Effect of Microcapsules of a Waterborne Core Material on the Properties of a Waterborne Primer Coating on a Wooden Surface
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Method
2.3. Testing and Characterization
3. Results and Discussion
3.1. Effect of the Microcapsules on the Optical Properties of the Coatings
3.1.1. Chromatic Aberration
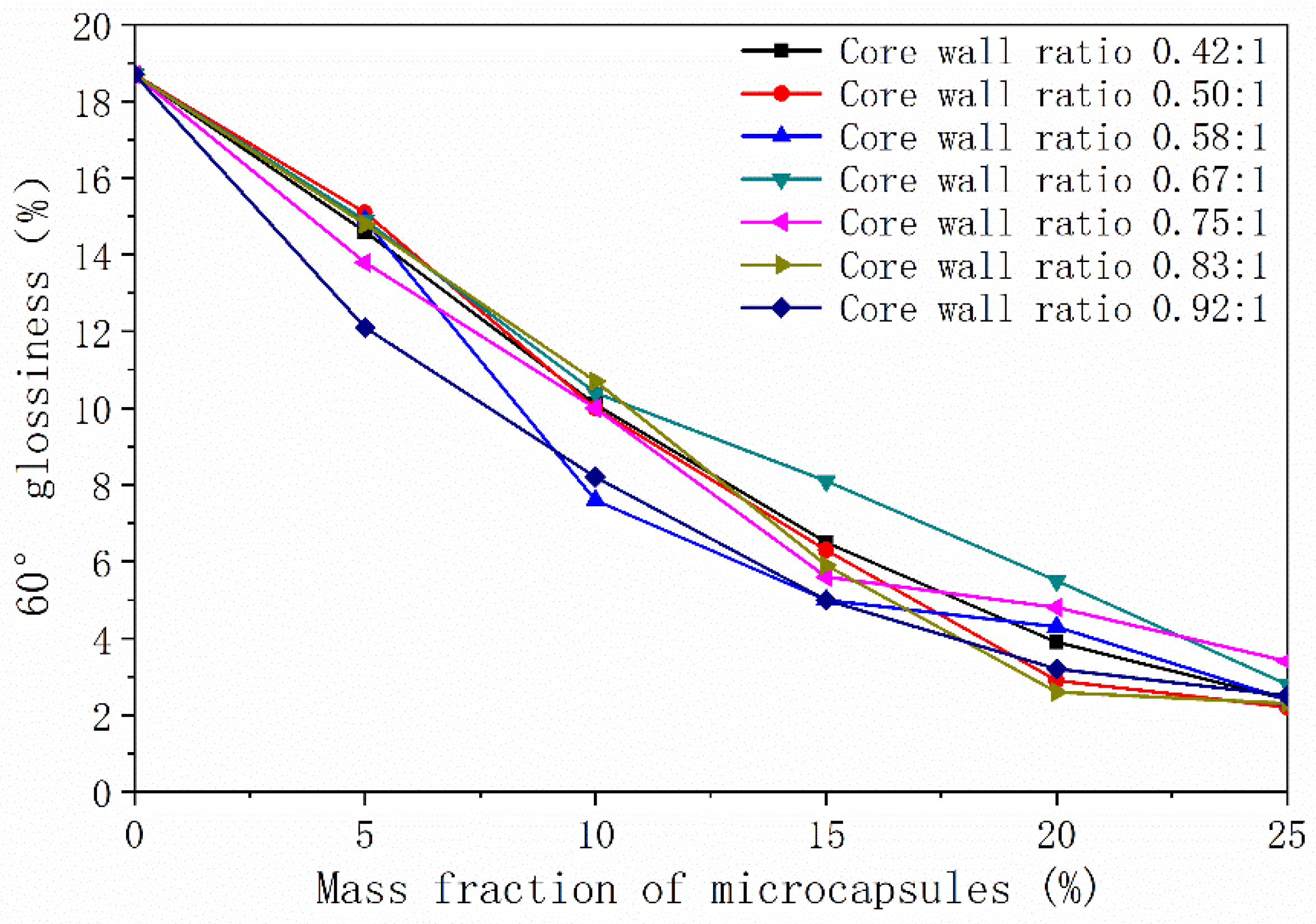
3.1.2. Glossiness
3.2. Effect of the Microcapsules on the Mechanical Properties of the Coatings
3.2.1. Hardness
3.2.2. Adhesion
3.2.3. Impact Resistance
3.2.4. Elongation at the Break
3.3. Effect of the Microcapsules on the Liquid Resistance of the Coatings
3.4. Microstructure Analysis
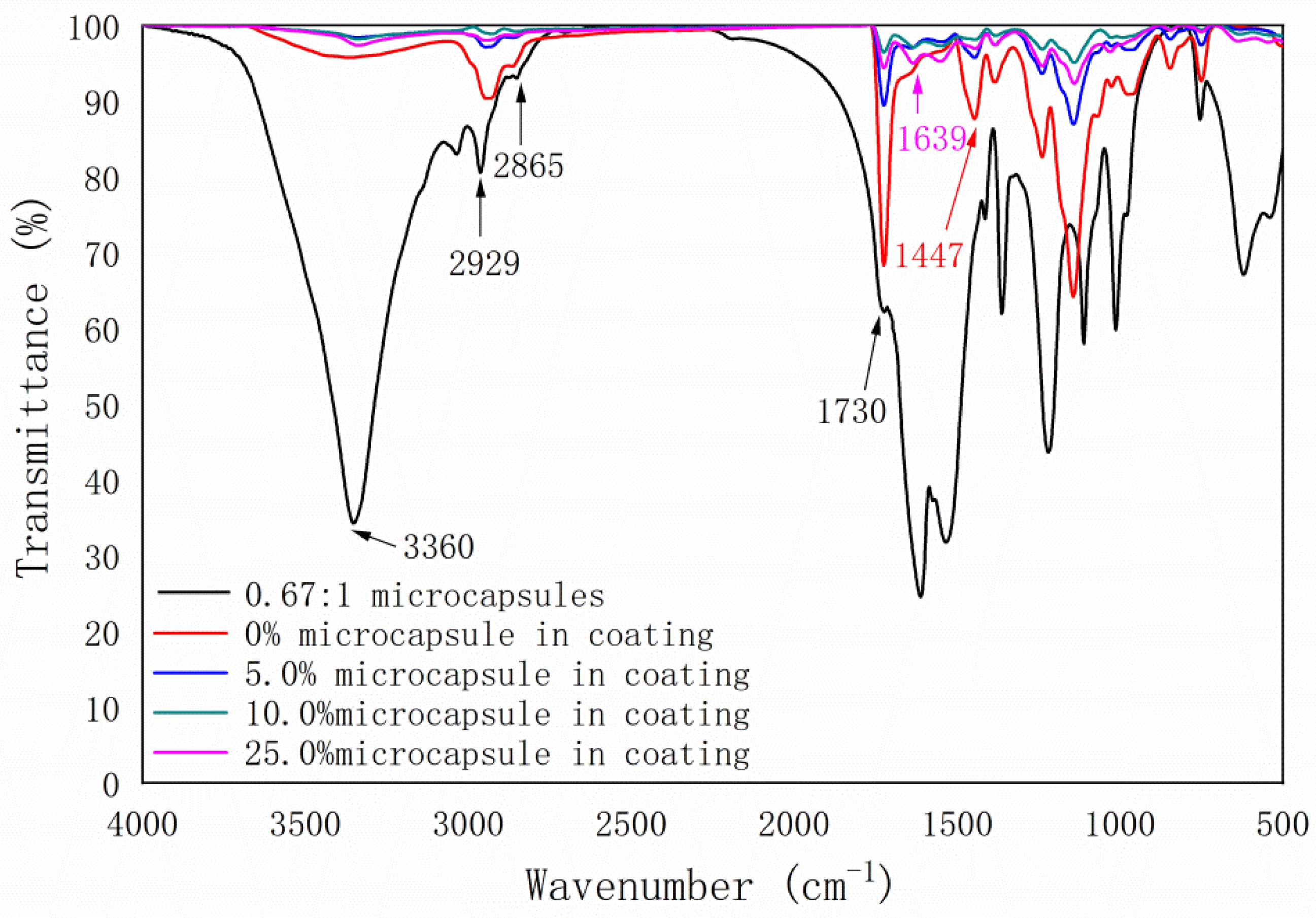
3.5. Infrared Spectroscopy Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeong, H.; Lim, H.; Lee, D.Y.; Song, Y.S.; Kim, B.Y. Preparation and drug release behavior of nifedipine-loaded poly(lactic acid)/polyethylene glycol microcapsules. J. Nanosci. Nanotechnol. 2021, 21, 3735–3741. [Google Scholar] [CrossRef]
- Li, Y.; Hao, P.W.; Zhang, M.Y. Fabrication, characterization and assessment of the capsules containing rejuvenator for improving the self-healing performance of asphalt materials: A review. J. Clean. Prod. 2021, 289, 125079. [Google Scholar] [CrossRef]
- Ryu, S.J.; Bae, H.S. Properties analysis of crosslinked chitosan microcapsules by multiple emulsification method. Fash. Text. 2021, 8, 1–14. [Google Scholar] [CrossRef]
- Cotting, F.; Koebsch, A.; Aoki, I.V. Epoxy self-healing coating by encapsulated epoxy ester resin in poly (urea-formaldehyde-melamine) microcapsules. Front. Mater. 2019, 6, 314. [Google Scholar] [CrossRef]
- Tzavidi, S.; Zotiadis, C.; Porfyris, A.; Korres, D.M.; Vouyiouka, S. Epoxy loaded poly(urea-formaldehyde) microcapsules via in situ polymerization designated for self-healing coatings. J. Appl. Polym. Sci. 2020, 137, 43. [Google Scholar] [CrossRef]
- Sun, D.W.; Yan, Z.; Lan, M.Z.; Wang, Z.M.; Cui, S.P.; Yang, J.L. Robust and impermeable metal shell microcapsules for one-component self-healing coatings. Appl. Surf. Sci. 2021, 546, 149114. [Google Scholar] [CrossRef]
- Jiang, W.J.; Zhou, G.; Duan, J.J.; Liu, D.; Zhang, Q.T.; Tian, F.C. Synthesis and characterization of a multifunctional sustained-release organic-inorganic hybrid microcapsule with self-healing and flame-retardancy properties. ACS Appl. Mater. Interfaces 2021, 13, 15668–15679. [Google Scholar] [CrossRef] [PubMed]
