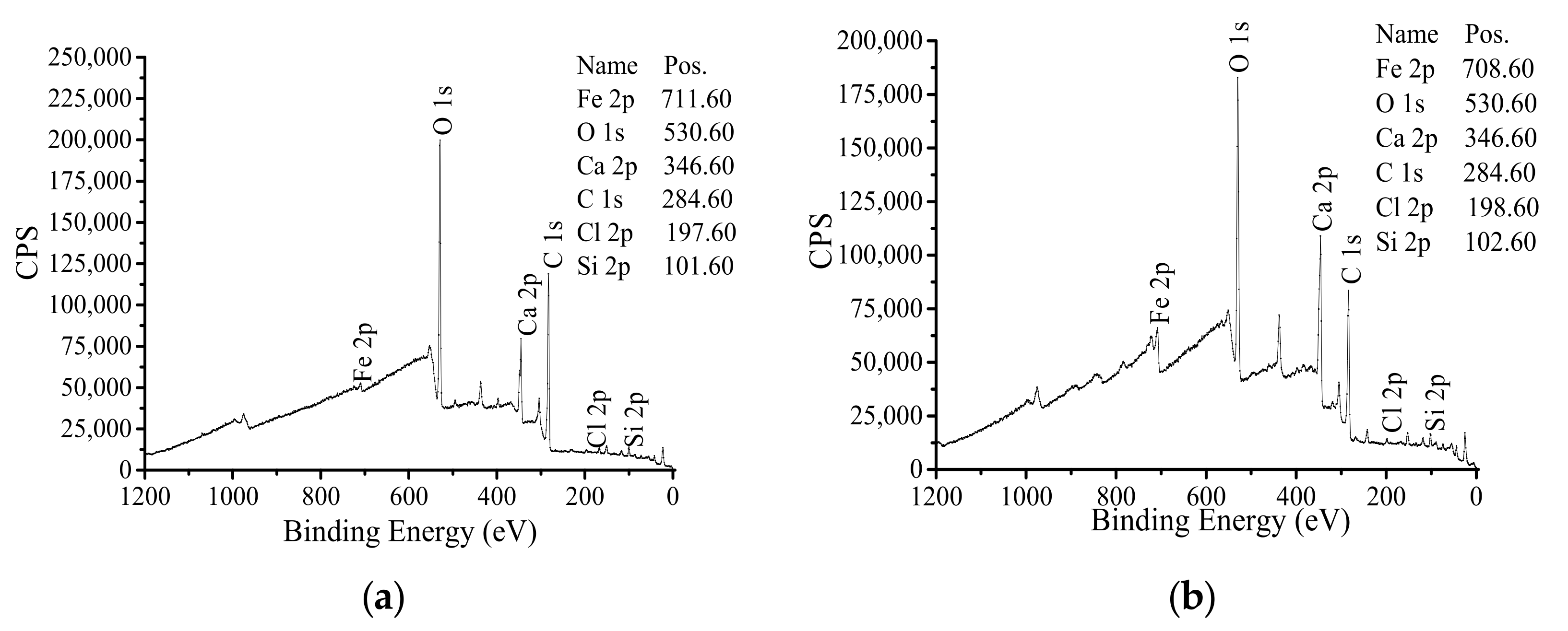
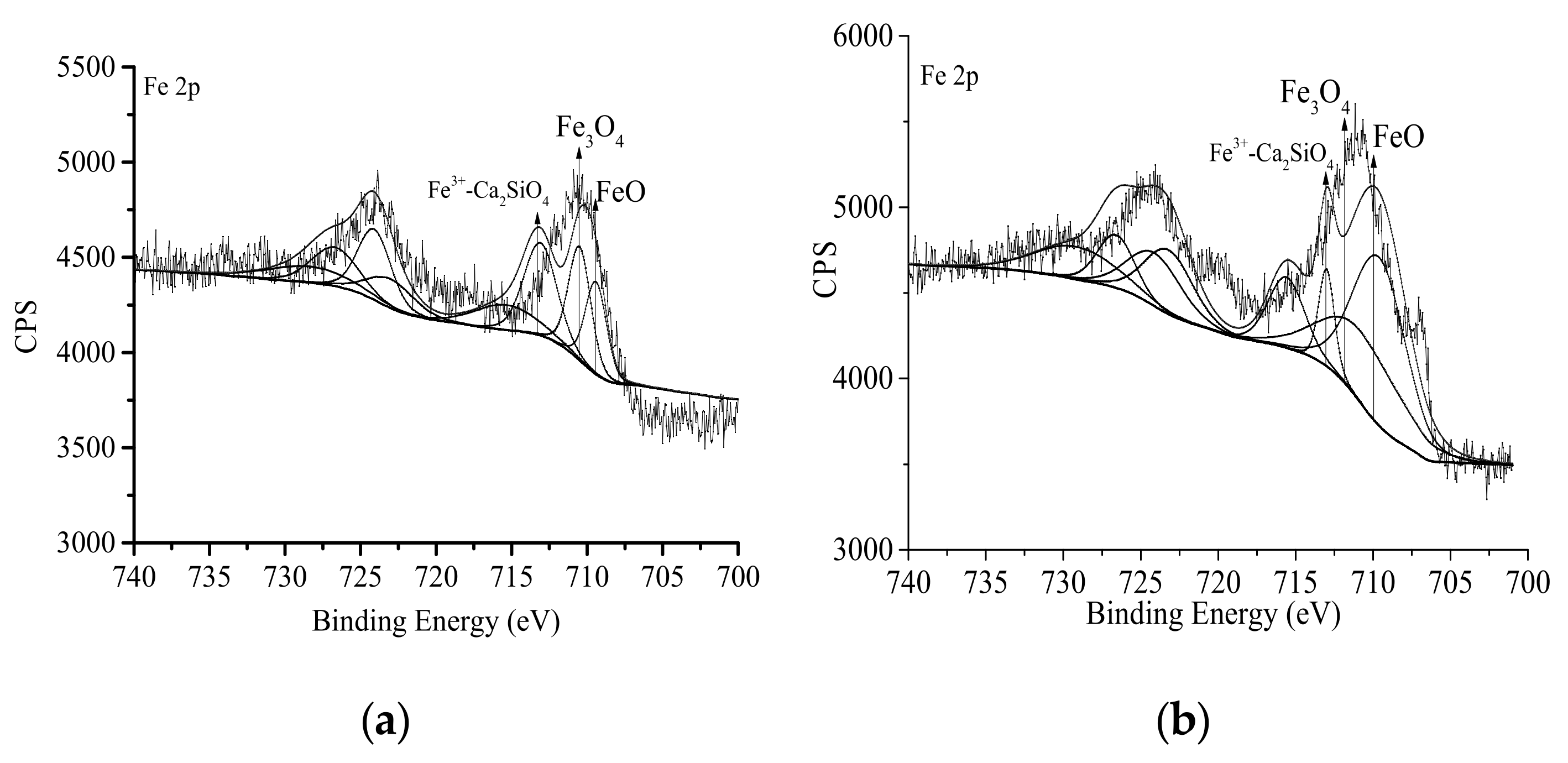
3.2. Composition of Passivation Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, B.; Gao, Y.; Qu, L.; Duan, K.; Zhou, W.; Pei, G. Characteristics analysis of self-luminescent cement-based composite materials with self-cleaning effect. J. Clean. Prod. 2019, 225, 1169–1183. [Google Scholar] [CrossRef]
- Mo, Z.; Gao, X.; Su, A. Mechanical performances and microstructures of metakaolin contained UHPC matrix under steam curing conditions. Constr. Build. Mater. 2021, 268, 121112. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Gao, X. Incorporation of self-ignited coal gangue in steam cured precast concrete. J. Clean. Prod. 2021, 292, 126004. [Google Scholar] [CrossRef]
- Ren, G.; Yao, B.; Huang, H.; Gao, X. Influence of sisal fibers on the mechanical performance of ultra-high performance concretes. Constr. Build. Mater. 2021, 286, 122958. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Su, Y.; He, X.; Tan, H.; Yang, W.; Strnadel, B. Eco-friendly treatment of ow-calcium coal fly ash for high pozzolanic reactivity: A step towards waste utilization in sustainable building material. J. Clean. Prod. 2019, 238, 117962. [Google Scholar] [CrossRef]
- He, X.; Zheng, Z.; Ma, M.; Su, Y.; Yang, J.; Tan, H.; Wang, Y.; Strnadel, B. New treatment technology: The use of wet-milling concrete slurry waste to substitute cement. J. Clean. Prod. 2020, 242, 118347. [Google Scholar] [CrossRef]
- Liu, X.; Ma, B.; Tan, H.; Gu, B.; Zhang, T.; Chen, P.; Li, H.; Mei, J. Effect of aluminum sulfate on the hydration of Portland cement, tricalcium silicate and tricalcium aluminate. Constr. Build. Mater. 2020, 232, 117179. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, X.; He, X.; Guo, Y.; Deng, X.; Su, Y.; Yang, J.; Wang, Y. Utilization of lithium slag by wet-grinding process to improve the early strength of sulphoaluminate cement paste. J. Clean. Prod. 2018, 205, 536–551. [Google Scholar] [CrossRef]
- Zhang, G.; Yu, H.; Li, H.; Yang, Y. Experimental study of deformation of early age concrete suffering from frost damage. Constr. Build. Mater. 2019, 215, 410–421. [Google Scholar] [CrossRef]
