Abstract
Titanium and titanium alloys have been extensively utilized in biomedical implants due to their excellent comprehensive mechanical properties and biocompatibility. In this study, a ZrN/Ag2O micro–nano gradient composite structure was prepared on the surface of pure Ti by multi-arc ion plating (MAIP) technique and metal vapor vacuum arc (MEVVA) ion implantation technology. This study indicated that a dramatic improvement in performance in the surface hardness (~1800 HV0.1) was attributed to the presence of the ceramic phase (ZrN) with high hardness included in composite structure. The relatively low wear rate of gradient composite structure confirmed its excellent performance in abrasion resistance and the abrasion mechanism of gradient composite structure was mainly abrasive wear. After the potentiodynamic polarization and electrochemical impedance spectroscopy (EIS) tests, because of the synergy effect of ZrN micron coating and Ag2O nanoparticles, the ZrN/Ag2O gradient coatings indicated the highest free corrosion potential (Ecorr) and lowest corrosion current density (icorr) in Ringer’s solution, and the polarization resistances of multilayer coatings were greater than that of the substrate, exhibiting positive effects on retarding localized corrosion tendency. Additionally, the suitable dose of ZrN/Ag2O gradient composite coating can obtain antibacterial ability, which exerts no significant cytotoxicity and even excellent cytocompatibility over a longer service process. Furthermore, this study is conducive to design and develop for multifunctional coatings of implant materials.
1. Introduction
In the modern medical field, biomedical materials have been widely used in substituting human organs and tissues and modifying their functions to satisfy diversified requirements [1,2,3]. Thereinto, titanium and its alloys, due to their adequate biocompatibility and comprehensive mechanical properties, have been the most frequently used metallic materials for surgical implantation, such as artificial joint and bone [4,5]. However, their application and development are restricted to a certain extent by their poor hardness, wear resistance and antibacterial properties. Caused by the wear and corrosion of metallic implants, the loss of asepsis is a main problem of artificial joint or bone for long-term clinical usage [6,7]. Meanwhile, the surface of titanium alloys does not have effective antibacterial properties, resulting in bacterial adhesion to form bacterial biofilm [8,9,10]. Hence, in their course of service, bacterial infection and the related risk of infection associated with the implants are counted among the most serious complications. Both of these issues would influence the service time and usage effect of insertion or implantation, or even its failure [11,12,13,14].
Therefore, in addition to improving the surface physical properties of the metallic implants to prevent the loss of asepsis as much as possible, it is also vital to prevent bacterial biofilm formation. Instead of exploiting novel biomaterials, the most frequently used method is on the basis of the surface modification for the biomaterials in existence by a coating [15,16,17]. Herein, zirconium nitride (ZrN) is a noteworthy biomedical coating to realize this purpose due to its properties such as high hardness, wear resistance, corrosion resistance and biocompatibility [18,19], whereas the ZrN coating has still not shown any antibacterial properties, so it is crucial to determine a method to restrain bacterial infection [20].
Silver ions (Ag+) are a notable broad-spectrum bactericide against different microorganisms exhibiting powerful antimicrobial activity at very low concentrations. It is possible to fabricate coatings with antibacterial characteristics by introducing Ag+ or silver nanoparticles (acting as a reservoir of Ag+). In fact, it has been reported that Ag-containing coatings can kill bacteria observably by the release of silver ions. The Ag-containing coatings can release silver ions and generate reactive oxygen species (ROS), which interact with membrane proteins affecting their correct function and facilitate the increase in membrane permeability and the loss of the proton motive force, inducing the de-energization of the cells and efflux of phosphate, leakage of cellular content, and disruption of DNA replication [21,22,23,24,25].
Previous investigations have shown that surface modification of biomedical metal materials is one of the main research fields for biomedical researchers and various antimicrobial methods have been proposed in the literature, such as the sol–gel method, magnetron sputtering, plasma deposition and ion implantation [26,27,28]. Baheiraei et al. reported that silver-doped silica thin films could be deposited on the surface of ceramic tiles by sol–gel method, which presented excellent antibacterial activity [29]. Kelly et al. produced CrN/Ag, ZrN/Ag, TiN/Ag and TiN/Cu nanocomposite coatings with different silver or copper contents by co-deposition in a dual pulsed magnetron sputtering system to achieve antibacterial activity and wear resistance [30]. Gao et al. explored that TiO2–NT arrays decorated with Ag2O nanoparticle were formed on titanium (Ti) by TiAg magnetron sputtering and anodization, which not only ensured the sufficient antibacterial activity of coating, but also controlled the release of Ag+ and showed no obvious biological toxicity [31]. Cao et al. reported that the silver and calcium ions were dual-ion implanted into titanium surface, which could generate the synergistic actions of Ag/Ca galvanic current for breaking the reactive oxygen species (ROS) homeostasis to achieve antibacterial effect [32]. Increasingly, researchers are focusing on innovative approaches to prepare antimicrobial coatings. At the same time, the promotion of these coatings in mechanical performance also should be valued as an important research subject of clinical field, which plays an important role in many practical applications.
Hence, in this work, we attempted to combine the Ag+ with the ZrN coating as a gradient composite structure in order to obtain a multifunctional coating. In consideration of the cytotoxicity which is observed due to the fast release of silver ions from metal agents [33,34], the metallic phase of silver cannot satisfy the request of the medical antibacterial materials for long-term service. Thus, a more positive structure containing Ag+ in cytocompatibility with controlled Ag+ release is therefore highly required. Recently, the metal oxide structure has attracted a great deal of attention in pursuit of an efficient antibacterial agent to satisfy this requirement [31,35]. Our project to synthesize ZrN/Ag2O micro–nano gradient composite structure was accomplished by two procedures empowered by MAIP and MEVVA ion implantation technology: (1) ZrN coating was deposited on the surface of substrate by multi-arc ion plating adopting a pure Zr target implemented in the Ar + N2 gas mixture and (2) different doses for silver ions were implanted on the surface of ZrN coating by MEVVA ion implantation in vacuum. The hardness, adhesion force and tribological properties of coatings were assessed by micro-hardness, scratching and wear tests, respectively. Their anti-corrosion properties were evaluated by the potentiodynamic polarization and EIS methods in Ringer’s solutions. Additionally, the antibacterial properties and the biocompatibility of the surface were then evaluated in a comprehensive and systematic manner. The newly developed ZrN/Ag2O multifunctional coating can improve the consumer properties of implants made of pure titanium or Ti–6Al–4V alloys.