- Ning, K.; Loomans, B.; Yeung, C.; Li, J.; Yang, F.; Leeuwenburgh, S. Influence of microcapsule parameters and initiator concentration on the self-healing capacity of resin-based dental composites. Dent. Mater. 2021, 37, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.X.; Chang, Y.J.; Qian, X.Y. Preparation and self-repairing properties of urea formaldehyde-coated epoxy resin microcapsules. Int. J. Polym. Sci. 2019, 2019, 7215783. [Google Scholar] [CrossRef]
- Xu, W.; Fang, X.Y.; Han, J.T.; Wu, Z.H.; Zhang, J.L. Effect of coating thickness on sound absorption property of four wood species commonly used for piano soundboards. Wood Fiber. Sci. 2020, 52, 28–43. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of sanding processes on the surface properties of modified Poplar coated by primer compared with Mahogany. Coatings 2020, 10, 856. [Google Scholar] [CrossRef]
- Herrera, R.; Muszynska, M.; Krystofiak, T.; Labidi, J. Comparative evaluation of different thermally modified wood samples finishing with UV-curable and waterborne coatings. Appl. Surf. Sci. 2015, 357, 1444–1453. [Google Scholar] [CrossRef]
- Yan, X.X.; Wang, L. Preparation of shellac resin microcapsules coated with urea formaldehyde resin and properties of waterborne paint films for Tilia Amurensis Rupr. Membranes 2020, 10, 278. [Google Scholar] [CrossRef]
- Bao, Y.; Yan, Y.; Chen, Y.; Ma, J.Z.; Zhang, W.B.; Liu, C. Facile fabrication of BTA@ZnO microcapsules and their corrosion protective application in waterborne polyacrylate coatings. Prog. Org. Coat. 2019, 136, 105233. [Google Scholar] [CrossRef]
- Chen, K.L.; Zhou, J.L.; Che, X.G.; Zhao, R.Y.; Gao, Q. One-step synthesis of core shell cellulose-silica/n-octadecane microcapsules and their application in waterborne self-healing multiple protective fabric coatings. J. Colloid Interface Sci. 2020, 566, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.B.; Wu, L.; Weng, M.J.; Tang, B.S.; Lai, P.F.; Chen, J.C. Effect of different encapsulating agent combinations on physicochemical properties and stability of microcapsules loaded with phenolics of plum (Prunus salicina lindl.). Powder Technol. 2018, 340, 459–464. [Google Scholar]
- Wang, L.; Yan, X.X. Mechanical and optical properties of thermochromic reversible waterborne primer film on tilia europaea with 1,2-benzo-6-diethylaminofluorane based microcapsules. Polymers 2020, 12, 2062. [Google Scholar] [CrossRef]
- Fu, H.; Gong, W.; Chen, B.B.; Chen, Y.X.; Ban, D.M.; Yin, X.G.; Pei, X.L.; He, L. Influence of electrolytes on thermal expansion microcapsules. J. Macromol. Sci. A 2019, 56, 104–114. [Google Scholar] [CrossRef]