- Zou, F.; Tan, H.; Guo, Y.; Ma, B.; He, X.; Zhou, Y. Effect of sodium gluconate on dispersion of polycarboxylate superplasticizer with different grafting density in side chain. J. Ind. Eng. Chem. 2017, 55, 91–100. [Google Scholar] [CrossRef]
- Shi, X.; Fay, L.; Peterson, M.M.; Yang, Z. Freeze–thaw damage and chemical change of a portland cement concrete in the presence of diluted deicers. Mater. Struct. 2009, 43, 933–946. [Google Scholar] [CrossRef]
- Xu, W.; Lo, Y.T.; Ouyang, D.; Memon, S.A.; Xing, F.; Wang, W.; Yuan, X. Effect of rice husk ash fineness on porosity and hydration reaction of blended cement paste. Constr. Build. Mater. 2015, 89, 90–101. [Google Scholar] [CrossRef]
- Al-Sibahy, A.; Sabhan, M. Corrosion effects on the bond behaviour of steel bars in self-compacting concrete. Constr. Build. Mater. 2020, 250, 118568. [Google Scholar] [CrossRef]
- Suzuki, Y.; Morishita, A. Influence of corrosion inhibitor in chemical conversion coatings on corrosion performance in scratches in zinc-coated steels. ISIJ Int. 2019, 59, 1878–1885. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; Ma, W.; Zhang, S.; Mu, Y.; Li, G. Effect of freeze-thaw cycles in mechanical behaviors of frozen loss. Cold Reg. Sci. Technol. 2018, 146, 9–18. [Google Scholar] [CrossRef]
- Etteyeb, N.; Dhouibi, L.; Takenouti, H.; Triki, E. Protection of reinforcement steel corrosion by phenylphosphonic acid pre-treatment PART II: Tests in mortar medium. Cem. Concr. Compos. 2016, 65, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Ba, M.-F.; Qian, C.-X. Hydration evolution of pre-cast concrete with steam and water curing. J. Cent. South Univ. 2013, 20, 2870–2878. [Google Scholar] [CrossRef]
- Paul, G.; Boccaleri, E.; Buzzi, L.; Canonico, F.; Gastaldi, D. Friedel’s salt formation in sulfoaluminate cements: A combined XRD and 27 Al MAS NMR study. Cem. Concr. Res. 2015, 67, 93–102. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; Khayat, K. Influence of silica fume content on microstructure development and bond to steel fiber in ultra-high strength cement-based materials (UHSC). Cem. Concr. Compos. 2016, 71, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Hilke, V.; Herman, T.; De Graeve, I. Inhibitor evaluation in different simulated concrete pore solution for the protection of steel rebars. Constr. Build. Mater. 2016, 124, 887–896. [Google Scholar]