2. Materials and Methods
2.1. Preparation and Modification of Specimens
Commercially pure titanium plates with sizes of 10 × 10 × 2 mm3 were sonicated in ethanol and deionized water, with every cleaning step taking 15 min. Then, the cleaned Ti plates were placed into the deposition chamber of the MAIP system (LZD1300, Beijing Technol Science Co., Ltd., Beijing, China), mounted on a rotational holder (rotating in the entire process) surrounding the center of the chamber. The deposition was conducted under a negative bias of −200 V at an operating temperature of 573 K. The DC arc sources were operated at 110 A, 23.5 V for Zr target and the flow rates of Ar and N2 were 80 sccm and 100 sccm, respectively. The deposited time of ZrN coating was approximately 100 min. After the MAIP process treatment, the high energy metal ion implanter with a MEVVA ion source (LZX-700, Beijing Technol Science Co., Ltd., Beijing, China) was used to implant different doses of silver ions into the ZrN coating obtained in previous step. The implantation chamber was vacuumed to a pressure of below 6.0 × 10−4 Pa. The cathode rod was composed of pure Ag metal with a purity of 99.99 wt.%. The accelerated voltage was 45 keV. The different implantation influences were 1 × 1016, 5 × 1016, 10 × 1016 and 15 × 1016 ions/cm2, from less to more.
2.2. Surface Characterization
Morphology examination for the surface and cross-sectional of coatings was characterized via scanning electron microscopy (SEM; S-4800, Hitachi Co., Ltd., Tokyo, Japan) incorporating an energy dispersive spectrometer (EDS). The crystal structures were determined by X-ray diffraction (XRD; XRD-6100, Shimadzu Co., Ltd., Kyoto, Japan) equipped with Cu Kα radiation (wavelength: 0.15406 nm). The diffraction angle measurement ranged from 20° to 80° at a scanning speed of 0.1°/s. The chemical compositions and chemical states of the specimen surfaces were explored by X-ray photoelectron spectroscopy (XPS; K-Alpha XPS system, Thermo Scientific Ltd., Waltham, MA, USA).
2.3. Mechanical Properties Tests
The surface microhardness of specimens was tested by a micro-hardness tester (HXS-1000AY, Shanghai Optical Instrument Co., Ltd., Shanghai, China) at the load rating of 100 g and a dwell time of 5 s. Each specimen was determined by ten measurements along the diagonal.
The scratch experiment was carried out by a micro-scratch tester (MST3, Anton Paar, Graz, Austria) in which a diamond cone indenter slides on the surface of specimen at a sliding speed of 166.7 μm/s with a loading rate of 1 N/s from 30 mN to 30 N during the test process. The critical scratch load was determined through acoustic emission and the scratch traces, evaluating the adhesion strength and the initial failure.
The wear tests against the Al2O3 ball (diameter of 5 mm) were implemented by the surface property tester (CFT-I, Lanzhou Zhongke Kaihua Technology Development, Co., Ltd., Lanzhou, China) via a fixing device in linear reciprocation mode. Experimental parameters: loading from 4.2 to 20 N at the constant speed of 4 m/min for the persistent period of 15 min. The wear rates of specimens were calculated from the formula presented below:
where K is the wear rate (mm3/Nm), V is the wear volume (mm3), F is the axial load (N) and L is the total sliding distance (m).
K = V/FL,
2.4. Electrochemical Experiments
Electrochemical corrosion tests were conducted by an electrochemical working station (Zennium E4, Zahner, Kronach, Germany) with a conventional three-electrode system. The specimens were employed as the working electrode, leaving 1.0 cm2 soaked in Ringer’s solution (c-NaCl = 8.61 g/L, c-CaCl2 = 0.49 g/L and c-KCl = 0.30 g/L). The reference electrode was the Ag/AgCl electrode, while the counter electrode was a platinum sheet. Potentiodynamic polarization tests were conducted at a sweep rate of 0.5 mV/s. The EIS tests were carried out at the respective EOCP, for frequencies ranging from 100 kHz to 10 mHz, with a 10 mV AC perturbation. The obtained data from EIS tests were interpreted and simulated using the electrical equivalent circuits (EEC) mode through the Zahner analysis software.
2.5. In Vitro Antibacterial Evaluation
The bactericidal properties of ZrN/Ag2O composite coatings were measured by a bacterial counting method [36] using Gram-positive bacteria Staphylococus aureus (S. aureus, ATCC 29213) and Gram-negative bacteria Escherichia coli (E. coli, ATCC 25922). The experiment details are as follows: S. aureus or E. coli were diluted and separated to single bacterium by phosphate buffer solution (PBS). A 60 μL bacterial suspension with a bacterial density of 107 cfu/mL was dropped on the surface of each specimen, cultured at 37 °C for 24 h into a constant temperature oven. Subsequently, the specimens with the bacterial suspension were immersed into aseptic centrifuge tubes with 4 mL physiological saline; subsequently, the tubes were vibrated drastically to separate the bacteria from specimens. These generating suspensions were attenuated 10, 100, and 1000 times by physiological saline in sequence. Thereafter, a 100 μL bacterial suspension was extracted from each centrifuge tube and dropped on agar culture plates. Finally, all plates were deposited in a 37 °C incubator for 24 h and the colonies that followed were counted. The bactericidal ratio K was calculated by the following formula according to the National Standard of China GB/T4789.2 protocol:
where A represents the number of bacteria colonies grown in the culture medium corresponding to the substrate specimen and B represents that of bacteria colonies about different composite coating specimens.
K = (A − B)/A × 100%,
2.6. Silver Ions Release
To verify the concentration of Ag+ release as safety permitted, the specimens were immersed in centrifuge tube containing 10 mL Ringer’s solution at 37 °C for 30 days. After that, the release concentration of silver ions for different doses of ZrN/Ag2O coatings was detected by inductively coupled plasma–mass spectrometry (ICP–MS) technique (Nexion 300X, PerkinElmer, Waltham, MA, USA) [37].
