Lubrication and Anti-Rust Properties of Jeffamine-Triazole Derivative as Water-Based Lubricant Additive
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
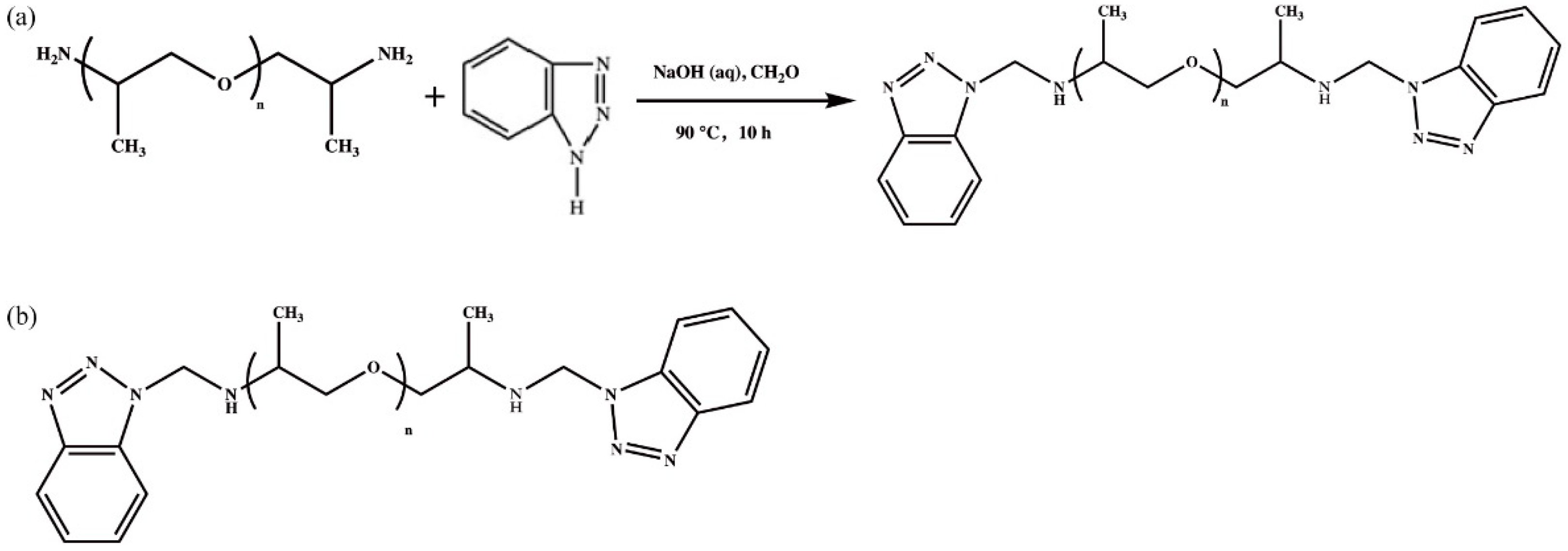
2.2. Preparation of the JD2000
2.3. Pretreatment of the Friction Pair
2.4. Friction and Wear Tests
2.5. Corrosion Tests
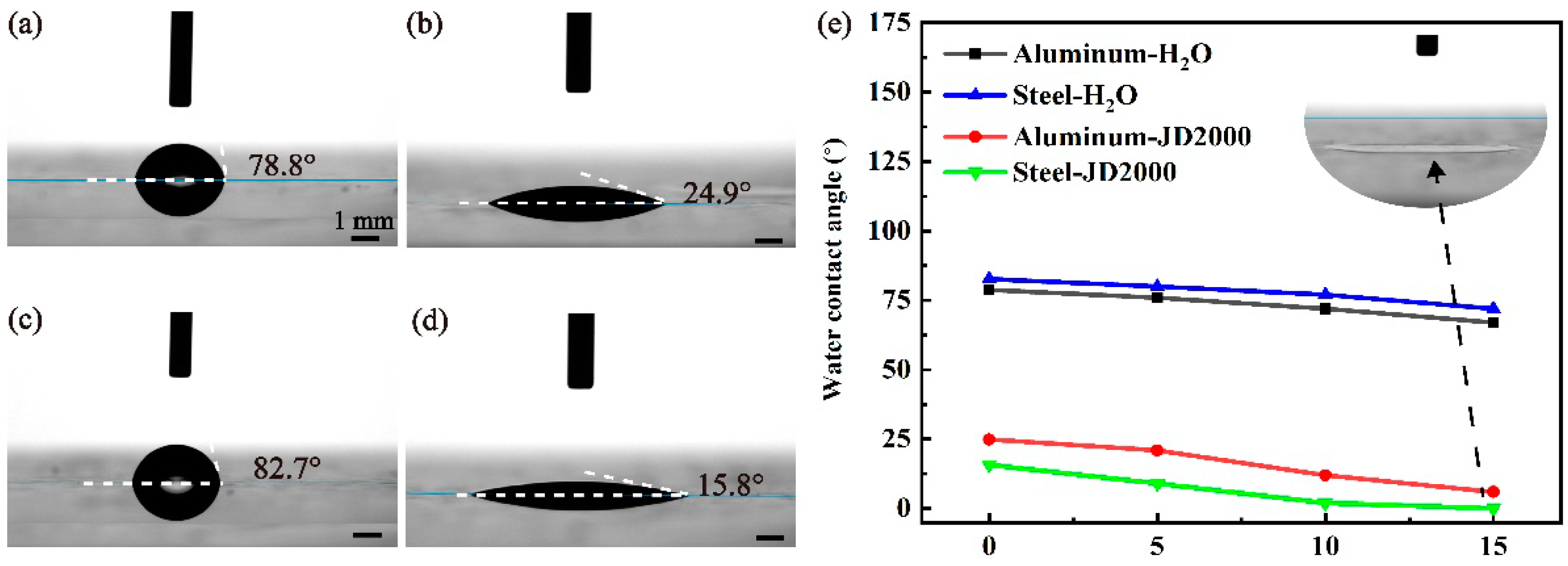
2.6. Water Contact Angle Measurements
2.7. Adsorption Behavior Test of the JD2000 on Metal Surface
