Characterization of Three Surface Treatments on TiZr—Coating Properies and Corrosion Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate Preparation
2.2. PLA Solution Preparation and Deposition of Nanofibers
2.3. Characterization of the Obtained Samples
3. Results and Discussion
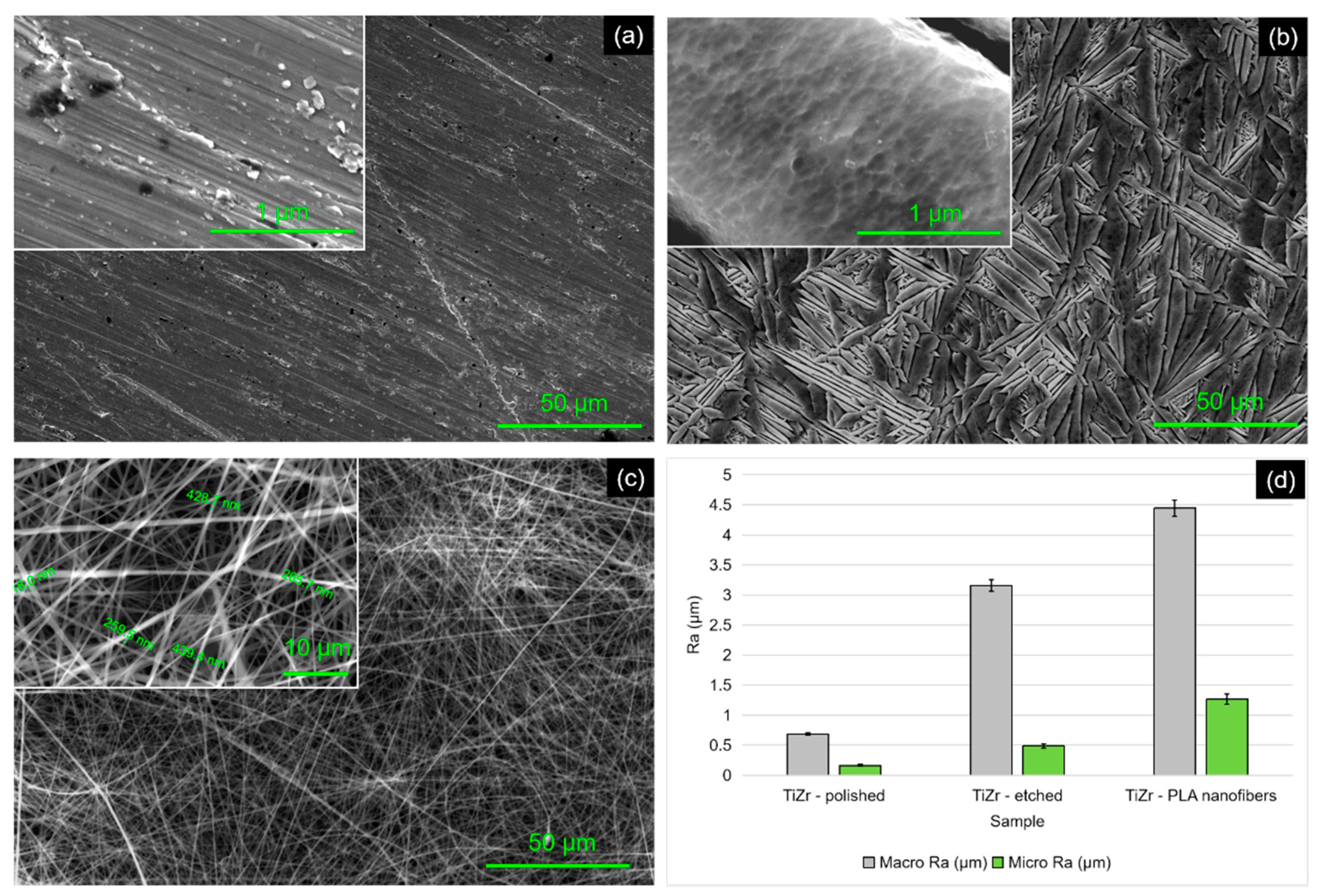
3.1. Sample Morphology
3.2. Roughness Measurements
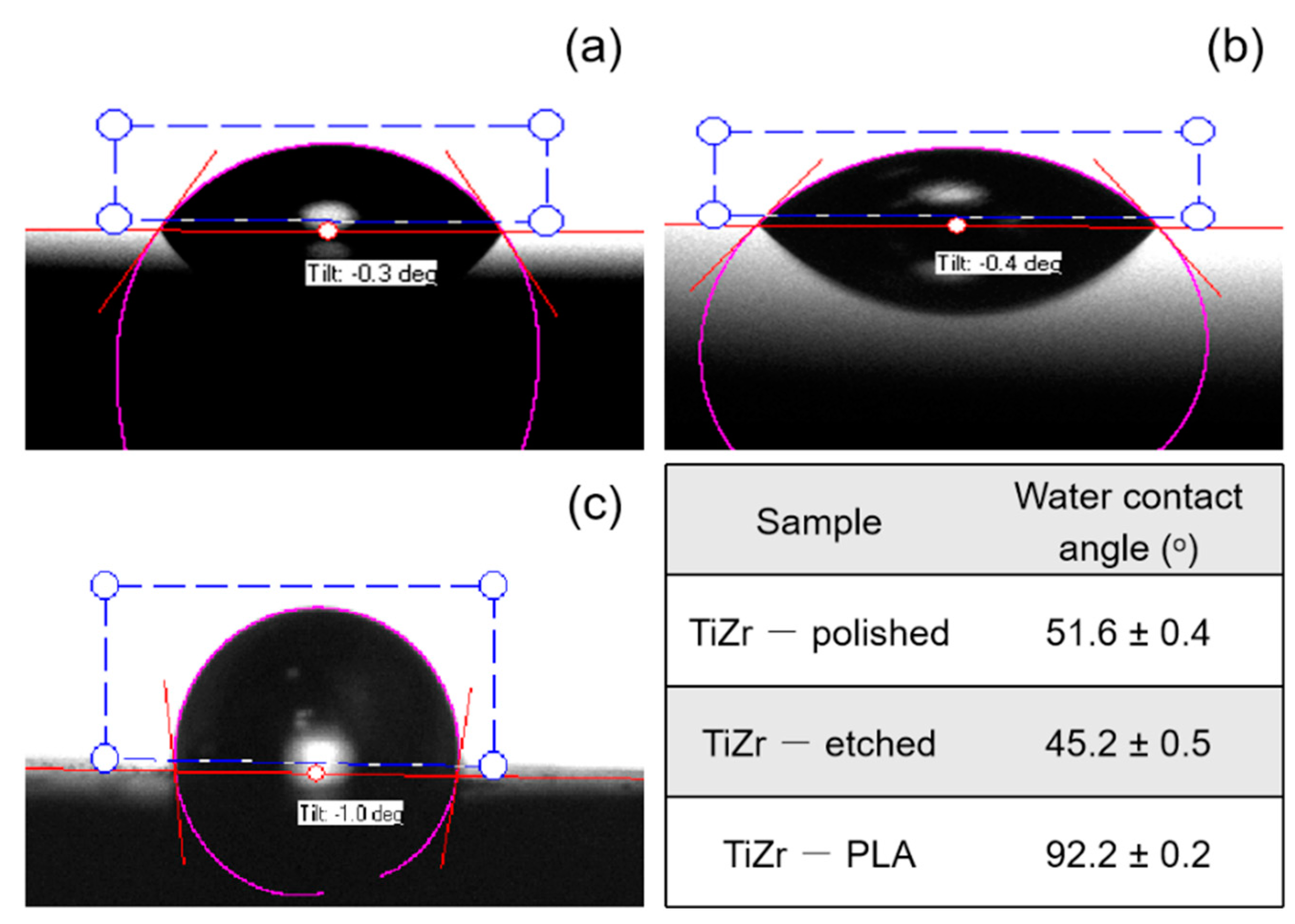
3.3. Contact Angle Measurements
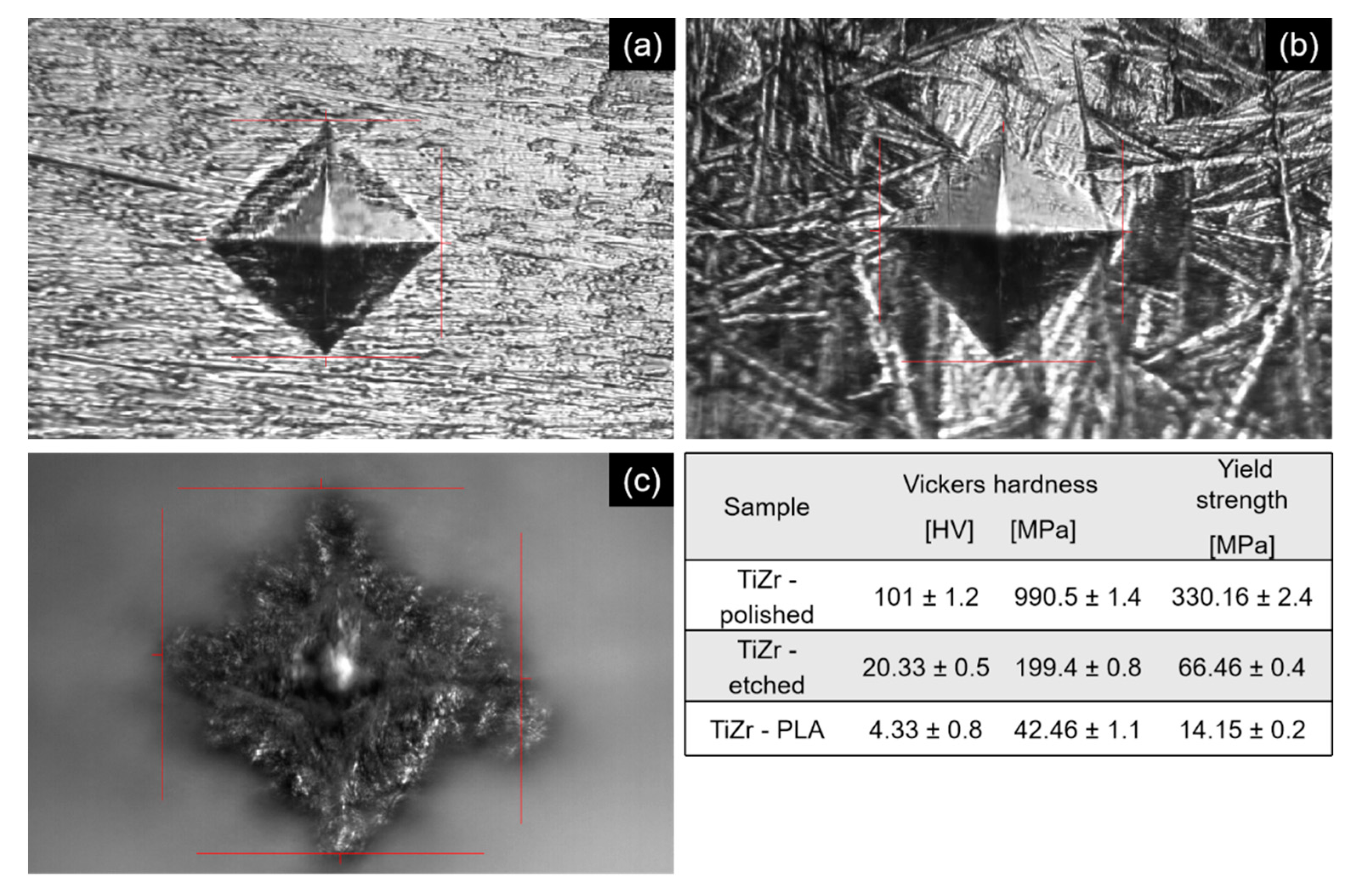
3.4. Micro Hardness Tests
3.5. Adhesion of Coatings
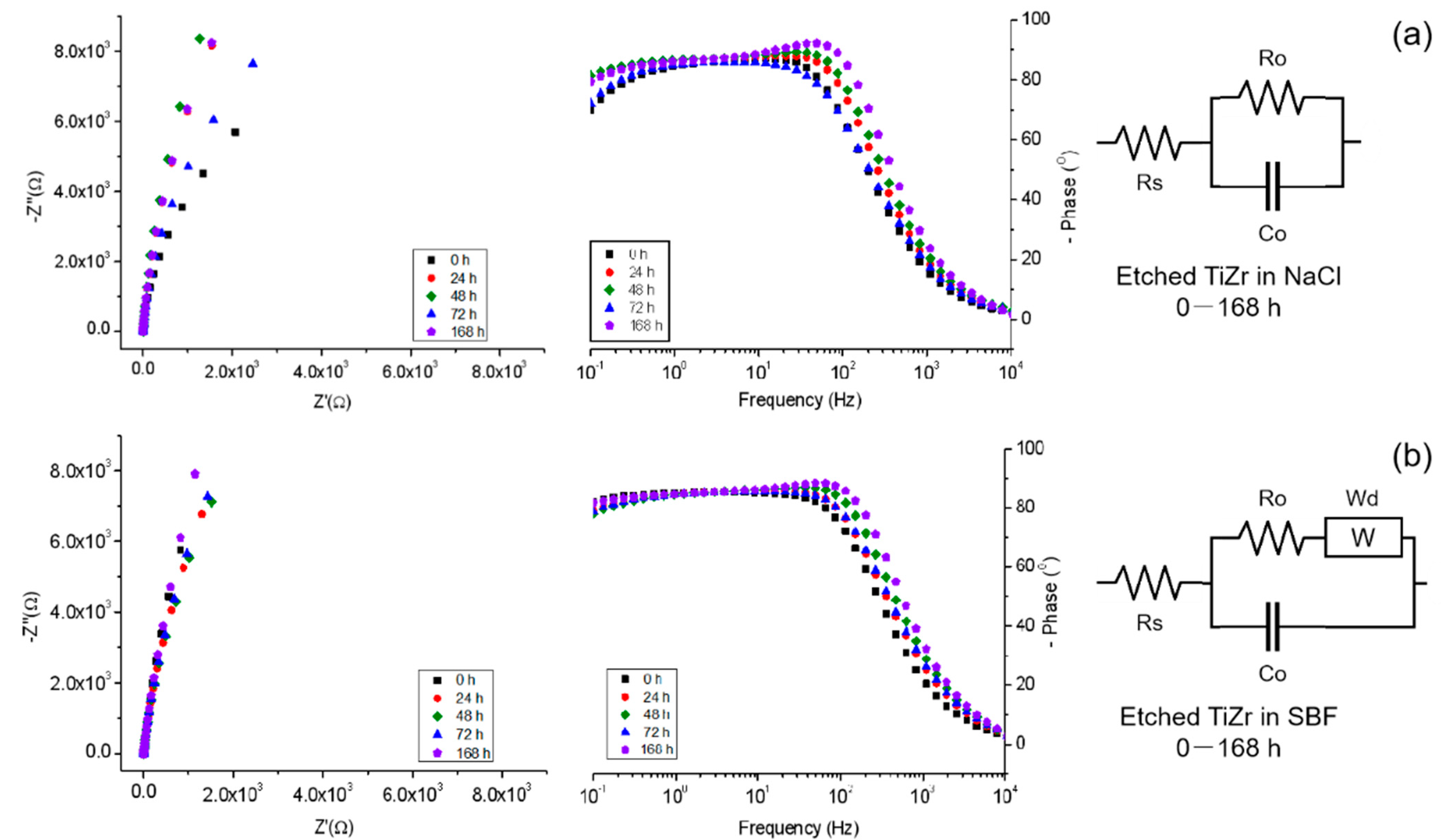
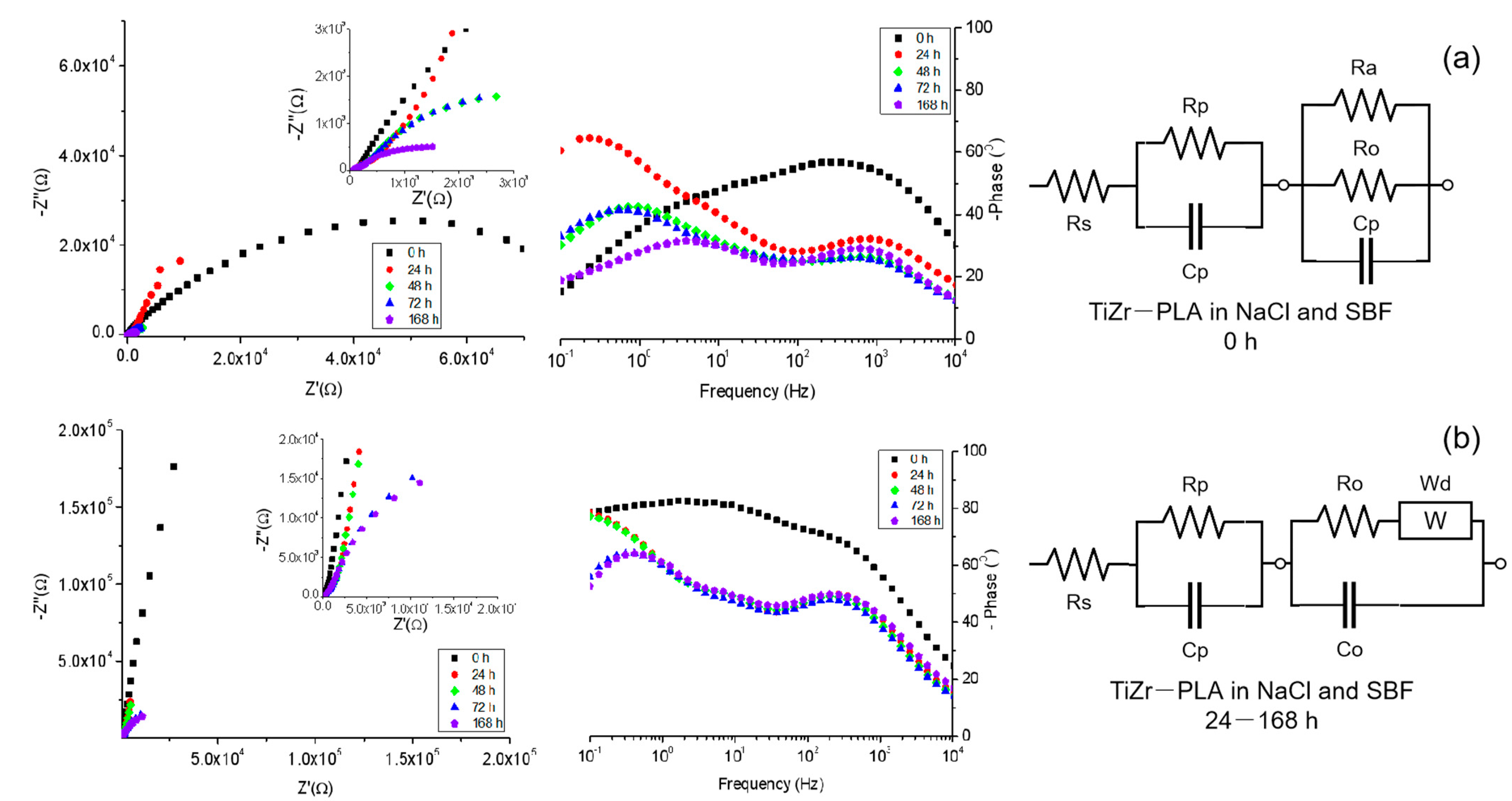
3.6. Electrochemical Impedance Spectroscopy
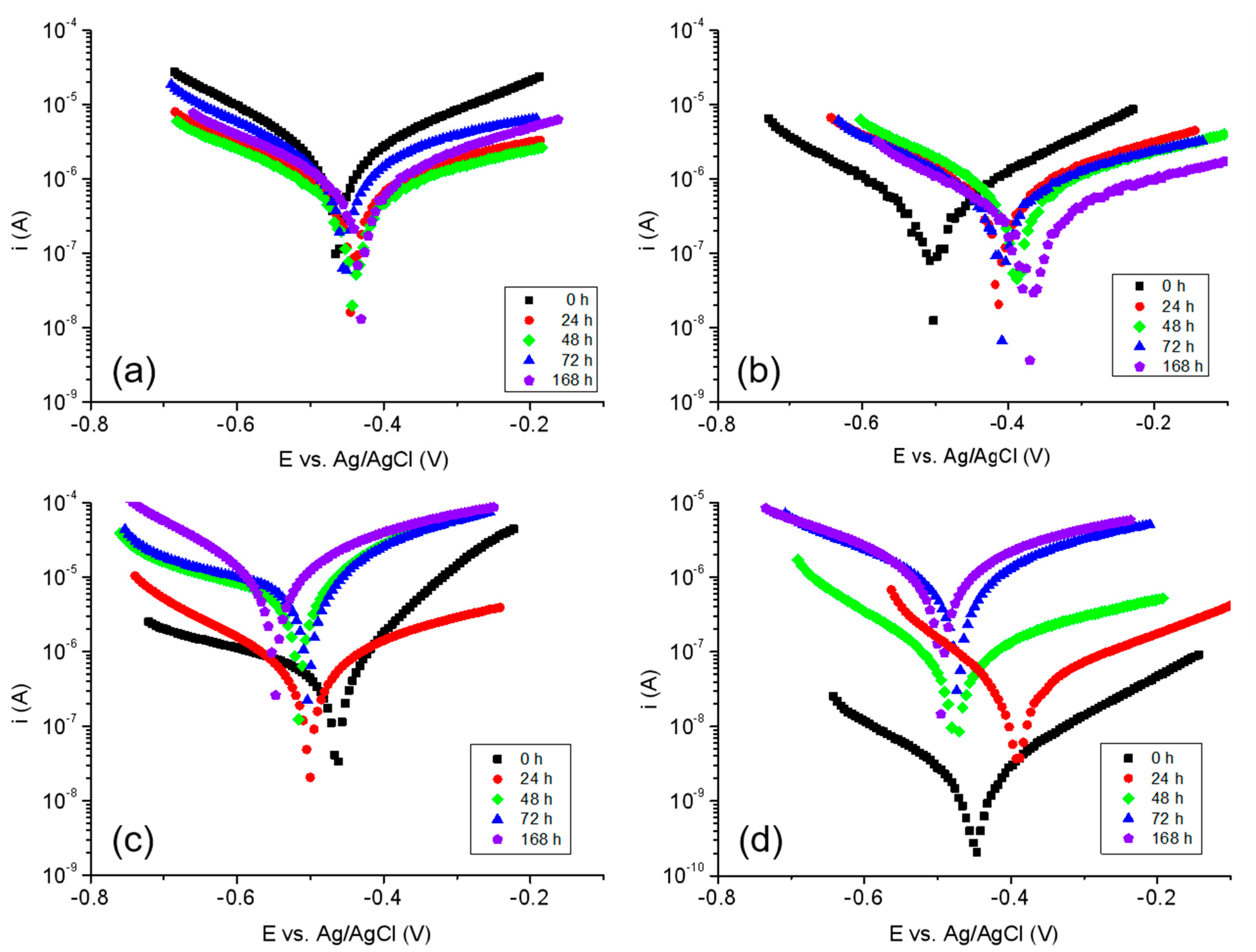
3.7. Corrosion Resistance Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Hengel, I.A.J.; Gelderman, F.S.A.; Athanasiadis, S.; Minneboo, M.; Weinans, H.; Fluit, A.C.; van der Eerden, B.C.J.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Functionality-packed additively manufactured porous titanium implants. Mater. Today Bio 2020, 7, 100060. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Hansson, B.O.; Adell, R.; Breine, U.; Lindström, J.; Hallén, O.; Ohman, A. Osseointegrated implants in the treatment of the edentulous jaw. Experience from a 10-year period. Scand. J. Plast. Reconstr. Surg. Suppl. 1977, 16, 1–132. [Google Scholar]