2.7. Cell Proliferation
L929 cells that grew well were inoculated onto pure titanium plate and ZrN/Ag2O-coated titanium plate. Cell counting kit-8 (cck-8, Dojindo, Kumamoto, Japan) was used to detect cell proliferation and cytotoxicity at 24, 72 h after inoculation. In brief, the L929 cells were inoculated on the surface of each specimen and cultivated following the above times. Then, the culture medium was eliminated and the 500 μL cck-8 measuring medium (contain 10% cck-8 standard reagent) was added into each pore. After incubation at 37 °C in a 5% CO2 incubator for 3 h, 100 μL of the supernatant from each pore was transferred in duplicates into a 96-well plate and the absorbance was measured at 450 nm using a microplate reader (ELX800, Biotek, Winooski, VT, USA). The relative ratio of cell proliferation Kc was calculated by the following formula:
Kc = ODgradient coating/ODsubstrate × 100%,
The calculated Kc was according to the level of national standard for assessing the toxicity of materials, where Kc ≥ 100% is defined as grade 0, 75%~99% is defined as grade 1, 50%~74% is defined as grade 2, 25%~49% is defined as grade 3, and 1%~24% is defined as grade 4 [38]. Therefore, grade 0 and grade 1 represent nearly no cytotoxicity, grade 2 represents slight cytotoxicity, grade 3 represents moderate cytotoxicity, and grade 4 represents noteworthy cytotoxicity.
3. Results
3.1. Coating Characterization
Figure 1 shows the surface and cross-sectional morphologies of the deposited ZrN coating and their corresponding EDS analyses. As presented in Figure 1a, the ZrN coating revealed the uniform dense surface produced by the MAIP technique. Additionally, the surface elements mapping of coating after MAIP technique confirmed the existence of ZrN phase, as shown in Figure 1b. Meanwhile, Figure 1c shows that the thickness of a ZrN coating is about 10 μm, and the ZrN coating is closely integrated with the substrate, whose interface is void of any defects (such as microcracks, fine holes, etc.). Combined with the EDS analysis of the cross-section in Figure 1d, it can be distinctly substantiated that the ZrN coating designed in micron has been successfully deposited by the MAIP technique.
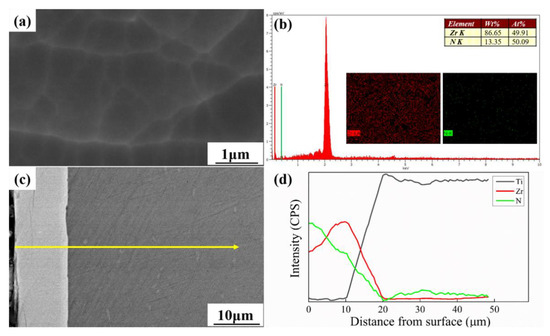
Figure 1.
(a,b) Surface morphology SEM images and EDS analyses of ZrN coating; (c,d) cross-sectional morphology SEM images and EDS analyses of ZrN coating.
Thereafter, in order to prepare the designed micro–nano gradient composite coating for antimicrobial application, different doses of silver ions were implanted on the surface of ZrN coating by MEVVA ion implantation in vacuum, and the surface morphologies of them are shown in Figure 2. From Figure 2a–d, it is clear that the dispersion of nanoparticles appear more and more obvious and the content of Ag element becomes increasingly high, rising to 4 wt.% at most, accompanied by increased doses of silver ions by MEVVA ion implantation. While attaining a quite high dose of 15 × 1016 icon/cm2, a mass of holes can be detected on the surface of composite coating, as shown in Figure 2d. Here, by means of two processes successively enabled by MAIP and MEVVA ion implantation technology, the micro–nano gradient composite structure was completely established.
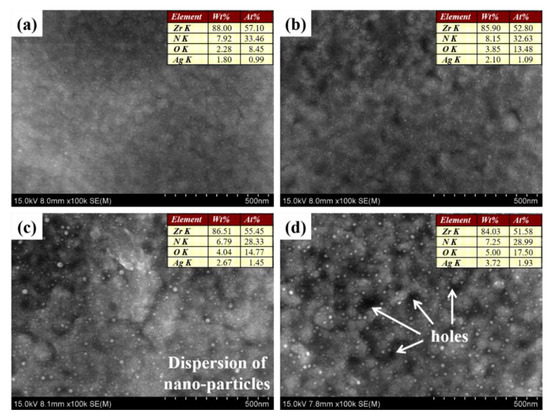
Figure 2.
Surface morphologies for different doses of silver ions implanted on the surface of ZrN coating: (a) ZrN/Ag2O (1 × 1016 icon/cm2); (b) ZrN/Ag2O (5 × 1016 icon/cm2); (c) ZrN/Ag2O (10 × 1016 icon/cm2); and (d) ZrN/Ag2O (15 × 1016 icon/cm2). EDS analyses for Zr, N, O and Ag elements are embodied in the images.
To ascertain the phase compositions of this gradient composite coating both in micron and nano dimension, XRD tests were used for analyses, as shown in Figure 3. There is only the ZrN phase (Space Group Fm-3m 225, FCC phase) diffraction peaks can be detected by the power diffraction files whether from separate ZrN coating or gradient composite coating. This is because the general XRD test has a depth range (about a few microns) from the surface, so the XRD test result in this article is about the micron dimension area, that is, the ZrN phase structure, as mentioned previously.
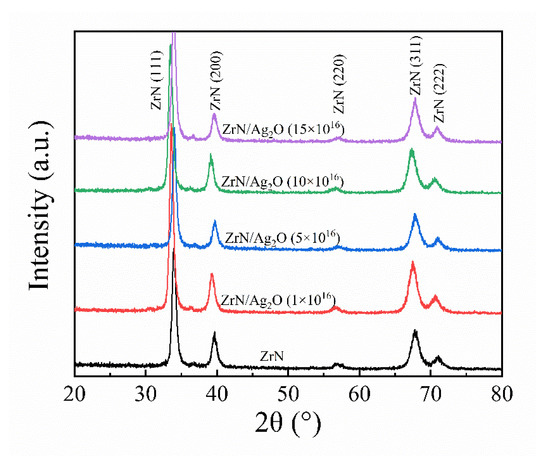
Figure 3.
X-ray diffraction pattern of the separate ZrN coating and different doses of ZrN/Ag2O gradient composite coatings.