2.8. Characterization of Product Structure and Investigation of Worn Surface
3. Results
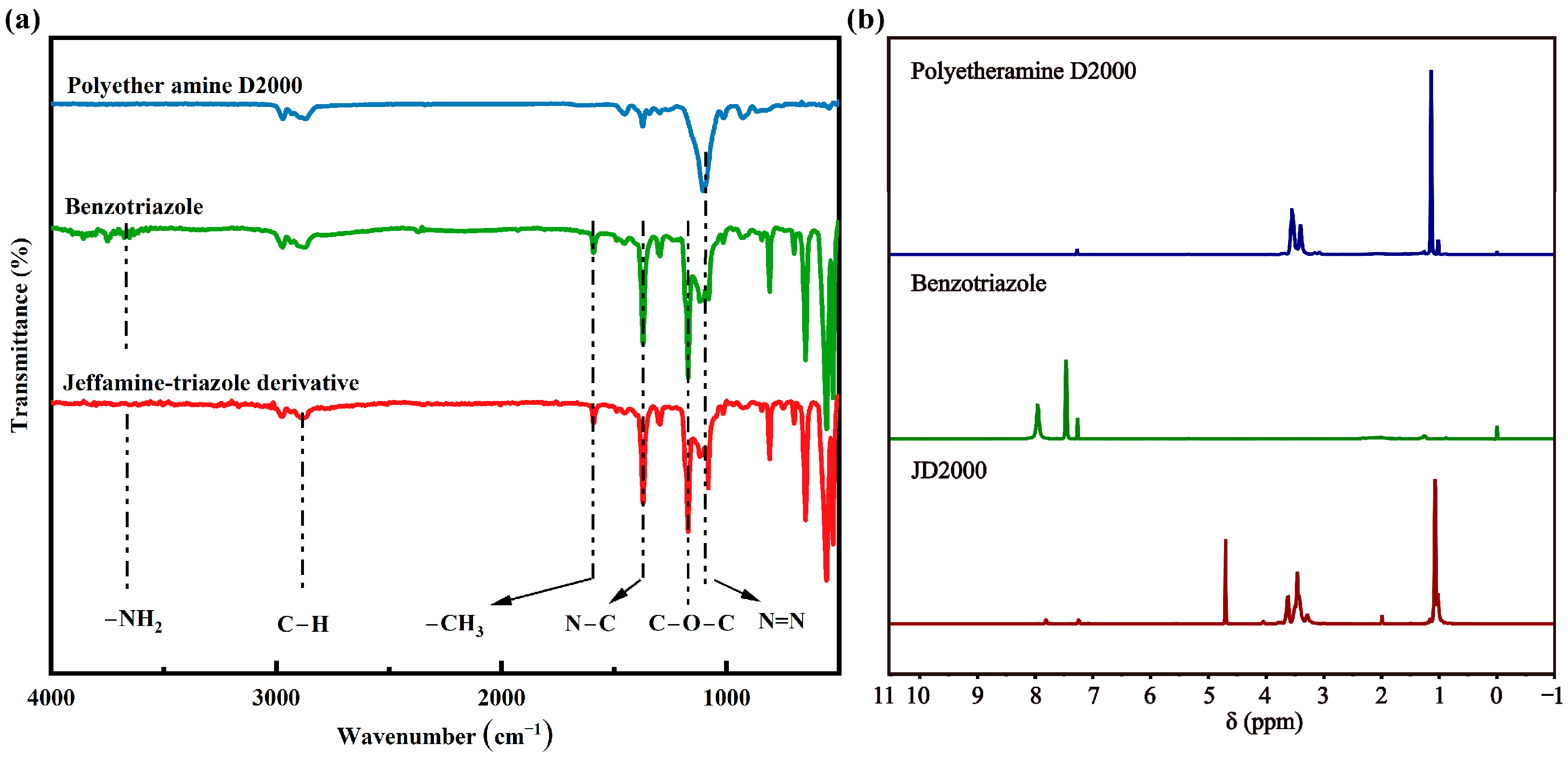
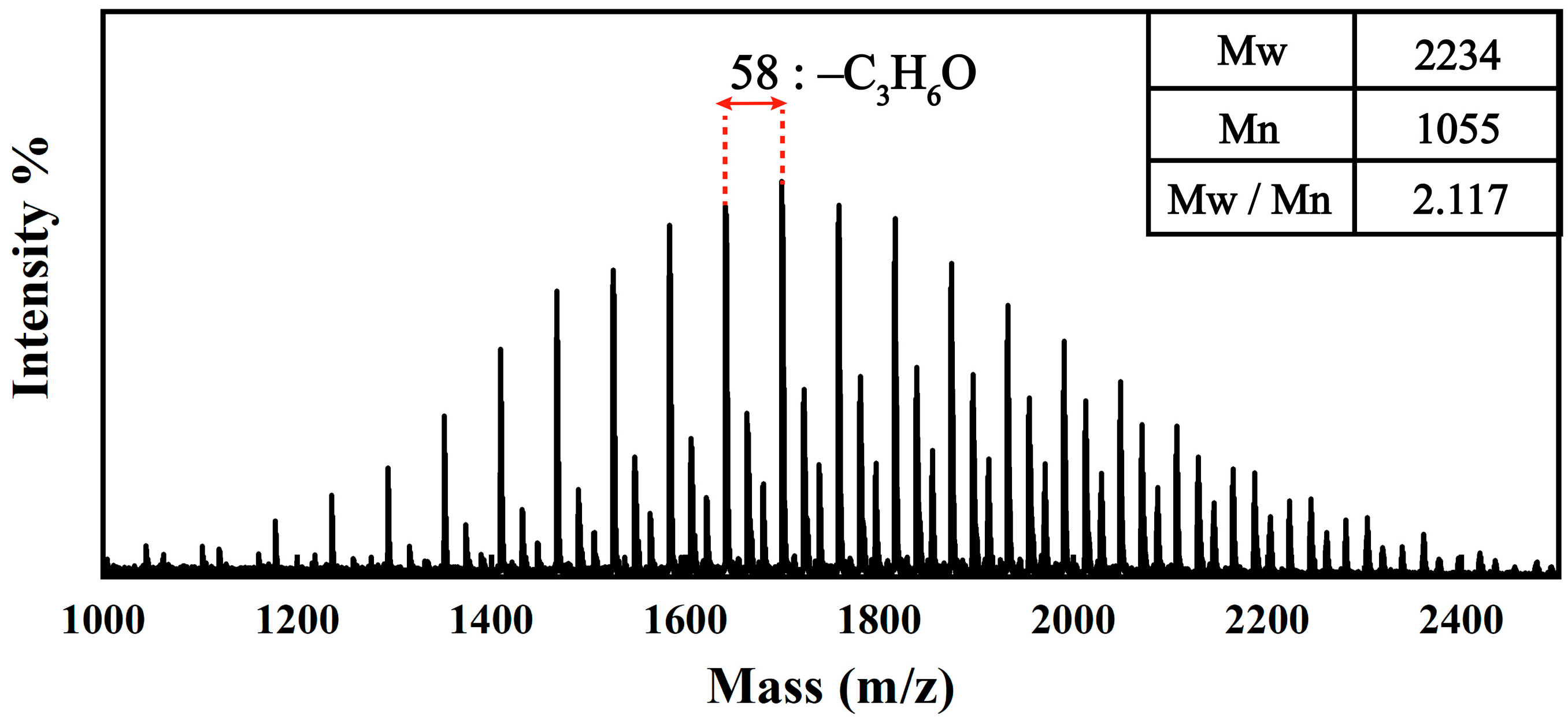
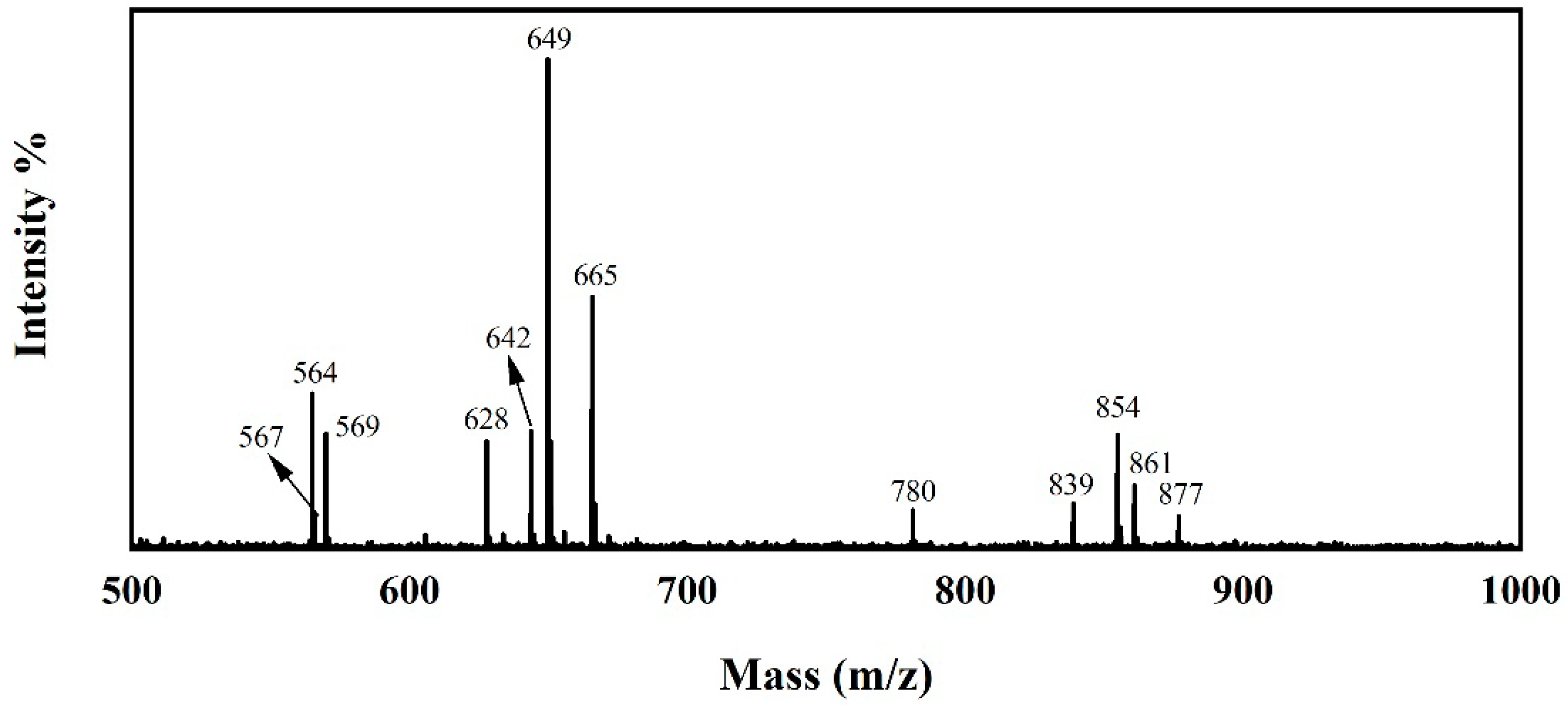
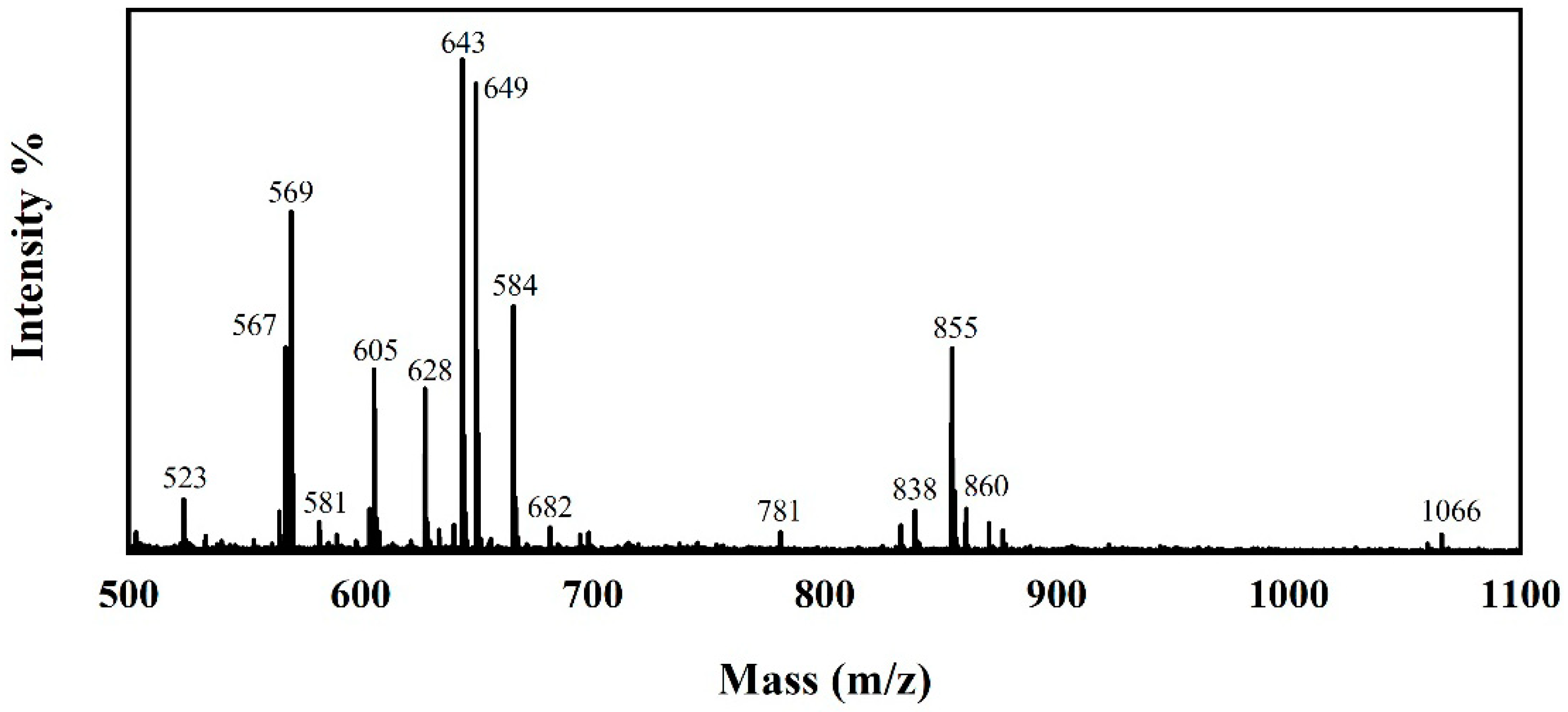
3.1. Chemical Structures of the JD2000
3.2. Configuration and Stability Test of the JD2000 Water-Based System
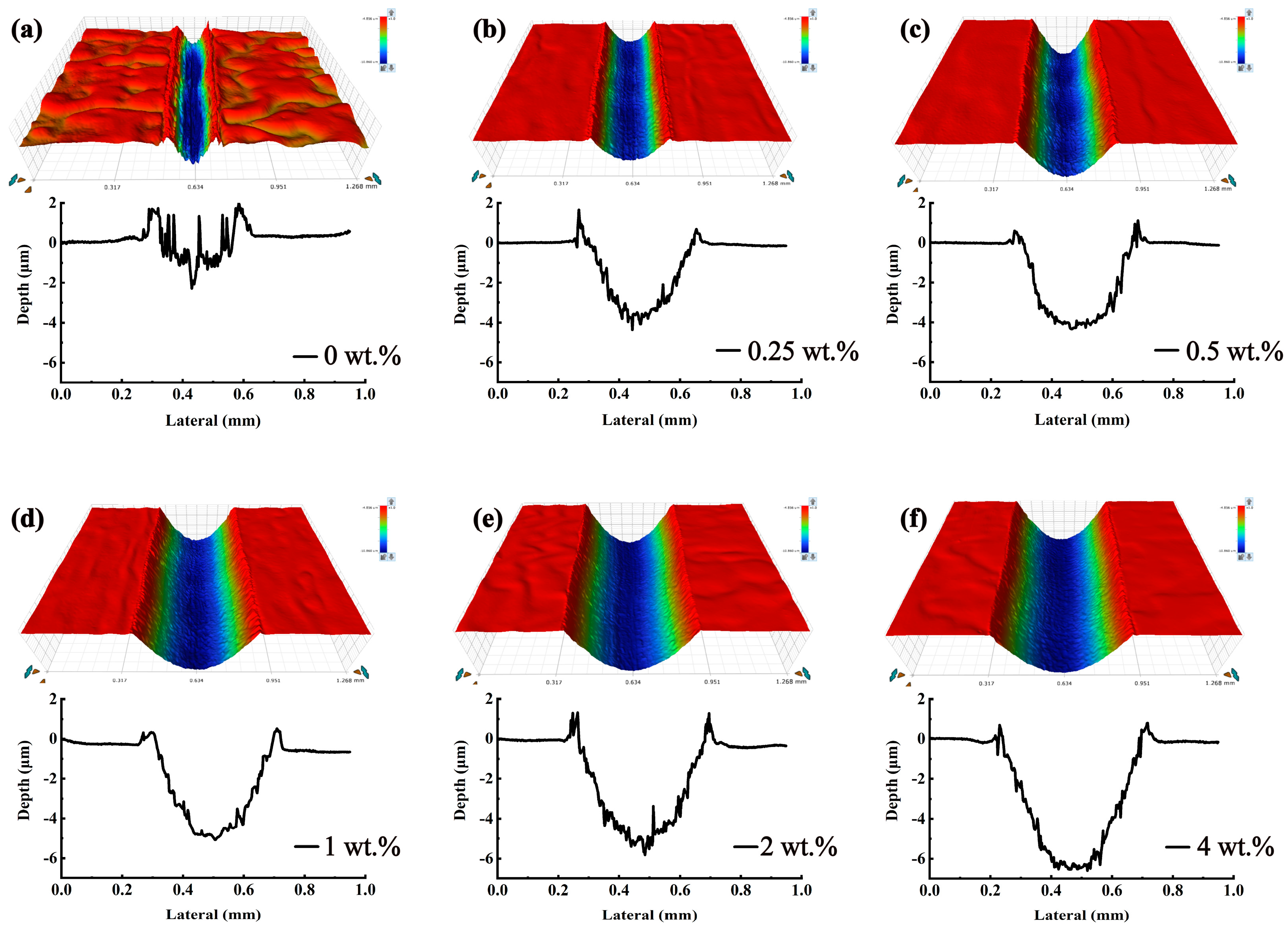
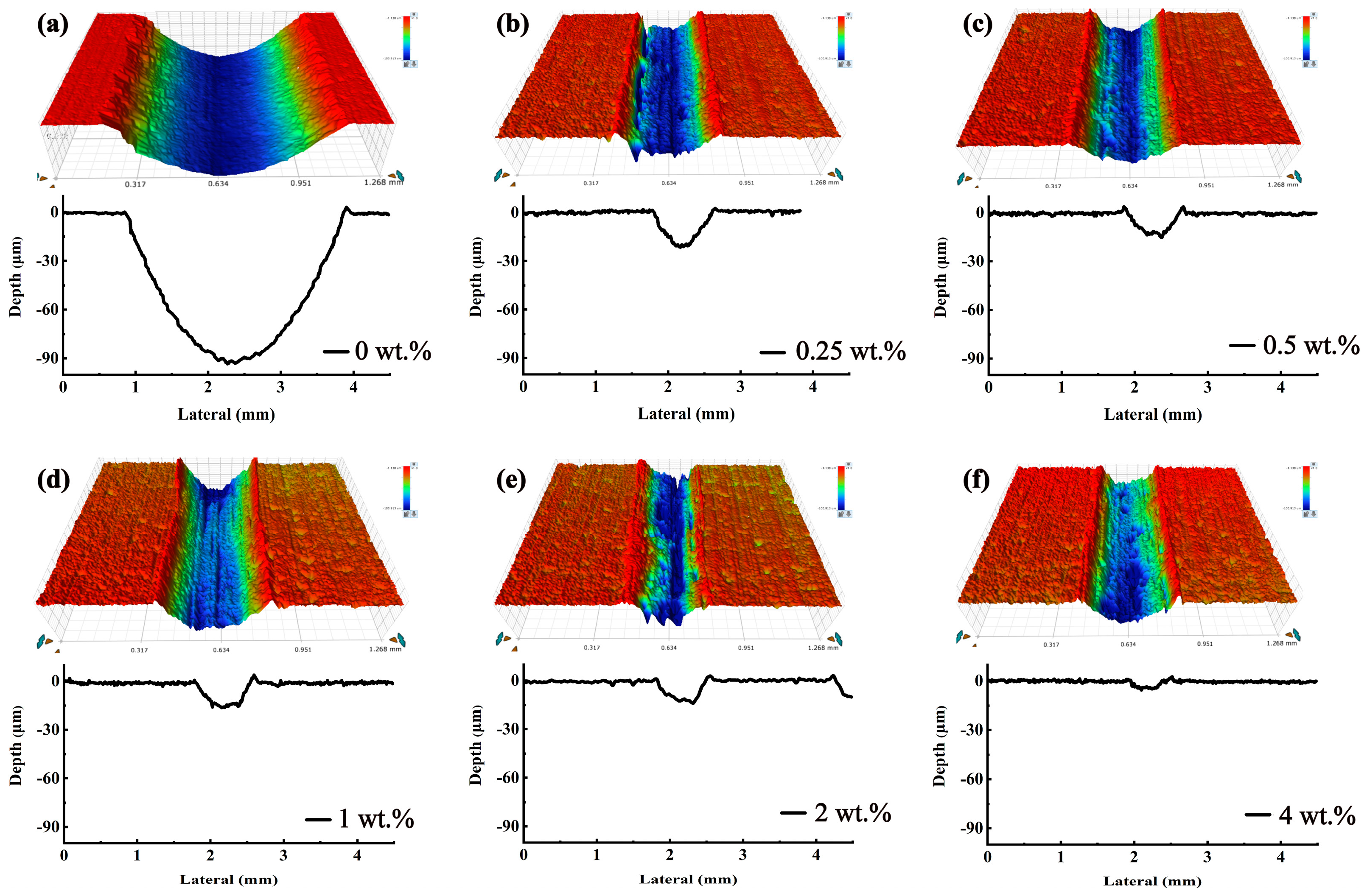