- Murphy, M.; Walczak, M.S.; Thomas, A.G.; Silikas, N.; Berner, S.; Lindsay, R. Toward optimizing dental implant performance: Surface characterization of Ti and TiZr implant materials. Dent. Mater. 2017, 33, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, S.; Tsoi, J.; Matinlinna, J.P. Binary titanium alloys as dental implant materials—A review. Regen. Biomater. 2017, 4, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Todai, M.; Nagase, T.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Li, F.; He, B.; Wang, J.; Sun, B. Air plasma sprayed thermal barrier coatings on titanium alloy substrates. Surf. Coat. Technol. 2007, 201, 7360–7367. [Google Scholar] [CrossRef]
- Prodana, M.; Nistor, C.E.; Stoian, A.B.; Ionita, D.; Burnei, C. Dual nanofibrous bioactive coatings on TiZr implants. Coatings 2020, 10, 526. [Google Scholar] [CrossRef]
- Ionita, D.; Grecu, M.; Ungureanu, C.; Demetrescu, I. Antimicrobial activity of the surface coatings on TiAlZr implant biomaterial. J. Biosci. Bioeng. 2011, 112, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Ungureanu, C.; Pirvu, C.; Mindroiu, M.; Demetrescu, I. Antibacterial polymeric coating based on polypyrrole and polyethylene glycol on a new alloy TiAlZr. Prog. Org. Coat. 2012, 75, 349–355. [Google Scholar] [CrossRef]
- Standard Specification for Wrought Titanium 6Al 4V ELI Alloy for Surgical Implants; ASTM Designation F136-82; ASTM: Philadelphia, PA, USA, 1994; pp. 19–20.