- Katoueizadeh, E.; Zebarjad, S.M.; Janghorban, K. Mechanical properties of epoxy composites embedded with functionalized urea-formaldehyde microcapsules containing an oxidizable oil. Mater. Chem. Phys. 2021, 260, 124106. [Google Scholar] [CrossRef]
- Han, T.L.; Wang, X.F.; Li, D.W.; Li, D.F.; Xing, F.; Han, N.X. Influence of strain rate on mechanical characteristic and pore structure of self-healing cementitious composites with epoxy/urea-formaldehyde microcapsules. Constr. Build. Mater. 2021, 268, 121138. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.C.; Chen, Z.Q.; Chen, Z.W.; Ni, L. Preparation and characterization of microencapsulated aluminum hypophosphite and its performance on the thermal, flame retardancy, and mechanical properties of epoxy resin. Polym. Compos. 2021, 42, 1818–1834. [Google Scholar] [CrossRef]
- Yan, X.X.; Zhao, W.T.; Qian, X.Y. Effect of urea-formaldehyde (UF) with waterborne emulsion microcapsules on properties of waterborne acrylic coatings based on coating process for American Lime. Appl. Sci. 2020, 10, 6341. [Google Scholar] [CrossRef]
- Liu, W.L.; Zhang, J.P.; Liu, Q.; Pei, J.Z.; Zhu, C.Z.; Liu, P. Effects of emulsifier dosage and curing time on self-healing microcapsules containing rejuvenator and optimal dosage in asphalt binders. J. Nanosci. Nanotechnol. 2019, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.D.; Li, C.N.; Wang, J.Y.; Jia, X.L.; Zhu, J.T.; Wang, Q.; Wang, H.; Yang, Y.J. Osmosis manipulable morphology and photonic property of microcapsules with colloidal nano-in-micro structure. J. Colloid Interface Sci. 2020, 574, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Wu, R.X.; Feng, M.C.; Zhang, G.X.; Zhang, C.C.; Hao, P.W. Synthesis of microcapsules with waste oil core and application in self-healing asphalt. Pet. Sci. Technol. 2019, 38, 203–209. [Google Scholar] [CrossRef]
- Zhao, M.X.; Li, M.K.; Wang, L.; Zhang, X.; Kong, X.F. Preparation and characterization of paraffin@CLPS/MS phase change microcapsules for thermal energy storage. ChemistrySelect 2020, 5, 7190–7196. [Google Scholar] [CrossRef]
- Neto, A.G.C.; Pellanda, A.C.; Jorge, A.R.D.; Floriano, J.B.; Berton, M.A.C. Preparation and evaluation of corrosion resistance of a self-healing alkyd coating based on microcapsules containing Tung oil. Prog. Org. Coat. 2020, 147, 105874. [Google Scholar] [CrossRef]
- Amor, G.; Sabbah, M.; Caputo, L.; Idbella, M.; De Feo, V.; Porta, R.; Fechtali, T.; Mauriello, G. Basil essential oil: Composition, antimicrobial properties, and microencapsulation to produce active chitosan films for food packaging. Foods 2021, 10, 121. [Google Scholar] [CrossRef]