To further clarify the phase composition of dispersive nanoparticles in the top-view SEM images of composite coating (Figure 2), the XPS methods were adopted to determine the elemental composition and chemical states of the nano dimension surface layer of the composite coating. The XPS high-resolution Zr 3d and Ag 3d spectra are presented in Figure 4. In Figure 4a, the Zr–N bond at 179.1 eV is equivalent to a non-saturated covalent compound ZrNx (x ≈ 0.68), so that the element O could readily supersede the element N and the ZrN phase of the surface layer was transformed to the oxidized ZrN phase. This result can be evidenced by the ZrO2 bond at 181.7 and 184.3 eV in Figure 4a, which consents adequately with the published consequences [39]. Figure 4b shows that the XPS Ag 3d spectra reveal unobvious binding energy shift among the different doses of silver ions specimens, illustrating that the existential form of silver element in these specimens is nearly consistent. The Ag 3d5/2 peak at 367.5 eV corresponding to the binding energy of Ag+ and no loss feature can be observed in Figure 4b, indicating that Ag exists exclusively in compound state on the surface [37]. Since the existence of an O element on the surface layer is confirmed through XPS Zr 3d spectra, it is reasonable to deduce that Ag exists exactly in the oxidized state (Ag2O phase) on the surface layer. Combined with the top-view SEM images of composite coating in Figure 2, the compositions and phases of nanoparticles can be accurately identified as the Ag2O nanoparticles. Here, the composite structure fabricated in this article was characterized as the micro–nano gradient composite patterns, containing the ZrN phase (micron dimension) as the main coating and the dispersion of Ag2O nanoparticles (nano dimension) on the surface layer.
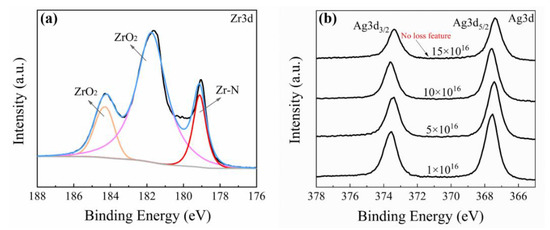
Figure 4.
(a) XPS spectra of Zr-3d from ZrN/Ag2O gradient composite coating; (b) XPS spectra of Ag-3d from different doses of ZrN/Ag2O gradient composite coatings.
3.2. Mechanical Properties
As shown in Figure 5a, for the existence of a ZrN ceramic phase with high hardness in the micron dimension, the micro hardness of both the separate ZrN coating or ZrN/Ag2O composite coatings increase to ~1800 HV0.1, almost the septuple of that of the pure Ti substrate. It is indicated that the improvement of mechanical properties is primarily contributed by the ZrN ceramic phase (micron dimension) as the main reinforced coating, while the Ag2O nanoparticles (nano dimension) have scarcely any degree of contribution on this part. Therefore, the chosen ZrN/Ag2O (10 × 1016 icon/cm2) composite coatings can represent different doses of silver ions specimens for further study about mechanics, such as adhesion and wear resistance, because they exhibit the features of unity in mechanical properties. The scratch appearance, the relevant acoustic emission spectra and penetration depth have been shown in Figure 5b. The critical load at the ZrN/Ag2O (10 × 1016 icon/cm2) composite coating stripped from the substrate is about 16~17 N. Simultaneously, the penetration depth precisely surpasses the thickness of the coating (about 10 μm), indicating that the composite coating has been worn through at this critical load.
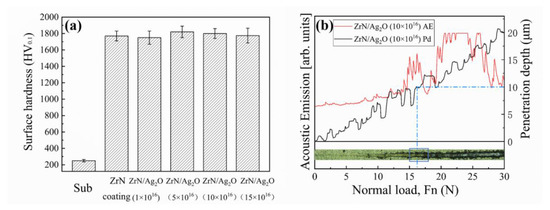
Figure 5.
(a) Micro-hardness of substrate, separate ZrN coating and ZrN/Ag2O composite coatings; (b) the scratched scar image, the corresponding acoustic emission spectra and penetration depth of ZrN/Ag2O composite coating.
Caused by the wear of metallic implants, the loss of asepsis is a main problem of artificial joint or bone for long-time clinical usage. Hence, the wear resistance of composite coatings is an issue worthy of deep study in the improvement of biomechanics in this article. Figure 6 shows the wear rates of different specimens (substrate, separate ZrN coating and ZrN/Ag2O composite coating) under different axial loads. It is clear that both the separate ZrN coating and ZrN/Ag2O composite coating exhibit extremely excellent abrasion resistance under axial load from 4.2 to 15 N, even the wear rate of ZrN/Ag2O composite coating decline to 37 × 10−6 mm3/Nm, significantly less than that of the substrate (498 × 10−6 mm3/Nm). Again, the ZrN ceramic phase with high hardness in micron dimension plays a major part in improving the wear resistance. However, at the axial load of 20 N, the wear rate of the composite coating specimen unexpectedly increases to 340 × 10−6 mm3/Nm, which is close to the wear rate of the substrate.
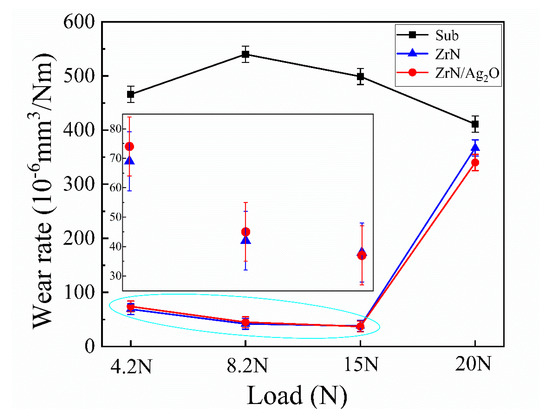
Figure 6.
Wear rates of different specimens (substrate, separate ZrN coating and ZrN/Ag2O composite coating) under axial load from 4.2 to 20 N.
The coefficients of friction (COFs) and wear scar morphologies of substrate and composite coating specimens under different axial loads are shown in Figure 7 and Figure 8, respectively, reflecting the representative characteristics of substrate and composite coating during the wear process. From 4.2 to 15 N, the COFs of composite coating specimens maintain at ~0.8 steadily. Particularly, a sudden sharp drop of the COF happened in composite coating at the axial load of 20 N, as shown in Figure 7d. As shown in Figure 8b,d,f, only the slight wear pits can be observed in the center, verifying that the plastic deformation is restricted to a low extent by reason of the ZrN ceramic phase with high hardness and the abrasion mechanism of the composite coating was mainly abrasive wear. While in Figure 8h, a mass of adhesive areas can be observed and its morphology features are approximate to the wear scar morphologies of the substrate, indicating that the hardened coating has been virtually destroyed without the protection from wear and the adhesive wear dominates the wear process. Combined with the analyses of the COF curve and the corresponding wear scar morphologies of composite coating at the load of 20 N, it is obvious that there exists a critical load (16~17 N) during the wear process, when the axial load exceeds the critical load, the reinforced composite coating suffered a great destruction, resulting in the failure of protection of the hardened coating from wear. These results are consistent with the result of an adhesion test, as is mentioned above. Actually, the implant materials in their course of service are general under the lower loads [40]. In conclusion, the composite coatings designed and prepared in this article could perfectly realize the requirements of biomechanical properties in implants without the loss of asepsis.