- Standard Specification for Wrought Titanium 6Al 7Nb Alloy for Surgical Implants; ASTM Designation F1295-92; ASTM: Philadelphia, PA, USA, 1994; pp. 687–689.
- Nartita, R.; Daniela, I.; Demetrescu, I. A combined scientometric and critical approach in reviewing TiZr implant alloys and coating performances. Coatings 2021, 11, 392. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Y. Fatigue behavior of Ti50Zr alloy for dental implant application. J. Alloys Compd. 2019, 793, 212–219. [Google Scholar] [CrossRef]
- Grigorescu, S.; Pruna, V.; Titorencu, I.; Jinga, V.V.; Mazare, A.; Schmuki, P.; Demetrescu, I. The two step nanotube formation on TiZr as scaffolds for cell growth. Bioelectrochemistry 2014, 98, 39–45. [Google Scholar] [CrossRef]
- Vardaki, M.; Mohajernia, S.; Pantazi, A.; Nica, I.C.; Enachescu, M.; Mazare, A.; Demetrescu, I.; Schmuki, P. Post treatments effect on TiZr nanostructures fabricated via anodizing. J. Mater. Res. Technol. 2019, 8, 5802–5812. [Google Scholar] [CrossRef]
- Gulati, K.; Kogawa, M.; Prideaux, M.; Findlay, D.M.; Atkins, G.J.; Losic, D. Drug-releasing nano-engineered titanium implants: Therapeutic efficacy in 3D cell culture model, controlled release and stability. Mater. Sci. Eng. C 2016, 69, 831–840. [Google Scholar] [CrossRef]