3.3. Friction Reduction and Anti-Wear Properties of the Steel Plates
3.4. Friction Reduction and Anti-Wear Properties of the Aluminum Plates
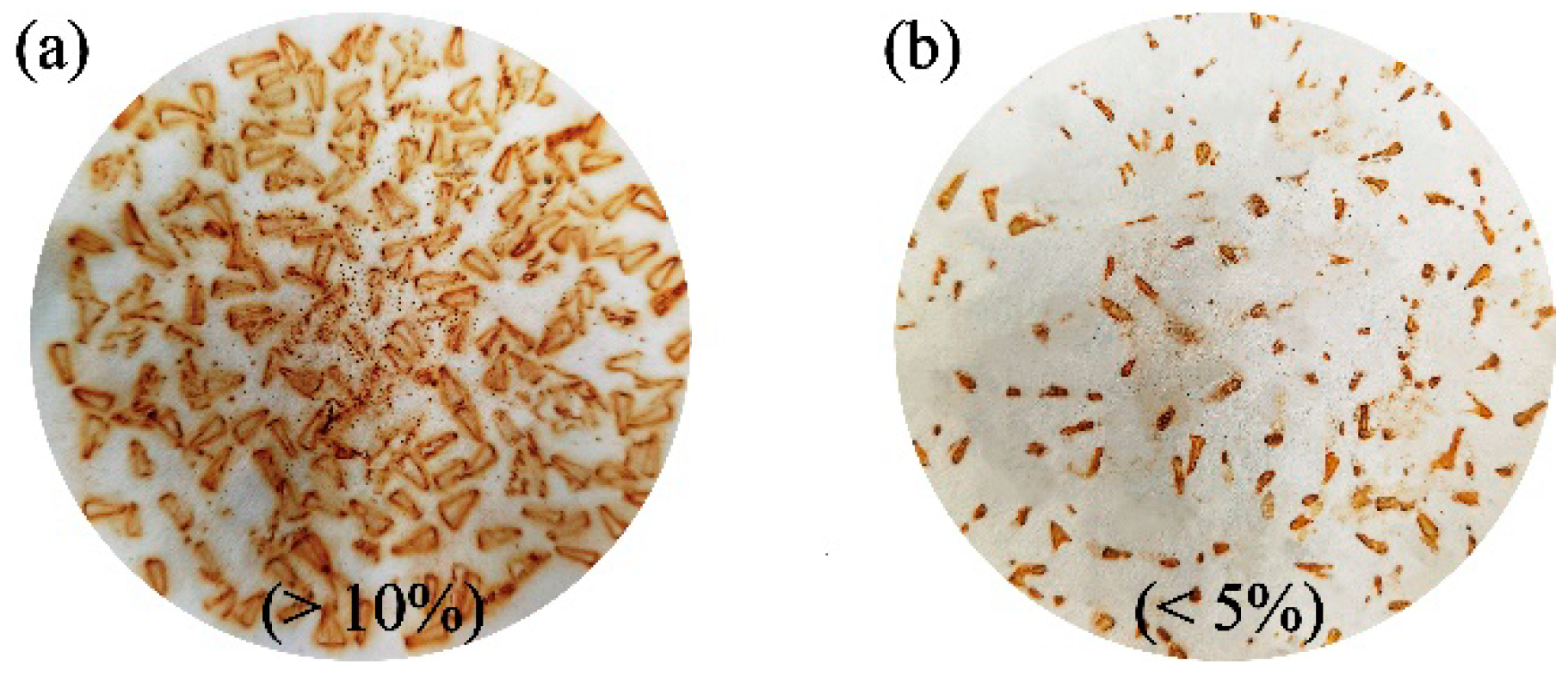
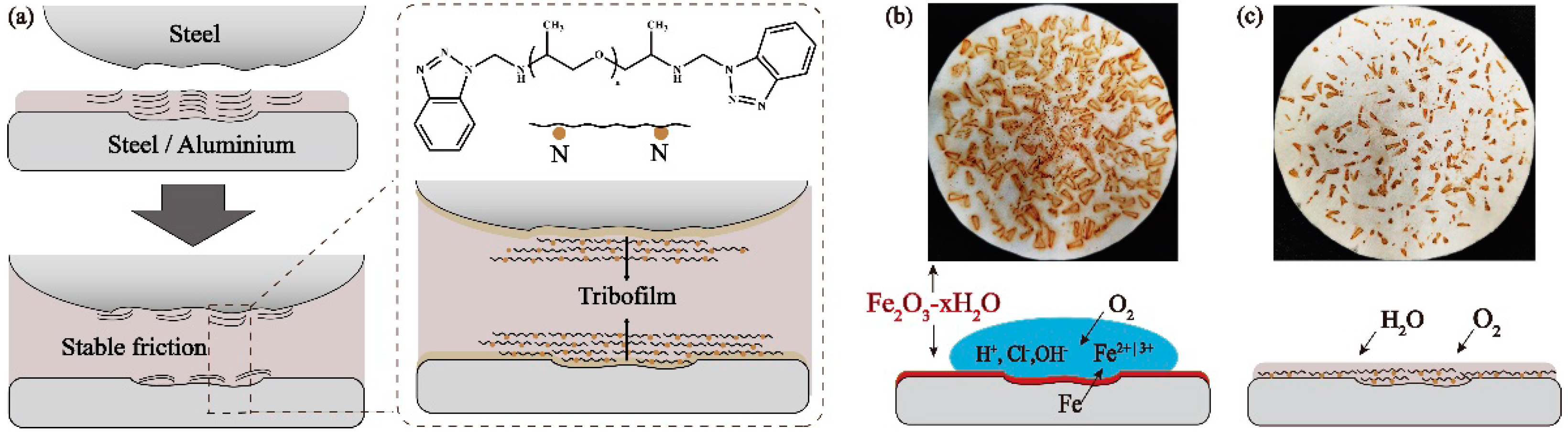
3.5. Anti-Corrosion and Anti-Rust Property
3.6. Lubrication Mechanisms
3.6.1. Analysis of Water Contact Angle Measurements Results
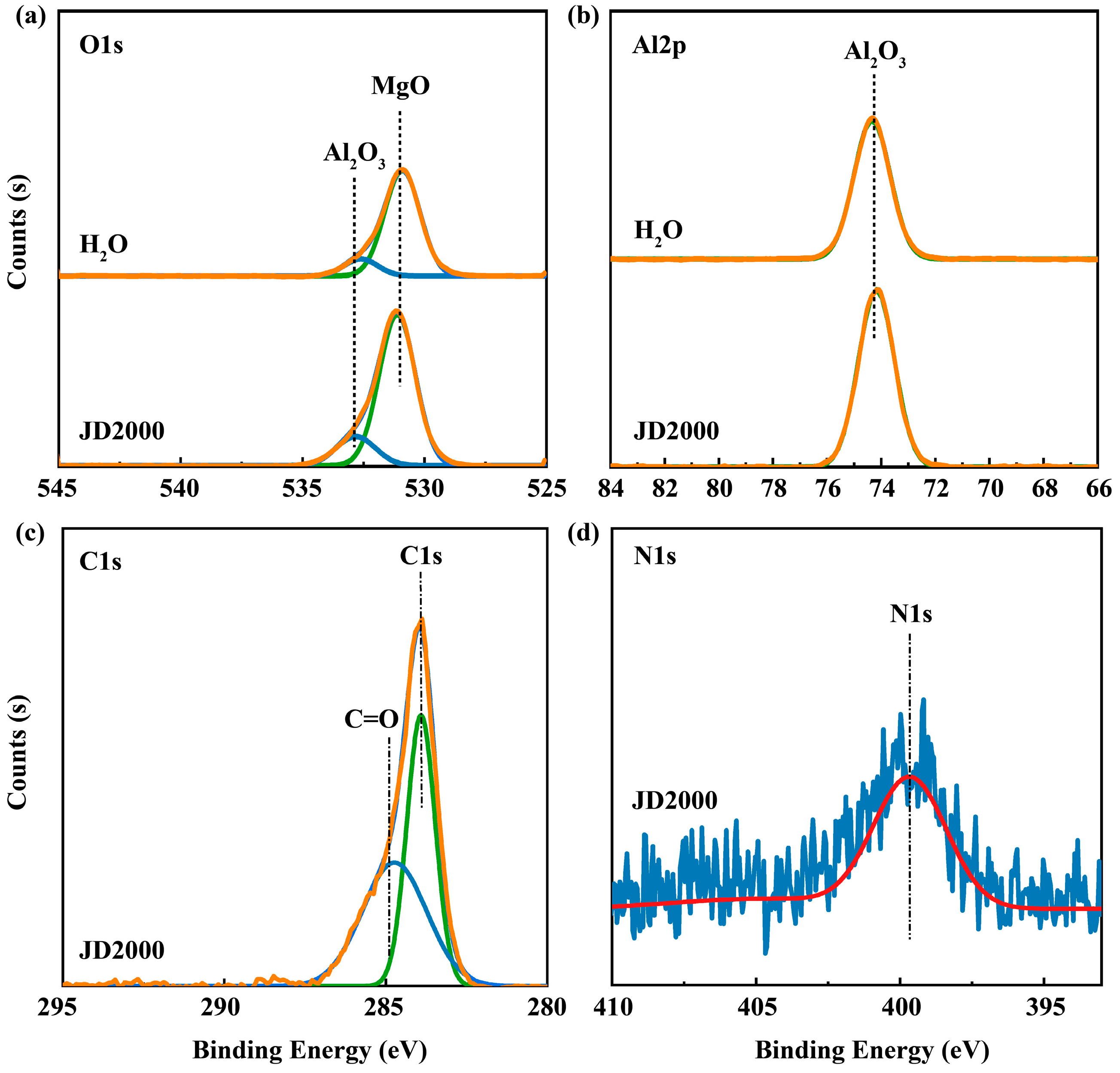
3.6.2. Analysis of Adsorption Behavior Results