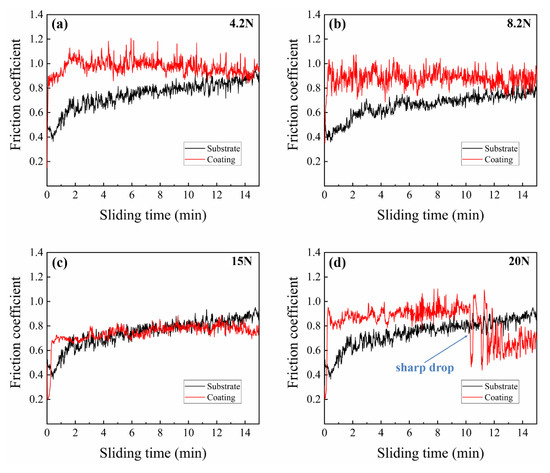
Figure 7.
Coefficients of friction of substrate and composite coating specimens under different axial loads: (a) 4.2 N; (b) 8.2 N; (c) 15 N; and (d) 20 N.
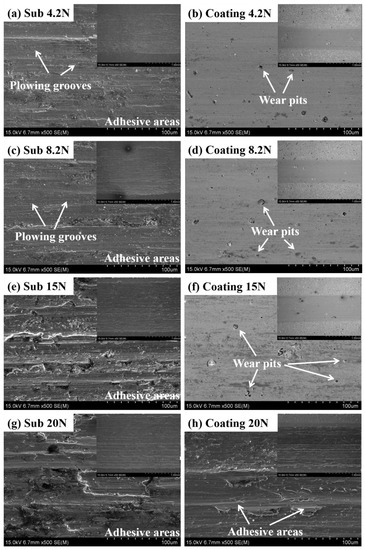
Figure 8.
Wear scar morphologies of substrate and composite coating specimens under different axial loads: (a,b) 4.2 N; (c,d) 8.2 N; (e,f) 15 N; and (g,h) 20 N.
3.3. Corrosion Resistance
The electrochemical performances of different specimens were evaluated by potentiodynamic polarization and EIS methods in Ringer’s solution. Figure 9a exhibits the characteristic potentiodynamic polarization curves of the substrate, separate ZrN coating and ZrN/Ag2O micro–nano gradient composite coatings with different doses of silver ions. The acquired values of Ecorr, icorr and the corrosion rate of all specimens are recorded in Table 1. Obviously, in comparison with the substrate, the separate ZrN coating has a slightly higher Ecorr value (−0.215 V) than that of the substrate (−0.266 V), while the Ecorr value of the gradient composite coatings could rise to the range of −0.098~−0.05 V. Synchronously, the gradient composite coatings present the lowest icorr values (i.e., icorr = 35.5, 31.8, 31.7, 29.5 nA/cm2 for composite coatings with different doses of silver ions, respectively) throughout the entire period of cathode and anode polarization, in contrast to that of the substrate (73.6 nA/cm2) and the separate ZrN coating (53.7 nA/cm2). Furthermore, the micro–nano gradient composite structure constructed on pure Ti in this article exhibits much lower corrosion rates (256~308 × 10−6 mm/year) than the uncoated substrate (638 × 10−6 mm/year) during the service. According to the above results from Figure 9a and Table 1, it can be deduced that not only can the ZrN ceramic phase structure in the micron dimension effectively reduce the corrosion during the test, but the Ag2O nanoparticles on the surface with excellent anticorrosion can also make an effective contribution in promoting the corrosion resistance, owing to the more noble corrosion potential of Ag2O nanoparticles relative to that of the ZrN phase.
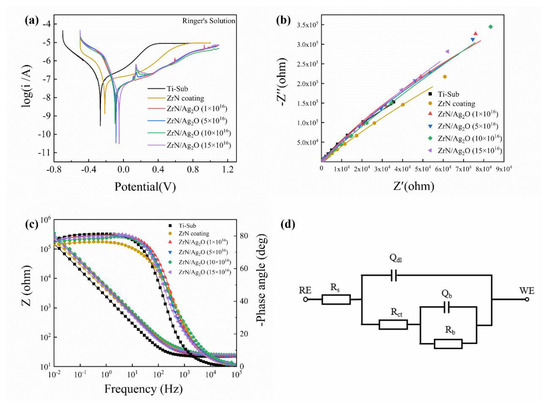
Figure 9.
Potentiodynamic polarization and EIS measurements of different specimens in Ringer’s solution: (a) the typical potentiodynamic polarization curves recorded on the substrate, separate ZrN coating and ZrN/Ag2O micro–nano gradient composite coatings with different doses of silver ions; (b,c) the representative Nyquist and Bode plots for the tested samples, respectively; and (d) the equivalent circuit (EC) model used to simulate the EIS data of the composite coating and substrate in Ringer’s solution.

Table 1.
The acquired values of Ecorr, icorr and the corrosion rate of all specimens.