- GB/T4893.4-2013 Physicochemical Performance Test of Furniture Surface Paint Film Part 4 Determination of Adhesion Cross-Cutting Method; Standardization Administration of the People’s Republic of China: Beijing, China, 2013; pp. 1–4. (In Chinese)
- GB/T4893.9-2013 Physical and Chemical Properties Test of Furniture Surface Paint Film Part 9 Determination of Impact Resistance; Standardization Administration of the People’s Republic of China: Beijing, China, 2013; pp. 1–4. (In Chinese)
- Du, W.; Yu, J.Y.; He, B.Y.; He, Y.H.; He, P.; Li, Y.; Liu, Q.T. Preparation and characterization of nano-SiO2/paraffin/PE wax composite shell microcapsules containing TDI for self-healing of cementitious materials. Constr. Build. Mater. 2020, 231, 117060. [Google Scholar] [CrossRef]
- Chen, T.Q.; Fang, L.; Li, X.; Gao, D.S.; Lu, C.H.; Xu, Z.Z. Self-healing polymer coatings of polyurea-urethane/epoxy blends with reversible and dynamic bonds. Prog. Org. Coat. 2020, 147, 105876. [Google Scholar] [CrossRef]
- Mohammadloo, H.E.; Mirabedini, S.M.; Pezeshk-Fallah, H. Microencapsulation of quinoline and cerium based inhibitors for smart coating application: Anti-corrosion, morphology and adhesion study. Prog. Org. Coat. 2019, 137, 105339. [Google Scholar] [CrossRef]
- Kim, Y.N.; Nam, K.H.; Jung, Y.C.; Han, H. Interfacial adhesion and self-healing kinetics of multi-stimuli responsive colorless polymer bilayers. Compos. Part B Eng. 2020, 203, 108451. [Google Scholar] [CrossRef]
- Han, T.L.; Wang, X.F.; Li, D.W.; Li, D.F.; Xing, F.; Han, N.X. Impermeability characteristics of cementitious materials with self-healing based on epoxy/urea-formaldehyde microcapsules using an immersion test. Constr. Build. Mater. 2020, 259, 119782. [Google Scholar] [CrossRef]
- Nassho, Y.; Sanada, K. Microstructure optimizations for improving interlaminar shear strength and self-healing efficiency of spread carbon fiber/epoxy laminates containing microcapsules. J. Compos. Mater. 2021, 55, 27–38. [Google Scholar] [CrossRef]
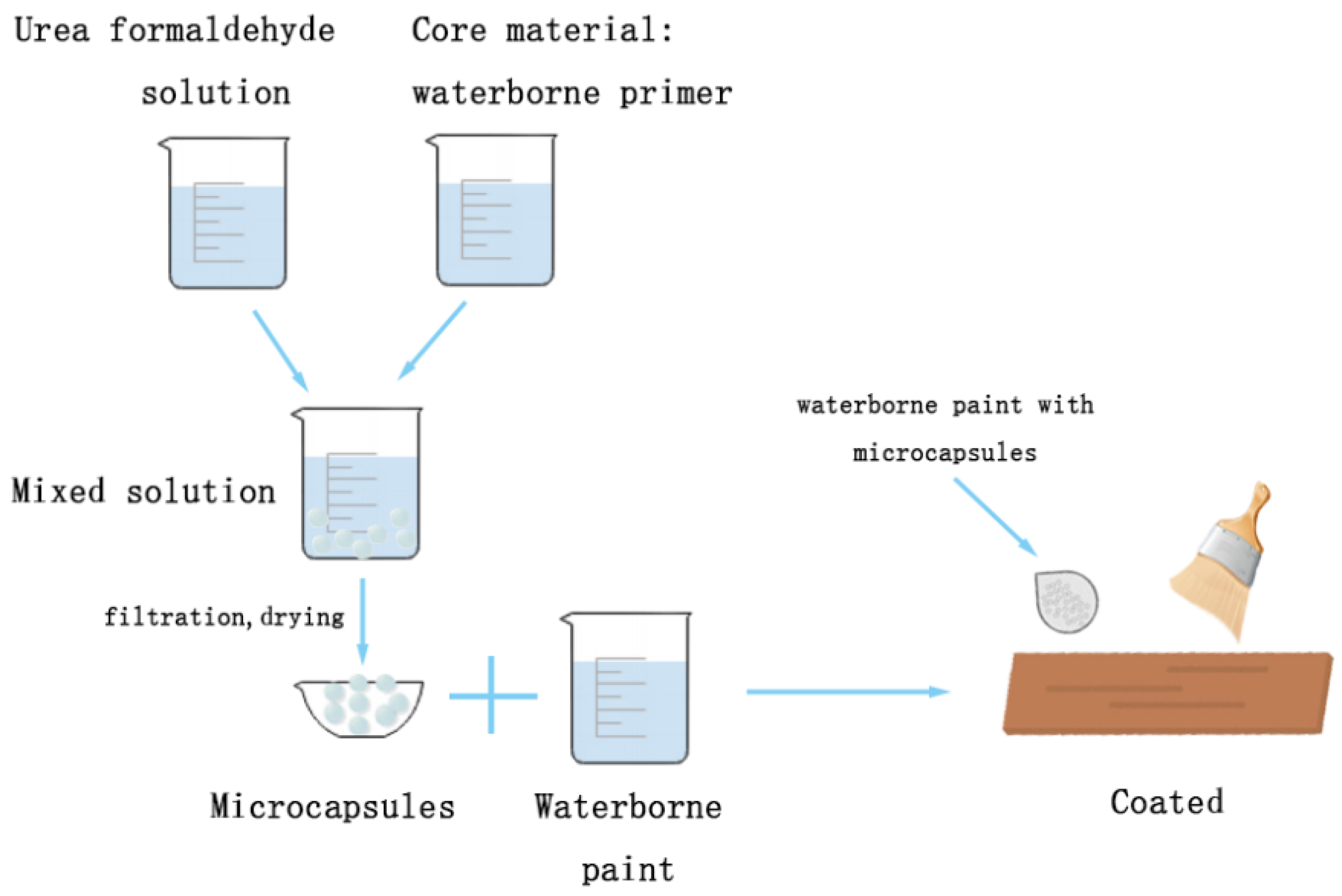




| Sample | Urea (g) | Formaldehyde Solution (g) | Waterborne Acrylic Coating (g) | Sodium Dodecyl Benzene Sulfonate (g) | Deionized Water (g) | Core-Shell Ratio |
|---|---|---|---|---|---|---|
| 1 | 20.0 | 27.0 | 12.5 | 0.98 | 97.02 | 0.42:1 |
| 2 | 20.0 | 27.0 | 15.0 | 1.17 | 115.83 | 0.50:1 |
| 3 | 20.0 | 27.0 | 17.5 | 1.37 | 135.60 | 0.58:1 |