- Cheewaket, T.; Jaturapitakkul, C.; Chalee, W. Initial corrosion presented by chloride threshold penetration of concrete up to 10 year-results under marine site. Constr. Build. Mater. 2012, 37, 693–698. [Google Scholar] [CrossRef]
- Ormellese, M.; Berra, M.; Bolzoni, F.; Pastore, T. Corrosion inhibitors for chlorides induced corrosion in reinforced concrete structures. Cem. Concr. Res. 2006, 36, 536–547. [Google Scholar] [CrossRef]
- Leon, C.; Val, D. Prediction of corrosion-induced cover cracking in reinforced concrete structures. Constr. Build. Mater. 2011, 25, 1854–1869. [Google Scholar]
- Tang, F.; Lin, Z.; Chen, G.; Yi, W. Three-dimensional corrosion pit measurement and statistical mechanical degradation analysis of deformed steel bars subjected to accelerated corrosion. Constr. Build. Mater. 2014, 70, 104–117. [Google Scholar] [CrossRef]
- Choi, Y.S.; Yi, S.-T.; Kim, M.Y.; Jung, W.Y.; Yang, E.I. Effect of corrosion method of the reinforcing bar on bond characteristics in reinforced concrete specimens. Constr. Build. Mater. 2014, 54, 180–189. [Google Scholar] [CrossRef]
- Kioumarsi, M.M.; Hendriks, M.A.; Kohler, J.; Geiker, M.R. The effect of interference of corrosion pits on the failure probability of a reinforced concrete beam. Eng. Struct. 2016, 114, 113–121. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Zhang, L.; Liu, J.; Han, Y.; Shu, H.; Wang, J. Study on the influence of compound rust inhibitor on corrosion of steel bars in chloride concrete by electrical parameters. Constr. Build. Mater. 2020, 262, 120763. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, A.; Zhang, L.; Liu, J.; Han, Y.; Wang, J. Research on the influence of carbonation on the content and state of chloride ions and the following corrosion resistance of steel bars in cement paste. Coatings 2020, 10, 1071. [Google Scholar] [CrossRef]