The representative Nyquist and Bode plots for the tested specimens are shown in Figure 9b,c, respectively. EIS spectra analyses were accomplished by fitting the experimental data via Zahner analysis equivalent circuit (EC) software. From Figure 9b, it is obvious that the separate ZrN coating and different doses of gradient composite coatings both have great improvement in capacitive reactance arc amplitude. Generally, under the same corrosion conditions, the greater representation in capacitive reactance arc amplitude from the Nyquist plot means the larger impedance in electrochemical reaction and the promotion in corrosion resistance. As shown in Figure 9c, the Bode plot for each coating exhibits characteristic of a thin passive layer, that is, a near capacitive response represented by a large capacitive arc and the phase angle closes to −80° over a wide range of frequency [41]. This indicates that the electrode reaction of coatings is deferred during EIS test course, exhibiting more favorable corrosion resistance. In addition, the impedance amplitude in the Bode plot reflects the density of protective film generated on the surface of different test electrodes. The larger the impedance amplitude is, the denser the protective passivation film is, and the better its protective effect on the material is, and the higher its corrosion resistance is. From Figure 9c, the impedance amplitudes of all the separate ZrN coating or composite coatings are increased in varying degrees compared to that of the substrate, illustrating that a more compact protective film is formed on the surface of substrate, and the reaction rate and corrosion rate of the electrode surface are lower than that of the pure titanium substrate. To make a deep investigation about the electrochemical characteristics of these specimens, the equivalent circuit in Figure 9d is used to explain the EIS fitting data. The impedance expression can be described as:
This is example 1 of an equation:
where Z is the impedance, Rs is the solution resistance, Rct and Qdl are the charge transfer resistance and capacitance of heterogeneity surface layer, respectively, and Rb and Qb are the polarization resistance and double layer capacitance, respectively. The fitted values for the different elements of the EC model are concluded in Table 2. It is apparent that the value of Rb is much larger than that of Rct, so Rct can be ignored, estimating that the corrosion resistance of different specimens is mainly determined by the value of Rb in the equivalent circuit diagram. The fitted Rb value of the substrate is 1.83 × 106 Ω·cm−2, while the Rb value of the separate ZrN coating increases to 4.65 × 106 Ω·cm−2, achieving a certain effect in corrosion resistance. Low dosage ion implantation (1 × 1016 and 5 × 1016 ions/cm2) of ZrN/Ag2O composite coatings’ Rb values are roughly equivalent to that of the separate ZrN coating, attributed to the unformed Ag2O nanoparticles on the ZrN coating based on the surface morphologies of ZrN/Ag2O composite coatings in Figure 2b. Along with the increase in the doses of silver ions by MEVVA ion implantation, the Ag2O nanoparticles were formed prominently and distributed uniformly on the ZrN coating. Owing to the more active anti-corrosion potential of Ag2O nanoparticles, the Rb values of high dosage ion implantation (10 × 1016 and 15 × 1016 ions/cm2) of ZrN/Ag2O composite coatings were further raised to 10.30 × 106 and 8.26 × 106 Ω·cm−2, obtaining more excellent performance in corrosion resistance.

Table 2.
Electrochemical parameters derived from impedance fitting for different specimens in Ringer’s solution.
3.4. Antimicrobial Performance and Cell Proliferation
To assess the antibacterial ability of the ZrN/Ag2O composite coatings, the adhesive bacteria were separated from the surfaces and recultivated on an agar medium for the plate count. Figure 10 shows the representative photos of the re-cultivated S. aureus and E. coli colonies on agar for different specimens and the antibacterial ratios were calculated according to these results in Figure 11a. It is evident that the promotion in antibacterial effect is accompanied by the increase in the doses of silver ions; indeed, the corresponding antibacterial rate is approximately 95% for S. aureus and 99% for E. coli as the dose of silver ions amount to 10 × 1016 ions/cm2, respectively. Generally, the antibacterial action of Ag-containing coatings depends on the effectiveness of the release of silver ions, exhibiting bactericidal or bacteriostatic activity as a well-known broad-spectrum bactericide. In this article, on account of the increase in the content of Ag element referring to the EDS analysis in Figure 2, combined with the Ag2O nanoparticles formed prominently and distributed uniformly on the surface, the performance of antimicrobial properties has been remarkably improved. Nevertheless, the biocompatibility of Ag-containing coatings is still suspectable, and the excessive amount of silver ions released may result in cytotoxicity during the implantation of biomaterials. In our approach, the release of silver ions in the ZrN/Ag2O gradient composite coating is limited by appropriate dosage of silver ions using MEVVA ion implantation. Furthermore, the release of silver ions can be effectively reduced by the metal oxide structure (Ag2O nanoparticles) in comparison to the metal structure (Ag nanoparticles), due to the more stable structure form covalent bond, providing a new method to solve the above problems. To verify the concentration of Ag+ release as safety permitted, the release concentration of silver ions for different doses of ZrN/Ag2O coatings in Ringer’s solution after 30 days was detected by ICP–MS technique, as shown in Figure 11b. The concentration of silver ions was indicated as ppb, typically in microgram per liter. It is shown that with the dose increase in silver ions beam, the release concentration of silver ions increased gradually, which reached 7.75, 9.17, 13.25, and 15.36 ppb, respectively. Combined with Figure 10, as a result, the evaluation of the antibacterial properties of ZrN/Ag2O gradient composite coatings are evidently affected by the release concentration of silver ions. Additionally, the increase in the quantities of the release of silver ions would further enhance the antibacterial properties during the implant service process. In addition, all of the release concentrations of silver ions for different gradient composite coatings are less than 20 ppb, indicating that the release amount of silver ions is slight and in a certain safe range. Furthermore, objectively, to assess the biocompatibility of ZrN/Ag2O gradient composite coatings, L929 cells were inoculated on the surface of pure titanium matrix and four different doses of ZrN/Ag2O coatings, and then the cell proliferation was detected by cck-8 kit after 24 and 72 h, respectively. According to national standards, grade 0 (Kc ≥ 100%) and grade 1 (75% ≤ Kc < 100%) are non-toxic, and grade 2 (50% ≤ Kc < 75%) is slight cytotoxicity. As shown in Table 3, by means of the in vitro cell culture assay on the surface of specimens, ZrN/Ag2O gradient composite coating at higher doses (exceed 5 × 1016 ions/cm2) would exhibit slight cytotoxicity after 24 h, however, the toxicity level of all the different doses of ZrN/Ag2O gradient composite coatings decreased to grade 0 or grade 1 with the passage of time to 72 h, proving their long-term biocompatibility and safety in clinical applications. Above all, it was deduced that the suitable ZrN/Ag2O gradient composite structure can obtain an antibacterial ability, which only shows a slight level of toxicity at the primary stage and over a longer service process exerts no significant cytotoxicity and even excellent cytocompatibility.
Figure 10.
Typical photos of the re-cultivated S. aureus and E. coli colonies on agar for different samples, respectively: (a1,a2) Ti-substrate; (b1,b2) ZrN/Ag2O (1 × 1016 ions/cm2); (c1,c2) ZrN/Ag2O (5 × 1016 ions/cm2); (d1,d2) ZrN/Ag2O (10 × 1016 ions/cm2); and (e1,e2) ZrN/Ag2O (15 × 1016 ions/cm2) gradient composite coatings.
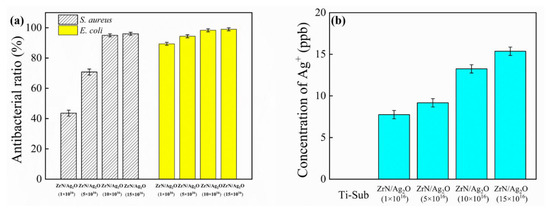
Figure 11.