- Ionita, D.; Bajenaru-Georgescu, D.; Totea, G.; Mazare, A.; Schmuki, P.; Demetrescu, I. Activity of vancomycin release from bioinspired coatings of hydroxyapatite or TiO2 nanotubes. Int. J. Pharm. 2017, 517, 296–302. [Google Scholar] [CrossRef]
- Ionita, D.; Pirvu, C.; Stoian, A.B.; Demetrescu, I. The Trends of TiZr Alloy Research as a Viable Alternative for Ti and Ti16 Zr Roxolid Dental Implants. Coatings 2010, 10, 422. [Google Scholar] [CrossRef]
- Yun, K.-D.; Yang, Y.; Lim, H.-P.; Oh, G.-J.; Koh, J.-T.; Bae, I.-H.; Kim, J.; Lee, K.-M.; Park, S.-W. Effect of nanotubular-micro-roughened titanium surface on cell response in vitro and osseointegration in vivo. Mater. Sci. Eng. C 2010, 30, 27–33. [Google Scholar] [CrossRef]
- Fattahi, F.; Khoddami, A.; Avinc, O. Poly(lactic acid) (PLA) Nanofibers for Bone Tissue Engineering. J. Text. Polym. 2019, 7, 47–64. [Google Scholar]
- Saldin, L.T.; Cramer, M.C.; Velankar, S.S.; White, L.J.; Badylak, S.F. Extracellular matrix hydrogels from decellularized tissues: Structure and function. Acta Biomater. 2017, 49, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Guo, Z.; Liu, W. Adhesion behaviors on superhydrophobic surfaces. Chem. Commun. 2019, 50, 3900–3913. [Google Scholar] [CrossRef] [PubMed]
- Manole, C.C.; Dinischiotu, A.; Nica, C.; Demetrescu, I.; Pirvu, C. Influence of electrospun TiO2 nanowires on corrosion resistance and cell response of Ti50Zr alloy. Mater. Corros. 2018, 69, 1609–1619. [Google Scholar] [CrossRef]