- Torres-Luque, M.; Bastidas-Arteaga, E.; Schoefs, F.; Sánchez-Silva, M.; Osma, J. Non-destructive methods for measuring chloride ingress into concrete: State-of-the-art and future challenges. Constr. Build. Mater. 2014, 68, 68–81. [Google Scholar] [CrossRef] [Green Version]
- Montemor, M.; Alves, J.; Simões, A.; Fernandes, J.; Lourenço, Z.; Costa, A.; Appleton, A.; Ferreira, M. Multiprobe chloride sensor for in situ monitoring of reinforced concrete structures. Cem. Concr. Compos. 2006, 28, 233–236. [Google Scholar] [CrossRef]
- Liu, J.-Z.; Ba, M.-F.; Du, Y.-G.; He, Z.-M.; Chen, J.-B. Effects of chloride ions on carbonation rate of hardened cement paste by X-ray CT techniques. Constr. Build. Mater. 2016, 122, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Maruthapandian, V.; Saraswathy, V.; Muralidharan, S. Development of solid state embeddable reference electrode for corrosion monitoring of steel in reinforced concrete structures. Cem. Concr. Compos. 2016, 74, 100–108. [Google Scholar] [CrossRef]
- Duffoa, G.; Farinab, S.; Giordano, C. Characterization of solid embeddable reference electrodes for corrosion monitoring in reinforced concrete structures. Electrochim. Acta 2009, 54, 1010–1020. [Google Scholar] [CrossRef]
- Qiao, G.; Hong, Y.; Song, G.; Li, H.; Ou, J. Electrochemical characterization of the solid-state reference electrode based on NiFe2O4 film for the corrosion monitoring of RC structures. Sens. Actuators B Chem. 2012, 168, 172–177. [Google Scholar] [CrossRef]
- Liu, M.; Tan, H.; He, X. Effects of nano-SiO2 on early strength and microstructure of steam-cured high volume fly ash cement system. Constr. Build. Mater. 2019, 194, 350–359. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, H.; Shen, W.; Xu, G.; Ma, B.; Ji, X. Nano-silica and silica fume modified cement mortar used as Surface Protection Material to enhance the impermeability. Cem. Concr. Compos. 2018, 92, 7–17. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, D.; Chen, H. The role of fly ash microsphere in the microstructure and macroscopic properties of high-strength concrete. Cem. Concr. Compos. 2017, 83, 125–137. [Google Scholar] [CrossRef]