| 4 | 20.0 | 27.0 | 20.0 | 1.56 | 154.44 | 0.67:1 |
| 5 | 20.0 | 27.0 | 22.5 | 1.76 | 174.24 | 0.75:1 |
| 6 | 20.0 | 27.0 | 25.0 | 1.95 | 193.05 | 0.83:1 |
| 7 | 20.0 | 27.0 | 27.5 | 2.15 | 212.85 | 0.92:1 |
| Mass Fraction of Microcapsules (%) | Mass of Microcapsules (g) | Mass of Waterborne Primer (g) | Mass of Self-Healing Waterborne Primer (g) |
|---|---|---|---|
| 0 | 0 | 4.0 | 4.0 |
| 5.0 | 0.2 | 3.8 | 4.0 |
| 10.0 | 0.4 | 3.6 | 4.0 |
| 15.0 | 0.6 | 3.4 | 4.0 |
| 20.0 | 0.8 | 3.2 | 4.0 |
| 25.0 | 1.0 | 3.0 | 4.0 |
| Mass Fraction of Microcapsules (%) | Hardness | ||||||
|---|---|---|---|---|---|---|---|
| Core–Shell Ratio 0.42:1 | Core–Shell Ratio 0.50:1 | Core–Shell Ratio 0.58:1 | Core–Shell Ratio 0.67:1 | Core–Shell Ratio 0.75:1 | Core–Shell Ratio 0.83:1 | Core–Shell Ratio 0.92:1 | |
| 0 | HB | HB | HB | HB | HB | HB | HB |
| 5.0 | 2H | 2H | 2H | 2H | H | 2H | 2H |
| 10.0 | 2H | 2H | 3H | 3H | 2H | 2H | 2H |
| 15.0 | 3H | 3H | 3H | 3H | 2H | 2H | 2H |
| 20.0 | 3H | 2H | 3H | 3H | 3H | 3H | 3H |
| 25.0 | 3H | 2H | 4H | 4H | 3H | 3H | 3H |
| Mass Fraction of Microcapsules (%) | Adhesion (Level) | ||||||
|---|---|---|---|---|---|---|---|
| Core–Shell Ratio 0.42:1 | Core–Shell Ratio 0.50:1 | Core–Shell Ratio 0.58:1 | Core–Shell Ratio 0.67:1 | Core–Shell Ratio 0.75:1 | Core–Shell Ratio 0.83:1 | Core–Shell Ratio 0.92:1 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5.0 | 0 | 1 | 0 | 0 | 1 | 0 | 1 |
| 10.0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 15.0 | 1 | 1 | 1 | 1 | 1 | 1 | 2 |
| 20.0 | 2 | 2 | 1 | 1 | 2 | 2 | 2 |
| 25.0 | 3 | 2 | 2 | 2 | 2 | 3 | 2 |
| Mass Fraction of Microcapsules (%) | Impact Resistance (kg·cm) | ||||||
|---|---|---|---|---|---|---|---|
| Core–Shell Ratio 0.42:1 | Core–Shell Ratio 0.50:1 | Core–Shell Ratio 0.58:1 | Core–Shell Ratio 0.67:1 | Core–Shell Ratio 0.75:1 | Core–Shell Ratio 0.83:1 | Core–Shell Ratio 0.92:1 | |
| 0 | 6.0 ± 0.5 | 6.0 ± 0.5 | 6.0 ± 0.5 | 6.0 ± 0.5 | 6.0 ± 0.5 | 6.0 ± 0.5 | 6.0 ± 0.5 |
| 5.0 | 8.0 ± 0.8 | 8.0 ± 0.5 | 8.0 ± 0.8 | 9.0 ± 0.2 | 9.0 ± 0.1 | 9.0 ± 0.9 | 8.0 ± 0.7 |
| 10.0 | 9.0 ± 0.1 | 12.0 ± 0.5 | 9.0 ± 0.8 | 10.0 ± 0.5 | 10.0 ± 0.8 | 12.0 ± 0.5 | 12.0 ± 0.5 |
| 15.0 | 10.0 ± 0.8 | 11.0 ± 0.5 | 10.0 ± 0.5 | 13.0 ± 0.5 | 10.0 ± 0.5 | 12.0 ± 0.5 | 10.0 ± 0.5 |
| 20.0 | 12.0 ± 0.5 | 10.0 ± 0.5 | 13.0 ± 0.5 | 11.0 ± 0.5 | 11.0 ± 0.5 | 10.0 ± 0.5 | 10.0 ± 0.5 |
| 25.0 | 9.0 ± 0.8 | 10.0 ± 0.5 | 13.0 ± 0.5 | 11.0 ± 0.5 | 11.0 ± 0.5 | 10.0 ± 0.8 | 10.0 ± 0.5 |
| Mass Fraction of Microcapsules (%) | Elongation at the Break (%) | |||
|---|---|---|---|---|