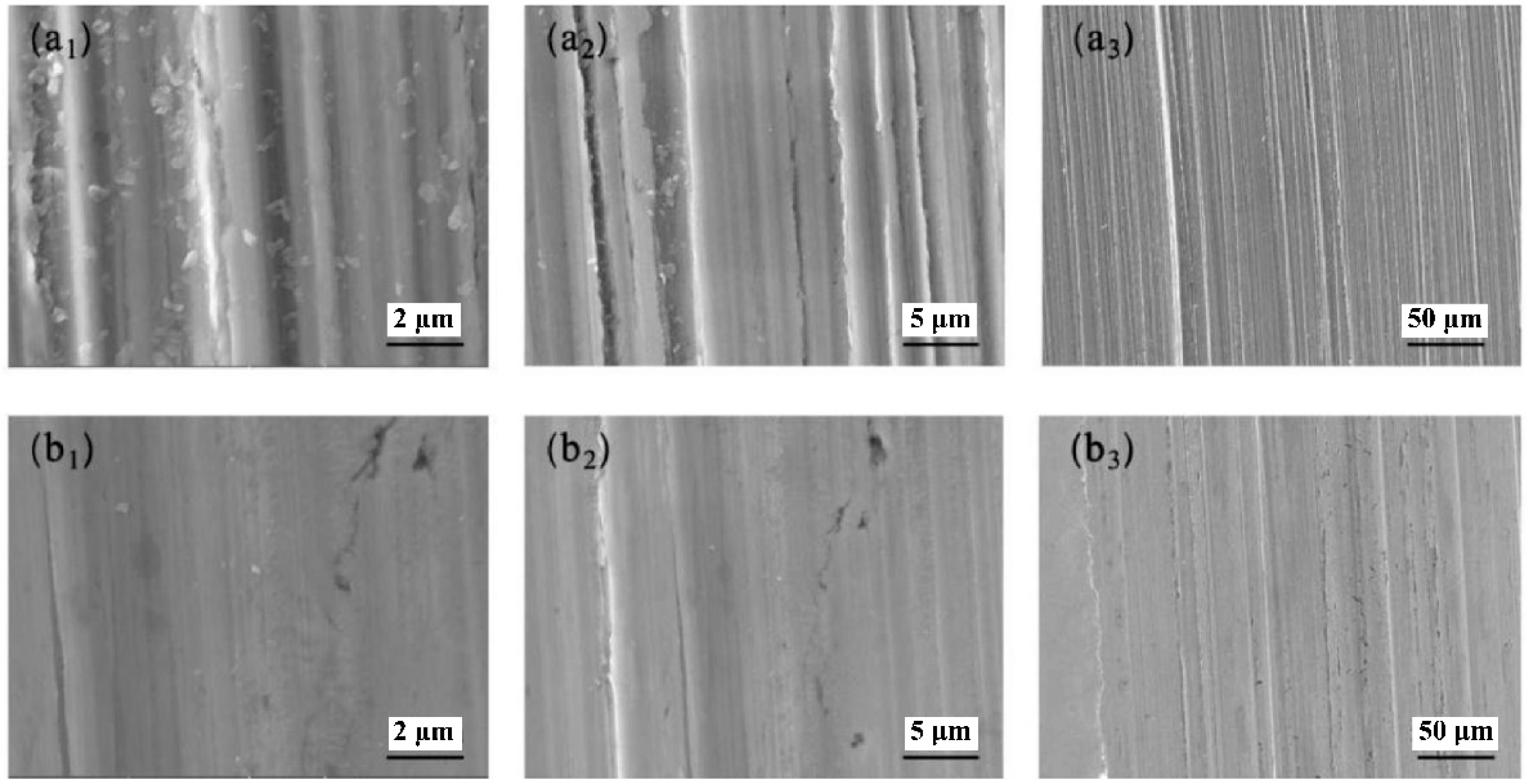
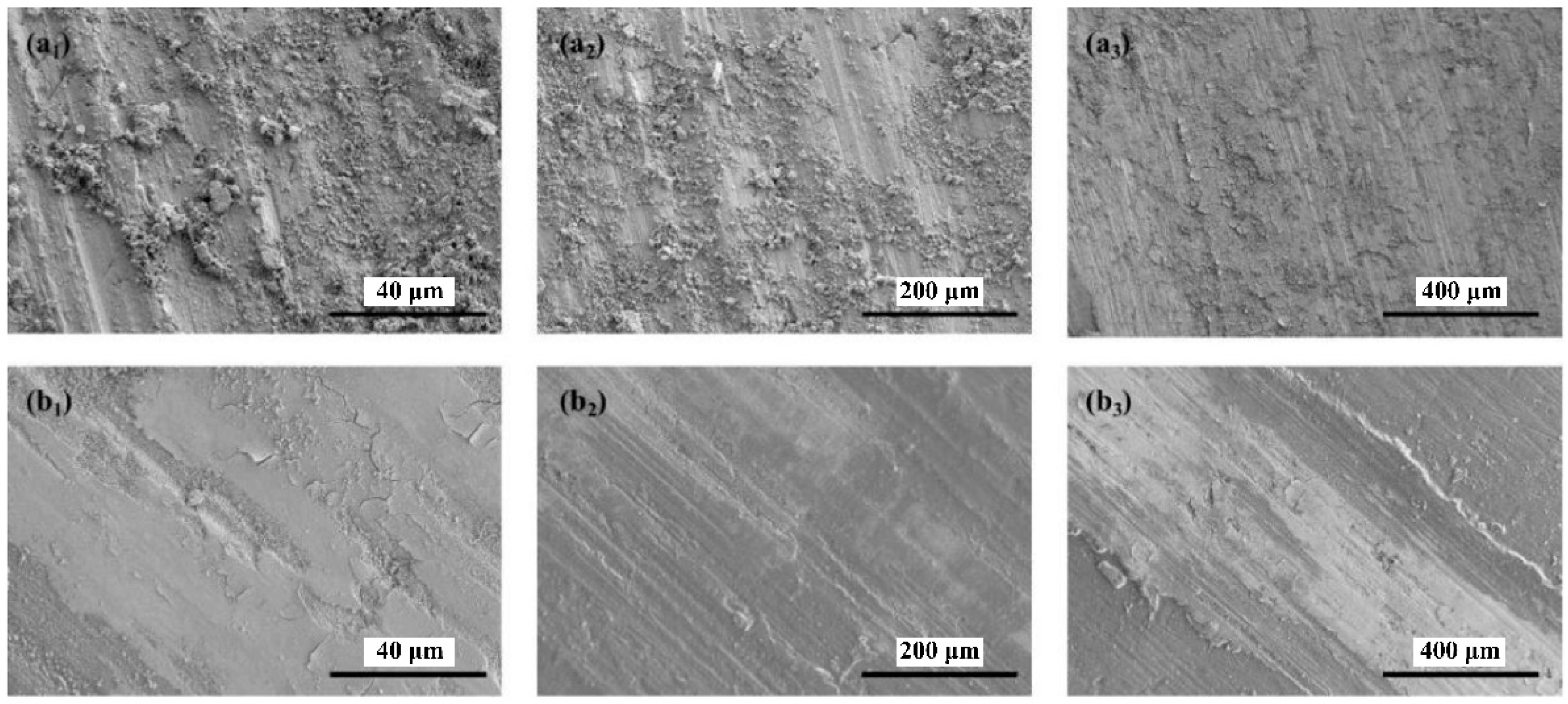
3.6.3. Analysis of the Worn Surfaces of Steel Plates
3.6.4. Analysis of the Worn Surfaces of Aluminum Plates
4. Discussion
5. Conclusions
- The preparation method is simple, and the raw materials are cheap and accessible. The product can exert the excellent properties of raw materials without environmental pollution. Therefore, it can be used as an ecofriendly water-based additive.
- The JD2000 was proved to be a multifunctional additive. It has excellent tribological properties compared with other water-based lubricants. The addition of the 2 wt.% JD2000 led to a reduction of 72.7% and 70.2% in friction for steel/steel and steel/aluminum contacts, respectively, relative to the distilled water, and the wear volume of the JD2000 reduced by 88.2% for steel/aluminum contact. The test also improved the anti-rust property of the JD2000 by 75.5%, while some water-based lubricants do not reduce or even enhance the degree of metal corrosion.
- The adsorption behavior of the JD2000 on metal surface and the formation of viscoelastic film were confirmed by adsorption behavior test and water contact angle tests. Worn surface analysis further determined the structure of tribofilms, which were composed of nitrogen-containing substances, metal oxides, and even polymer deposition. It is speculated that the nitrogen atoms in the JD2000 are first adsorbed on the metal surfaces and then form tribofilms. As the third body, the tribofilms improve the tribological properties by preventing straight asperity contact from the sliding surfaces. Meanwhile, this stable viscoelastic film can resist the corrosion of the metal from the external environment and reduce the corrosion degree of the metal.