- Rivera-Corral, J.; Fajardo, G.; Arliguie, G.; Orozco-Cruz, R.; Deby, F.; Valdez, P. Corrosion behavior of steel reinforcement bars embedded in concrete exposed to chlorides: Effect of surface finish. Constr. Build. Mater. 2017, 147, 815–826. [Google Scholar] [CrossRef]
- El Haleem, S.A.; El Wanees, S.A.; El Aal, E.A.; Diab, A. Environmental factors affecting the corrosion behavior of reinforcing steel. IV. Variation in the pitting corrosion current in relation to the concentration of the aggressive and the inhibitive anions. Corros. Sci. 2010, 52, 1675–1683. [Google Scholar] [CrossRef]
- Gustavo, S.D.; Silvia, B.F. Development of an embeddable sensor to monitor the corrosion process of new and existing reinforced concrete structures. Constr. Build. Mater. 2009, 23, 2746–2751. [Google Scholar]






| Particlesize /µm | 0.3 | 0.6 | 1 | 4 | 8 | 16 | 32 | 64 | |
|---|---|---|---|---|---|---|---|---|---|
| Type | |||||||||
| Cement | 0 | 0.33 | 2.66 | 15.01 | 28.77 | 46.64 | 72.73 | 93.59 | |
| Types | Chemical Composition/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MgO | CaO | SO3 | R2O | MnO | H2O | |
| Cement | 20.86 | 5.47 | 3.94 | 1.73 | 62.23 | 2.66 | 0.48 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhang, A.; Li, K.; Wang, Q.; Liu, J.; Wang, H. Influence of Rust Inhibitor on the Corrosion Resistance of Reinforcement in Cement Paste with Chloride. Coatings 2021, 11, 606. https://doi.org/10.3390/coatings11050606
Zhang L, Zhang A, Li K, Wang Q, Liu J, Wang H. Influence of Rust Inhibitor on the Corrosion Resistance of Reinforcement in Cement Paste with Chloride. Coatings. 2021; 11(5):606. https://doi.org/10.3390/coatings11050606
Chicago/Turabian StyleZhang, Linchun, Ailian Zhang, Ke Li, Qian Wang, Junzhe Liu, and Hui Wang. 2021. "Influence of Rust Inhibitor on the Corrosion Resistance of Reinforcement in Cement Paste with Chloride" Coatings 11, no. 5: 606. https://doi.org/10.3390/coatings11050606
APA StyleZhang, L., Zhang, A., Li, K., Wang, Q., Liu, J., & Wang, H. (2021). Influence of Rust Inhibitor on the Corrosion Resistance of Reinforcement in Cement Paste with Chloride. Coatings, 11(5), 606. https://doi.org/10.3390/coatings11050606