- Yin, L.; Chang, Y.; You, Y.; Liu, C.; Li, J.; Lai, H.-C. Biological responses of human bone mesenchymal stem cells to Ti and TiZr implant materials. Clin. Implant Dent. Relat. Res. 2019, 21, 550–564. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Villagran, H.; Romo-Uribe, A.; Teran-Salgado, E.; Domínguez Díaz, M.; Flores, A. Electrospun polylactic acid non-woven mats incorporating silver nanoparticles. Polym. Bull. 2014, 71, 2437–2452. [Google Scholar] [CrossRef]
- Yuan, Q.; Qin, C.; Wu, J.; Xu, A.; Zhang, Z.; Liao, J.; Lin, S.; Ren, X.; Zhang, P. Synthesis and characterization of Cerium-doped hydroxyapatite/polylactic acid composite coatings on metal substrates. Mater. Chem. Phys. 2016, 182, 365–371. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Barakat, N.A.M.; Lim, J.K. Hydroxyapatite-doped poly(lactic acid) porous film coating for enhanced bioactivity and corrosion behavior of AZ31 Mg alloy for orthopedic applications. Ceram. Int. 2013, 39, 183–195. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Ion, R.; Mazare, A.; Dumitriu, C.; Pirvu, C.; Schmuki, P.; Cimpean, A. Nanochannelar topography positively modulates osteoblast differentiation and inhibits osteoclastogenesis. Coatings 2018, 8, 294. [Google Scholar] [CrossRef] [Green Version]