| Core–Shell Ratio 0.42:1 | Core–Shell Ratio 0.50:1 | Core–Shell Ratio 0.58:1 | Core–Shell Ratio 0.67:1 | |
| 0 | 7.63 ± 0.10 | 7.63 ± 0.10 | 7.63 ± 0.10 | 7.63 ± 0.10 |
| 5.0 | 8.20 ± 0.30 | 7.91 ± 0.30 | 8.47 ± 0.10 | 9.03 ± 0.20 |
| 10.0 | 11.09 ± 0.10 | 8.88 ± 0.20 | 10.16 ± 0.10 | 12.21 ± 0.10 |
| 15.0 | 12.25 ± 0.20 | 10.25 ± 0.20 | 12.18 ± 0.20 | 16.59 ± 0.10 |
| 20.0 | 9.47 ± 0.10 | 13.56 ± 0.40 | 14.18 ± 0.10 | 9.90 ± 0.10 |
| 25.0 | 7.52 ± 0.30 | 9.28 ± 0.20 | 6.84 ± 0.20 | 9.21 ± 0.10 |
| Core–Shell Ratio of Microcapsules | Mass Fraction of Microcapsules (%) | Chromatic Aberration | |||
|---|---|---|---|---|---|
| NaCl | Ethanol | Detergent | Red Ink | ||
| 0.42:1 | 0 | 1.0 | 0.9 | 1.1 | 3.0 |
| 5.0 | 1.0 | 0.7 | 0.6 | 1.4 | |
| 10.0 | 1.1 | 0.6 | 1.1 | 0.8 | |
| 15.0 | 0.7 | 0.8 | 1.0 | 0.8 | |
| 20.0 | 1.1 | 0.4 | 0.8 | 1.1 | |
| 25.0 | 0.7 | 8.8 ± 0.1 | 1.5 | 3.6 ± 0.1 | |
| 0.50:1 | 0 | 1.0 | 0.9 | 1.1 | 3.0 ± 0.1 |
| 5.0 | 0.9 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.1 | |
| 10.0 | 1.1 ± 0.1 | 0.3 | 0.9 | 0.8 | |
| 15.0 | 0.6 | 1.0 | 1.0 | 1.1 ± 0.1 | |
| 20.0 | 1.0 | 1.7 ± 0.1 | 1.4 ± 0.1 | 2.9 ± 0.1 | |
| 25.0 | 0.9 | 4.9 ± 0.1 | 1.5 ± 0.1 | 4.0 ± 0.1 | |
| 0.58:1 | 0 | 1.0 | 0.9 | 1.1 | 3.0 ± 0.1 |
| 5.0 | 0.7 | 0.8 | 1.1 | 0.4 | |
| 10.0 | 0.6 | 1.1 | 0.7 | 1.9 ± 0.1 | |
| 15.0 | 0.7 | 1.0 | 1.0 | 1.0 | |
| 20.0 | 1.0 | 0.5 | 0.7 | 2.5 ± 0.1 | |
| 25.0 | 1.1 | 2.8 ± 0.1 | 2.4 ± 0.1 | 3.4 ± 0.1 | |
| 0.67:1 | 0 | 1.0 | 0.9 | 1.1 | 3.0 ± 0.1 |
| 5.0 | 0.4 | 0.9 | 1.1 ± 0.1 | 1.5 ± 0.1 | |
| 10.0 | 1.0 | 0.8 | 1.1 | 1.1 | |
| 15.0 | 0.6 | 1.1 | 1.1 | 1.3 ± 0.1 | |
| 20.0 | 0.9 | 0.4 | 1.0 | 0.6 | |
| 25.0 | 1.0 | 2.7 ± 0.1 | 0.9 | 2.1 ± 0.1 | |
| 0.75:1 | 0 | 1.0 | 0.9 | 1.1 | 3.0 ± 0.2 |
| 5.0 | 1.0 | 1.0 | 1.1 | 2.2 ± 0.1 | |
| 10.0 | 0.7 | 0.4 | 0.4 | 2.3 ± 0.1 | |
| 15.0 | 1.1 ± 0.1 | 0.6 | 0.5 | 2.2 ± 0.1 | |
| 20.0 | 0.9 | 0.6 | 0.4 | 2.6 ± 0.2 | |
| 25.0 | 1.0 | 1.0 | 1.0 | 2.8 ± 0.1 | |
| 0.83:1 | 0 | 1.0 ± 0.1 | 0.9 | 1.1 | 3.0 ± 0.1 |
| 5.0 | 0.4 | 0.9 | 0.7 | 2.1 ± 0.2 | |
| 10.0 | 0.9 | 0.5 | 0.4 | 2.1 ± 0.1 | |
| 15.0 | 1.1 ± 0.1 | 0.3 | 0.2 | 2.2 ± 0.1 | |
| 20.0 | 1.1 ± 0.1 | 4.3 ± 0.2 | 2.4 ± 0.1 | 2.2 ± 0.2 | |
| 25.0 | 1.3 ± 0.1 | 8.2 ± 0.1 | 1.7 ± 0.1 | 3.4 ± 0.1 | |
| 0.92:1 | 0 | 1.0 | 0.9 | 1.1 ± 0.1 | 3.0 ± 0.1 |
| 5.0 | 0.6 | 1.9 ± 0.1 | 1.1 ± 0.1 | 2.1 ± 0.1 | |
| 10.0 | 0.6 | 1.6 ± 0.1 | 0.4 | 2.0 ± 0.1 | |
| 15.0 | 0.4 | 0.7 | 0.4 | 2.2 ± 0.1 | |
| 20.0 | 0.6 | 1.2 ± 0.1 | 0.9 | 2.5 ± 0.2 | |
| 25.0 | 0.8 | 2.3 ± 0.1 | 0.9 | 3.1 ± 0.1 | |