(a) Antibacterial ratios of the re-cultivated S. aureus and E. coli colonies on agar for different doses of ZrN/Ag2O gradient composite coatings; (b) the release concentration of silver ions for different doses of ZrN/Ag2O coatings in Ringer’s solution after 30 days.

Table 3.
The absorbance at wavelengths of 450 nm and the toxicity grade of ZrN/Ag2O gradient composite coatings.
4. Conclusions
In this article, the composite structure was fabricated by MAIP and MEVVA ion implantation technology, characterized by the micro–nano gradient composite patterns, containing the ZrN phase (micron dimension) as the main coating and the dispersion of Ag2O nanoparticles (nano dimension) on the surface layer. Its comprehensive mechanical properties (high hardness and excellent wear resistance) could perfectly realize the requirements of biomechanical properties in implants without the loss of asepsis. Meanwhile, through the electrochemistry corrosion test, the ZrN/Ag2O gradient composite structure exhibit the favorable corrosion resistance not only because the ZrN ceramic phase structure in micron dimension can effectively reduce the corrosion, but also the Ag2O nanoparticles on the surface with excellent anticorrosion can make an effective contribution in promoting the corrosion resistance. Furthermore, the suitable ZrN/Ag2O gradient composite structure designed in this paper can obtain an antibacterial ability by the accurate release of silver ions, which only shows a slight level of toxicity at the primary stage and over a longer service process exerts no significant cytotoxicity and even excellent cytocompatibility, finally satisfying the requirement of the metallic implants in clinical application.
Author Contributions
Conceptualization, T.T.; methodology, T.T. and F.D.; validation, F.L., K.Y. and J.Y.; investigation, F.D.; resources, P.Z. and D.W.; writing—original draft preparation, T.T.; writing—review and editing, B.D., K.Y. and F.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Natural Science Foundation for Excellent Young Scientists of Jiangsu Province, China (Grant No. BK20180068); China Postdoctoral Science Foundation funded project (Grant No. 2018M630555); Opening Project of Materials Preparation and Protection for Harsh Environment Key Laboratory of Ministry of Industry and Information Technology (Grant No. XCA20013-1); Opening Project of Jiangsu Key Laboratory of Advanced Structural Materials and Application Technology (Grant No. ASMA201701); the Fundamental Research Funds for the Central Universities, China (Grant No. NS2018039).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Francesca, V.; Melania, M.; Silvia, B.; Milena, F. In vivo studies on osteoinduction: A systematic review on animal models, implant site, and type and postimplantation investigation. J. Biomed. Mater. Res. Part A 2020, 108, 1834–1866. [Google Scholar]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloys Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Geetha, M.; Singh, A.K.; Asokamani, R.; Gogia, A.K. Ti based biomaterials, the ultimate choice for orthopaedic implants—A review. Prog. Mater. Sci. 2009, 54, 397–425. [Google Scholar] [CrossRef]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Bitar, D.; Parvizi, J. Biological response to prosthetic debris. World J. Orthop. 2015, 6, 172–189. [Google Scholar] [CrossRef]
- Sundfeldt, M.; Carlsson, L.V.; Johansson, C.B.; Thomsen, P.C.; Gretzer, C. Aseptic loosening, not only a question of wear: A review of different theories. Acta Orthop. 2006, 77, 177–197. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; Ruvollo-Filho, A.C.; Camargo, E.R.D.; Barbosa, D.B. The growing importance of materials that prevent microbial adhesion: Antimicrobial effect of medical devices containing silver. Int. J. Antimicrob. Agents 2009, 34, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osteointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef]
- Delanois, R.E.; Mistry, J.B.; Gwam, C.U.; Mohamed, N.S.; Choksi, U.S.; Mont, M.A. Current epidemiology of revision total knee arthroplasty in the united states. J. Arthroplast. 2017, 32, 2663–2668. [Google Scholar] [CrossRef]
- Patel, A.; Pavlou, G.; Mujica-Mota, R.E.; Toms, A.D. The epidemiology of revision total knee and hip arthroplasty in England and Wales: A comparative analysis with projections for the United States. A study using the National Joint Registry dataset. Bone Joint J. 2015, 97, 1076–1081. [Google Scholar] [CrossRef]
- Bozic, K.J.; Kurtz, S.M.; Lau, E.; Ong, K.; Chiu, V.; Vail, T.P.; Rubash, H.E.; Berry, D.J. The epidemiology of revision total knee arthroplasty in the United States. Clin. Orthop. Relat. Res. 2010, 468, 45–51. [Google Scholar] [CrossRef]
- Fernandes, A.; Dias, M. The Microbiological profiles of infected prosthetic implants with an emphasis on the organisms which form biofilms. J. Clin. Diagn. Res. 2013, 7, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Paul, K.C.; Ding, C.X. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. R 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Aranya, A.K.; Pushalkar, S.; Zhao, M.L.; Legeros, R.Z.; Zhang, Y.; Saxena, D. Antibacterial and bioactive coatings on titanium implant surfaces. J. Biomed. Mater. Res. Part A 2017, 105, 2218–2227. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Bose, S.; Bandyopadhyay, A.; Karandikar, B.; Gibbins, B.L. Surface coatings for improvement of bone cell materials and antimicrobial activities of Ti implants. J. Biomed. Mater. Res. Part B 2008, 87B, 455–460. [Google Scholar] [CrossRef]