- Frank, M.J.; Walter, M.S.; Lyngstadaas, S.P.; Wintermantel, E.; Haugen, H.J. Hydrogen content in titanium and a titanium–zirconium alloy after acid etching. Mater. Sci. Eng. C 2013, 33, 1282–1288. [Google Scholar] [CrossRef] [Green Version]
- Baran, E.H.; Erbil, H.Y. Surface Modification of 3D Printed PLA Objects by Fused Deposition Modeling: A Review. Colloids Interfaces 2019, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Li, S.X.; Zhang, Z.F. General relationship between strength and hardness. Mater. Sci. Eng. A 2011, 529, 62–73. [Google Scholar] [CrossRef]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Chung, M.K. Study of Anodic Oxide Films on Titanium and Titanium-Zirconium Alloys and Their Potential for Capacitive Energy Storage. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2016. [Google Scholar]
- Szewczyk, P.K.; Ura, D.P.; Metwally, S.; Knapczyk-Korczak, J.; Gajek, M.; Marzec, M.M.; Bernasik, A.; Stachewicz, U. Roughness and Fiber Fraction Dominated Wetting of Electrospun Fiber-Based Porous Meshes. Polymers 2018, 11, 34. [Google Scholar] [CrossRef] [Green Version]
| Component | Concentration [g/L] |
|---|---|
| NaCl | 7.996 |
| NaHCO3 | 0.35 |
| KCl | 0.224 |
| K2HPO4·3H2O | 0.228 |
| MgCl2·6H2O | 0.305 |
| CaCl2 | 0.278 |
| Na2SO4 | 0.071 |
| (CH2OH)3CNH2 | 6.057 |
| Sample | Adhesion Force [MPa] |
|---|---|
| TiZr—polished | 32.2 ± 0.6 |
| TiZr—etched | 21.4 ± 0.3 |
| TiZr—PLA | 4.3 ± 0.4 |