| Core–Shell Ratio of Microcapsules | Mass Fraction of Microcapsules (%) | Glossiness (%) | |||
|---|---|---|---|---|---|
| NaCl | Ethanol | Detergent | Red Ink | ||
| 0.42:1 | 0 | 18.5 ± 0.4 | 18.4 ± 0.2 | 18.7 ± 0.3 | 18.6 ± 0.2 |
| 5.0 | 14.2 ± 0.2 | 14.3 ± 0.5 | 14.8 ± 0.5 | 14.4 ± 0.4 | |
| 10.0 | 9.6 ± 0.3 | 9.9 ± 0.3 | 9.8 ± 0.2 | 10.2 ± 0.3 | |
| 15.0 | 5.7 ± 0.1 | 6.7 ± 0.2 | 6.2 ± 0.3 | 5.6 ± 0.2 | |
| 20.0 | 3.5 ± 0.1 | 3.4 ± 0.1 | 3.7 ± 0.1 | 3.3 ± 0.2 | |
| 25.0 | 2.1 ± 0.1 | 2.7 ± 0.1 | 2.3 ± 0.1 | 1.9 ± 0.1 | |
| 0.50:1 | 0 | 18.5 ± 0.5 | 18.4 ± 0.4 | 18.7 ± 0.5 | 18.6 ± 0.3 |
| 5.0 | 15.2 ± 0.3 | 15.3 ± 0.2 | 15.1 ± 0.3 | 14.8 ± 0.2 | |
| 10.0 | 10.1 ± 0.2 | 9.8 ± 0.1 | 10.2 ± 0.1 | 9.6 ± 0.2 | |
| 15.0 | 6.1 ± 0.1 | 6.2 ± 0.1 | 6.0 ± 0.1 | 6.0 ± 0.2 | |
| 20.0 | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.7 ± 0.1 | 2.2 ± 0.1 | |
| 25.0 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 | 1.9 ± 0.1 | |
| 0.58:1 | 0 | 18.5 ± 0.3 | 18.4 ± 0.4 | 18.7 ± 0.5 | 18.6 ± 0.5 |
| 5.0 | 14.6 ± 0.7 | 14.6 ± 0.5 | 14.9 ± 0.4 | 14.6 ± 0.6 | |
| 10.0 | 7.9 ± 0.2 | 7.8 ± 0.1 | 7.5 ± 0.1 | 7.5 ± 0.1 | |
| 15.0 | 5.0 ± 0.0 | 4.7 ± 0.1 | 4.9 ± 0.1 | 4.4 ± 0.1 | |
| 20.0 | 4.0 ± 0.1 | 4.1 ± 0.2 | 4.3 ± 0.2 | 3.7 ± 0.1 | |
| 25.0 | 2.2 ± 0.1 | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.1 | |
| 0.67:1 | 0 | 18.5 ± 0.5 | 18.4 ± 0.3 | 18.7 ± 0.4 | 18.6 ± 0.4 |
| 5.0 | 13.3 ± 0.3 | 13.7 ± 0.2 | 13.5 ± 0.3 | 12.5 ± 0.3 | |
| 10.0 | 9.3 ± 0.2 | 9.3 ± 0.3 | 9.2 ± 0.3 | 8.8 ± 0.1 | |
| 15.0 | 8.1 ± 0.2 | 8.2 ± 0.2 | 8.2 ± 0.3 | 7.7 ± 0.2 | |
| 20.0 | 5.4 ± 0.1 | 5.5 ± 0.1 | 5.5 ± 0.2 | 5.1 ± 0.2 | |
| 25.0 | 2.5 ± 0.2 | 2.6 ± 0.1 | 2.8 ± 0.2 | 2.8 ± 0.1 | |
| 0.75:1 | 0 | 18.5 ± 0.3 | 18.4 ± 0.3 | 18.7 ± 0.4 | 18.6 ± 0.5 |
| 5.0 | 13.3 ± 0.2 | 13.3 ± 0.3 | 13.2 ± 0.1 | 13.4 ± 0.2 | |
| 10.0 | 9.6 ± 0.2 | 9.9 ± 0.1 | 9.3 ± 0.3 | 9.7 ± 0.2 | |
| 15.0 | 5.6 ± 0.1 | 5.8 ± 0.1 | 5.5 ± 0.2 | 5.4 ± 0.1 | |
| 20.0 | 4.4 ± 0.1 | 4.6 ± 0.3 | 4.4 ± 0.2 | 4.4 ± 0.1 | |
| 25.0 | 3.4 ± 0.1 | 3.3 ± 0.1 | 3.2 ± 0.1 | 3.3 ± 0.2 | |
| 0.83:1 | 0 | 18.5 ± 0.2 | 18.4 ± 0.3 | 18.7 ± 0.3 | 18.6 ± 0.4 |
| 5.0 | 14.8 ± 0.3 | 14.8 ± 0.5 | 14.3 ± 0.4 | 14.2 ± 0.3 | |
| 10.0 | 10.2 ± 0.3 | 10.8 ± 0.2 | 10.4 ± 0.2 | 10.0 ± 0.4 | |
| 15.0 | 5.6 ± 0.1 | 5.5 ± 0.1 | 5.8 ± 0.1 | 5.7 ± 0.2 | |
| 20.0 | 2.5 ± 0.1 | 2.7 ± 0.1 | 2.5 ± 0.1 | 2.7 ± 0.1 | |
| 25.0 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 1.8 ± 0.1 | |
| 0.92:1 | 0 | 18.5 ± 0.4 | 18.4 ± 0.3 | 18.7 ± 0.4 | 18.6 ± 0.3 |