- This research can broaden the application of this Jeffamine-triazole derivative in the additives field and validates its application potential as a water-based lubricating and anti-rust additive on different metal surfaces. Concurrently, it also provides an effective method for the preparation and application of an ecofriendly water-soluble polymer-based additive.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raviv, U.; Giasson, S.; Kampf, N.; Gohy, J.F.; Jérôme, R.; Klein, J. Lubrication by charged polymers. Nature 2003, 425, 163–165. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Shinjo, K.; Kaneko, R.; Murata, Y. Observation of super lubricity by scanning tunneling microscopy. Phys. Rev. Lett. 2007, 78, 1448–1451. [Google Scholar] [CrossRef]
- Hu, W.J.; Zhang, Z.J.; Zeng, X.Q.; Li, J.S. Correlation between the degree of alkylation and tribological properties of amino-PEG2-amine-based organic friction modifiers. Ind. Eng. Chem. Res. 2020, 59, 215–225. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Zhang, C.H.; Luo, J.B. Superlubricity of a mixed aqueous solution. Chin. Phys. Lett. 2011, 28, 056201. [Google Scholar]
- Shen, M.W.; Luo, J.B.; Wen, S.Z. The tribological properties of oils added with diamond nano-particles. Tribol. Trans. 2001, 44, 494–498. [Google Scholar] [CrossRef]
- Peng, D.X.; Kang, Y.; Chen, C.H.; Chen, S.K.; Shu, F.C. The tribological behavior of modified diamond nanoparticles in liquid paraffin. Ind. Lubr. Tribol. 2009, 61, 213–219. [Google Scholar] [CrossRef]
- Cheng, G.G.; Jiang, S.Y.; Khosla, T.; Pesika, N.S.; Ding, J.N.; Zhang, Y.H. Synthesis of hard carbon iron microspheres and their aqueous based tribological performance under magnetic field. Tribol. Lett. 2016, 64, 48. [Google Scholar] [CrossRef]
- Hu, W.J.; Xu, Y.H.; Zeng, X.Q.; Li, J.S. Alkyl-ethylene amines as effective organic friction modifiers for the boundary lubrication regime. Langmuir 2020, 36, 6716–6727. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Hsu, W.C.; Lin, J.F. The anti-scuffing performance of diamond nano-particles as an oil additive. Wear. 2010, 268, 960–967. [Google Scholar] [CrossRef]
- Ghosh, S.; Rao, P.V. Comparison between sustainable cryogenic techniques and nano-MQL cooling mode in turning of nickel-based alloy. Clean. Prod. 2019, 231, 1036–1049. [Google Scholar]
- Desari, B.Z.; Davoobi, D. Assessing the lubrication performance of vegetable oil-based nano-lubricants for environmentally conscious metal forming processe. Clean. Prod. 2016, 135, 1198–1209. [Google Scholar] [CrossRef]
- Huang, S.Q.; Wang, Z.; Yao, W.Q.; Xu, X.F. Tribological evaluation of contact- charged electrostatic spray lubrication as a new near-dry machining technique. Tribol. Int. 2015, 91, 74–84. [Google Scholar] [CrossRef]
- Desanker, M.; He, X.; Lu, J.; Liu, P.; Pickens, D.B.; Delferro, M.; Marks, T.J.; Chung, Y.W.; Wang, Q.J. Alkyl-cyclens as effective sulfur- and phosphorus-free friction modifiers for boundary lubrication. ACS Appl. Mater. Interfaces 2017, 9, 9118–9125. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Lv, T.; Wang, M.; Xu, X. Effects of machining and oil mist parameters on electrostatic minimum quantity lubrication–EMQL turning process. Int. J. Pr. Eng. Man-GT. 2018, 5, 317–326. [Google Scholar] [CrossRef]
- Waits, C.M.; Geil, B.; Ghodssi, R. Encapsulated ball bearings for rotary micromachines. Micromech. Microeng. 2007, 17, 224–229. [Google Scholar] [CrossRef]
- Alazemi, A.A.; Etacheri, V.; Dysart, A.D.; Stacke, L.E.; Pol, V.G.; Sadeghi, F. Ultrasmooth submicrometer carbon spheres as lubricant additives for friction and wear reduction. Appl. Mater. Int. 2015, 7, 5514–5521. [Google Scholar] [CrossRef] [PubMed]
- Soltanahmadi, S.; Esfahani, E.A.; Nedelcu, I.; Morina, A.; van Eijk, M.C.P.; Neville, A. Surface reaction films from amine-based organic friction modifiers and their influence on surface fatigue and friction. Tribol. Lett. 2019, 67, 1–15. [Google Scholar] [CrossRef] [Green Version]
- FShin, Y.; Wang, L.Q.; Bae, I.T.; Arey, B.W.; Exarhos, G.J. Hydrothermal syntheses of colloidal carbon spheres from cyclodextrins. Phys. Chem. 2008, 12, 14236–14239. [Google Scholar]
- Xiang, Y.Y.; Li, M.M.; Zhi, G.Y.; Jin, Q.W.; Hong, G.W.; Sheng, R.Y. Covalent functionalization of fluorinated graphene and subsequent application as water based lubricant additive. Appl. Mater. Interfaces 2016, 8, 7483–7488. [Google Scholar]
- Xiao, W.P.; Li, T.H.; Wei, M.L.; Jing, C.H. Synthesis of water-soluble carbon nanotubes via surface initiated redox polymerization and their tribological properties as water-based lubricant additive. Eur. Polym. J. 2008, 44, 2458–2464. [Google Scholar]






| Statistics (wt.%) | C | N | O | Si | Cr | Ni | Mn | Fe |
|---|---|---|---|---|---|---|---|---|
| worn surfaces under distilled water | 1.08 | 0 | 0 | 0.23 | 16.62 | 8.74 | 1.14 | 72.19 |
| worn surfaces under 2 wt.% JD2000 aqueous solution | 1.7 | 7.64 | 3.89 | 0.58 | 17.16 | 6.36 | 0 | 62.67 |
| Statistics (wt.%) | O | Mg | Al | C |
|---|---|---|---|---|
| worn surfaces under distilled water | 23.25 | 2.56 | 74.20 | 0 |
| worn surfaces under 2 wt.% JD2000 aqueous solution | 30.33 | 1.40 | 44.35 | 23.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Hu, W.; Li, J. Lubrication and Anti-Rust Properties of Jeffamine-Triazole Derivative as Water-Based Lubricant Additive. Coatings 2021, 11, 679. https://doi.org/10.3390/coatings11060679
Wang J, Hu W, Li J. Lubrication and Anti-Rust Properties of Jeffamine-Triazole Derivative as Water-Based Lubricant Additive. Coatings. 2021; 11(6):679. https://doi.org/10.3390/coatings11060679
Chicago/Turabian StyleWang, Jiabei, Wenjing Hu, and Jiusheng Li. 2021. "Lubrication and Anti-Rust Properties of Jeffamine-Triazole Derivative as Water-Based Lubricant Additive" Coatings 11, no. 6: 679. https://doi.org/10.3390/coatings11060679
APA StyleWang, J., Hu, W., & Li, J. (2021). Lubrication and Anti-Rust Properties of Jeffamine-Triazole Derivative as Water-Based Lubricant Additive. Coatings, 11(6), 679. https://doi.org/10.3390/coatings11060679