| Sample | Time [h] | Rs [Ω] | Ro [kΩ] | Co [μF] | Wd [μMho] |
|---|---|---|---|---|---|
| TiZr—etched in 0.9% NaCl | 0 | 1.25 | 46.8 | 150 | - |
| 24 | 1.38 | 55.5 | 148 | - | |
| 48 | 1.26 | 58.3 | 143 | - | |
| 72 | 1.63 | 67.4 | 128 | - | |
| 168 | 1.58 | 61 | 116 | - | |
| TiZr—etched in SBF | 0 | 1.4 | 563 | 131 | 82.7 |
| 24 | 1.12 | 495 | 144 | 80.7 | |
| 48 | 2.05 | 14.4 | 126 | 70.3 | |
| 72 | 1.7 | 5.1 | 162 | 66 | |
| 168 | 3.4 | 1.42 | 116 | 62.1 |
| Sample | Time [h] | Rs [Ω] | Rp [kΩ] | Cp [μF] | Ro [kΩ] | Co [μF] | Ra [kΩ] | Wd [μMho] |
|---|---|---|---|---|---|---|---|---|
| TiZr—etched in 0.9% NaCl | 0 | 250 | 48.8 | 2.18 | 480 | 0.49 | 2.62 | - |
| 24 | 122 | 22.5 | 53.2 | 482 | 1.72 | - | 91.2 | |
| 48 | 72.7 | 2.16 | 144 | 204 | 3.73 | - | 77.4 | |
| 72 | 71.4 | 1.97 | 179 | 207 | 4.81 | - | 72.1 | |
| 168 | 62.5 | 0.93 | 195 | 176 | 5.7 | - | 57.3 | |
| TiZr—etched in SBF | 0 | 111 | 52.2 | 1.52 | 1.92 | 1.27 | 3.02 | - |
| 24 | 51 | 48.3 | 4.1 | 0.61 | 4.1 | - | 74.4 | |
| 48 | 51.3 | 42.8 | 4.75 | 0.59 | 4.75 | - | 68 | |
| 72 | 50.1 | 20.7 | 5.03 | 0.51 | 5.03 | - | 62.5 | |
| 168 | 38 | 19.7 | 5.34 | 0.46 | 5.34 | - | 57.1 |
| Sample | Time [h] | Ecor [V] | Icor [μA] | Vcor [μm/year] |
|---|---|---|---|---|
| TiZr—etched in 0.9% NaCl | 0 | −0.463 | 2.42 | 7.43 |
| 24 | −0.444 | 0.574 | 1.76 | |
| 48 | −0.439 | 0.182 | 0.558 | |