| 5.0 | 13.1 ± 0.2 | 12.3 ± 0.3 | 12.4 ± 0.4 | 12.3 ± 0.5 | |
| 10.0 | 8.4 ± 0.2 | 8.2 ± 0.1 | 8.4 ± 0.1 | 8.3 ± 0.2 | |
| 15.0 | 5.1 ± 0.2 | 4.9 ± 0.2 | 5.0 ± 0.3 | 5.1 ± 0.3 | |
| 20.0 | 3.1 ± 0.1 | 3.3 ± 0.1 | 3.1 ± 0.1 | 2.8 ± 0.1 | |
| 25.0 | 2.2 ± 0.1 | 2.3 ± 0.1 | 2.5 ± 0.1 | 2.4 ± 0.1 | |
| Core–Shell Ratio of Microcapsules | Mass Fraction of Microcapsules (%) | Liquid Resistance (Level) | |||
|---|---|---|---|---|---|
| NaCl | Ethanol | Detergent | Red Ink | ||
| 0.42:1 | 0 | 1 | 1 | 1 | 3 |
| 5.0 | 1 | 1 | 1 | 1 | |
| 10.0 | 1 | 1 | 1 | 1 | |
| 15.0 | 1 | 1 | 1 | 1 | |
| 20.0 | 1 | 1 | 1 | 1 | |
| 25.0 | 1 | 3 | 1 | 3 | |
| 0.50:1 | 0 | 1 | 1 | 1 | 3 |
| 5.0 | 1 | 1 | 1 | 1 | |
| 10.0 | 1 | 1 | 1 | 1 | |
| 15.0 | 1 | 1 | 1 | 1 | |
| 20.0 | 1 | 1 | 1 | 2 | |
| 25.0 | 1 | 3 | 1 | 3 | |
| 0.58:1 | 0 | 1 | 1 | 1 | 3 |
| 5.0 | 1 | 1 | 1 | 1 | |
| 10.0 | 1 | 1 | 1 | 1 | |
| 15.0 | 1 | 1 | 1 | 1 | |
| 20.0 | 1 | 1 | 1 | 2 | |
| 25.0 | 1 | 2 | 2 | 3 | |
| 0.67:1 | 0 | 1 | 1 | 1 | 3 |
| 5.0 | 1 | 1 | 1 | 1 | |
| 10.0 | 1 | 1 | 1 | 1 | |
| 15.0 | 1 | 1 | 1 | 1 | |
| 20.0 | 1 | 1 | 1 | 1 | |
| 25.0 | 1 | 2 | 1 | 3 | |
| 0.75:1 | 0 | 1 | 1 | 1 | 3 |
| 5.0 | 1 | 1 | 1 | 2 | |
| 10.0 | 1 | 1 | 1 | 2 | |
| 15.0 | 1 | 1 | 1 | 2 | |
| 20.0 | 1 | 1 | 1 | 2 | |
| 25.0 | 1 | 1 | 1 | 2 | |
| 0.83:1 | 0 | 1 | 1 | 1 | 3 |
| 5.0 | 1 | 1 | 1 | 2 | |
| 10.0 | 1 | 1 | 1 | 2 | |
| 15.0 | 1 | 1 | 1 | 2 | |
| 20.0 | 1 | 3 | 2 | 2 | |
| 25.0 | 1 | 3 | 1 | 3 | |
| 0.92:1 | 0 | 1 | 1 | 1 | 3 |
| 5.0 | 1 | 1 | 1 | 2 | |
| 10.0 | 1 | 1 | 1 | 2 | |
| 15.0 | 1 | 1 | 1 | 2 | |
| 20.0 | 1 | 1 | 1 | 2 | |
| 25.0 | 1 | 2 | 1 | 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Peng, W. Effect of Microcapsules of a Waterborne Core Material on the Properties of a Waterborne Primer Coating on a Wooden Surface. Coatings 2021, 11, 657. https://doi.org/10.3390/coatings11060657
Yan X, Peng W. Effect of Microcapsules of a Waterborne Core Material on the Properties of a Waterborne Primer Coating on a Wooden Surface. Coatings. 2021; 11(6):657. https://doi.org/10.3390/coatings11060657
Chicago/Turabian StyleYan, Xiaoxing, and Wenwen Peng. 2021. "Effect of Microcapsules of a Waterborne Core Material on the Properties of a Waterborne Primer Coating on a Wooden Surface" Coatings 11, no. 6: 657. https://doi.org/10.3390/coatings11060657
APA StyleYan, X., & Peng, W. (2021). Effect of Microcapsules of a Waterborne Core Material on the Properties of a Waterborne Primer Coating on a Wooden Surface. Coatings, 11(6), 657. https://doi.org/10.3390/coatings11060657