- Kumar, D.D.; Kaliaraj, G.S. Multifunctional zirconium nitride/copper gradient coatings on medical grade 316L SS and titanium substrates for biomedical applications. J. Mech. Behav. Biomed. Mater. 2018, 77, 106–115. [Google Scholar] [CrossRef]
- Bai, W.Q.; Li, L.L.; Li, R.L.; Gu, C.D.; Wang, X.L.; Jin, G.; Liu, D.G.; Tu, J.P. Deposition and characterization of a ZrN/Zr/a-C gradient: Implication on bio-tribological and corrosion behaviors. Surf. Coat. Technol. 2017, 324, 509–517. [Google Scholar] [CrossRef]
- Rizzi, M.; Gatti, G.; Migliario, M.; Marchese, L.; Rocchetti, V.; Reno, F. Effect of zirconium nitride physical vapor deposition coating on preosteoblast cell adhesion and proliferation onto titanium screws. J. Prosthet. Dent. 2014, 112, 1103–1110. [Google Scholar] [CrossRef]
- Chernousova, S.; Epple, M. Silver as antibacterial agent: Ion, nanoparticle, and metal. Angew. Chem. Int. Ed. 2013, 52, 1636–1653. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.S.; Jin, W.X.; Chen, Q.Y.; Cai, Y.C.; Zhu, Q.H.; Zhang, W.Z. Antibacterial activity of silver nanoparticles with different morphologies as well as their possible antibacterial mechanism. Appl. Phys. A Mater. Sci. Process. 2016, 122, 874. [Google Scholar] [CrossRef]
- Qin, Y.; Cheng, L.; Li, R.Y.; Liu, G.C.; Zhang, Y.B.; Tang, X.F.; Wang, J.C.; Liu, H.; Qin, Y.G. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Silver nanoparticle-mediated cellular responses in various cell lines: An in vitro model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Karns, M.; Goodson, M.; Rowe, J.; Hussain, S.M.; Schlager, J.J.; Hong, Y.L. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol. Appl. Pharmacol. 2008, 233, 404–410. [Google Scholar] [CrossRef]
- Xu, J.; Peng, S.; Guo, B.; Zhao, Y.T.; Fu, T.; Jiang, S.Y.; Munroe, P.; Xie, Z.H.; Lu, H. Influence of Ag alloying on the antibacterial properties, bio-corrosion resistance and biocompatibility of α-Nb5Si3 nanocrystalline coating. Appl. Surf. Sci. 2020, 503, 144082. [Google Scholar] [CrossRef]
- Ghaseminezhad, S.M.; Shojaosadati, S.A. Evaluation of the antibacterial activity of Ag/Fe3O4 nanocomposites synthesized using starch. Carbohydr. Polym. 2016, 144, 454–463. [Google Scholar] [CrossRef]
- Qin, H.; Cao, H.L.; Zhao, Y.C.; Zhu, C.; Cheng, T.; Wang, Q.J.; Peng, X.C.; Cheng, M.Q.; Wang, J.X.; Jin, G.D.; et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials 2014, 35, 9114–9125. [Google Scholar] [CrossRef]
- Baheiraei, N.; Moztarzadeh, F.; Hedayati, M. Preparation and Antibacterial Activity of Ag/SiO2 Thin Film on Glazed Ceramic Tiles by Sol-Gel Method. Ceram. Int. 2012, 38, 2921–2925. [Google Scholar] [CrossRef]
- Kelly, P.J.; Li, H.; Benson, P.S.; Whitehead, K.A.; Verran, J.; Arnell, R.D.; Iordanova, I. Comparison of the tribological and antimicrobial properties of CrN/Ag, ZrN/Ag, TiN/Ag, and TiN/Cu nanocomposite coatings. Surf. Coat. Technol. 2010, 205, 1606–1610. [Google Scholar] [CrossRef]
- Gao, A.; Hang, R.Q.; Huang, X.B.; Zhao, L.Z.; Zhang, X.Y.; Wang, L.; Tang, B.; Ma, S.L.; Chu, P.K. The effects of titania nanotubes with embedded silver oxide nanoparticles on bacteria and osteoblasts. Biomaterials 2014, 35, 4223–4235. [Google Scholar] [CrossRef]
- Cao, H.L.; Tang, K.W.; Liu, X.Y. Bifunctional galvanics mediated selective toxicity on titanium. Mater. Horiz. 2018, 5, 264–267. [Google Scholar] [CrossRef]
- Li, Y.; Qin, T.C.; Ingle, T.; Yan, J.; He, W.W.; Yin, J.J.; Chen, T. Differential genotoxicity mechanisms of silver nanoparticles and silver ions. Arch. Toxicol. 2017, 91, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Amberg, M.; Vandenbossche, M.; Hegemann, D. Controlled Ag release from electrically conductive coating systems. Surf. Coat. Technol. 2018, 336, 29–33. [Google Scholar] [CrossRef]
- Sonia, S.; Jayram, N.D.; Kumar, P.S.; Mangalaraj, D.; Ponpandian, N.; Viswanathan, C. Effect of NaOH concentration on structural, surface and antibacterial activity of CuO nanorods synthesized by direct sonochemical method. Superlattices Microstruct. 2014, 66, 1–9. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, Z.; Zara, J.N.; Hsu, C.; Soofer, D.E.; Lee, K.S.; Siu, R.K.; Miller, L.S.; Zhang, X.; Carpenter, D.; et al. The antimicrobial and osteoinductive properties of silver nanoparticle/poly (DL-lactic-co-glycolic acid)-coated stainless steel. Biomaterials 2012, 33, 8745–8756. [Google Scholar] [CrossRef]
- Li, J.H.; Qiao, Y.Q.; Zhu, H.Q.; Meng, F.H.; Liu, X.Y. Existence, release, and antibacterial actions of silver nanoparticles on Ag–PIII TiO2 films with different nanotopographies. Int. J. Nanomed. 2014, 9, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wan, R.X.; Gong, H.H.; Lv, X.F.; Chu, S.S.; Li, D.J.; Gu, H.Q.; Peng, C. Study on the in vitro and in vivo antibacterial activity and biocompatibility of novel TiN/Ag gradients immobilized onto biomedical titanium. J. Nanosci. Nanotechnol. 2019, 19, 3777–3791. [Google Scholar] [CrossRef]
- Lei, Z.F.; Zhang, Q.Q.; Zhu, X.D.; Ma, D.Y.; Ma, F.; Song, Z.X.; Fu, Y.Q. Corrosion performance of ZrN/ZrO2 gradient coatings deposited on 304 stainless steel using multi-arc ion plating. Appl. Surf. Sci. 2018, 431, 170–176. [Google Scholar] [CrossRef]
- Herbster, M.; Doring, J.; Nohava, J.; Lohmann, C.H.; Halle, T.; Bertrand, J. Retrieval study of commercially available knee implant coatings TiN, TiNbN and ZrN on TiAl6V4 and CoCr28Mo6. J. Mech. Behav. Biomed. Mater. 2020, 112, 104034. [Google Scholar] [CrossRef]
- Xu, J.; Sun, T.T.; Jiang, S.Y.; Munroe, P.; Xie, Z.H. Antimicrobial and biocorrosion-resistant MoO3-SiO2 nanocomposite coating prepared by double cathode glow discharge technique. Appl. Surf. Sci. 2018, 447, 500–511. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).