| 72 | −0.454 | 0.581 | 1.78 | |
| 168 | −0.488 | 0.286 | 0.879 | |
| TiZr—etched in SBF | 0 | −0.509 | 0.529 | 1.35 |
| 24 | −0.416 | 0.317 | 0.988 | |
| 48 | −0.388 | 0.164 | 0.419 | |
| 72 | −0.409 | 0.386 | 0.812 | |
| 168 | −0.369 | 0.330 | 0.239 | |
| TiZr—PLA in 0.9% NaCl | 0 | −0.462 | 0.217 | 2.22 |
| 24 | −0.499 | 0.272 | 2.79 | |
| 48 | −0.514 | 1.80 | 18.46 | |
| 72 | −0.501 | 2.30 | 23.56 | |
| 168 | −0.546 | 3.17 | 32.46 | |
| TiZr—PLA in SBF | 0 | −0.450 | 0.721 × 10−3 | 0.011 |
| 24 | −0.394 | 0.012 | 0.188 | |
| 48 | −0.472 | 0.028 | 0.436 | |
| 72 | −0.468 | 0.257 | 3.95 | |
| 168 | −0.492 | 0.305 | 4.68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voicu, M.E.; Stoian, A.B.; Demetrescu, I.; Ionita, D. Characterization of Three Surface Treatments on TiZr—Coating Properies and Corrosion Behavior. Coatings 2021, 11, 615. https://doi.org/10.3390/coatings11060615
Voicu ME, Stoian AB, Demetrescu I, Ionita D. Characterization of Three Surface Treatments on TiZr—Coating Properies and Corrosion Behavior. Coatings. 2021; 11(6):615. https://doi.org/10.3390/coatings11060615
Chicago/Turabian StyleVoicu, Manuela Elena, Andrei Bogdan Stoian, Ioana Demetrescu, and Daniela Ionita. 2021. "Characterization of Three Surface Treatments on TiZr—Coating Properies and Corrosion Behavior" Coatings 11, no. 6: 615. https://doi.org/10.3390/coatings11060615






