Powder Diffraction Data of Aluminum-Rich FCC-Ti1−xAlxN Prepared by CVD
Abstract
1. Introduction
2. Materials and Methods
2.1. Coating Deposition and Powder Preparation
2.2. Coating and Powder Analysis
3. Results and Discussion
3.1. Analysis of the Ti1−xAlxN Coating
3.1.1. Thickness of the Ti1−xAlxN Coating
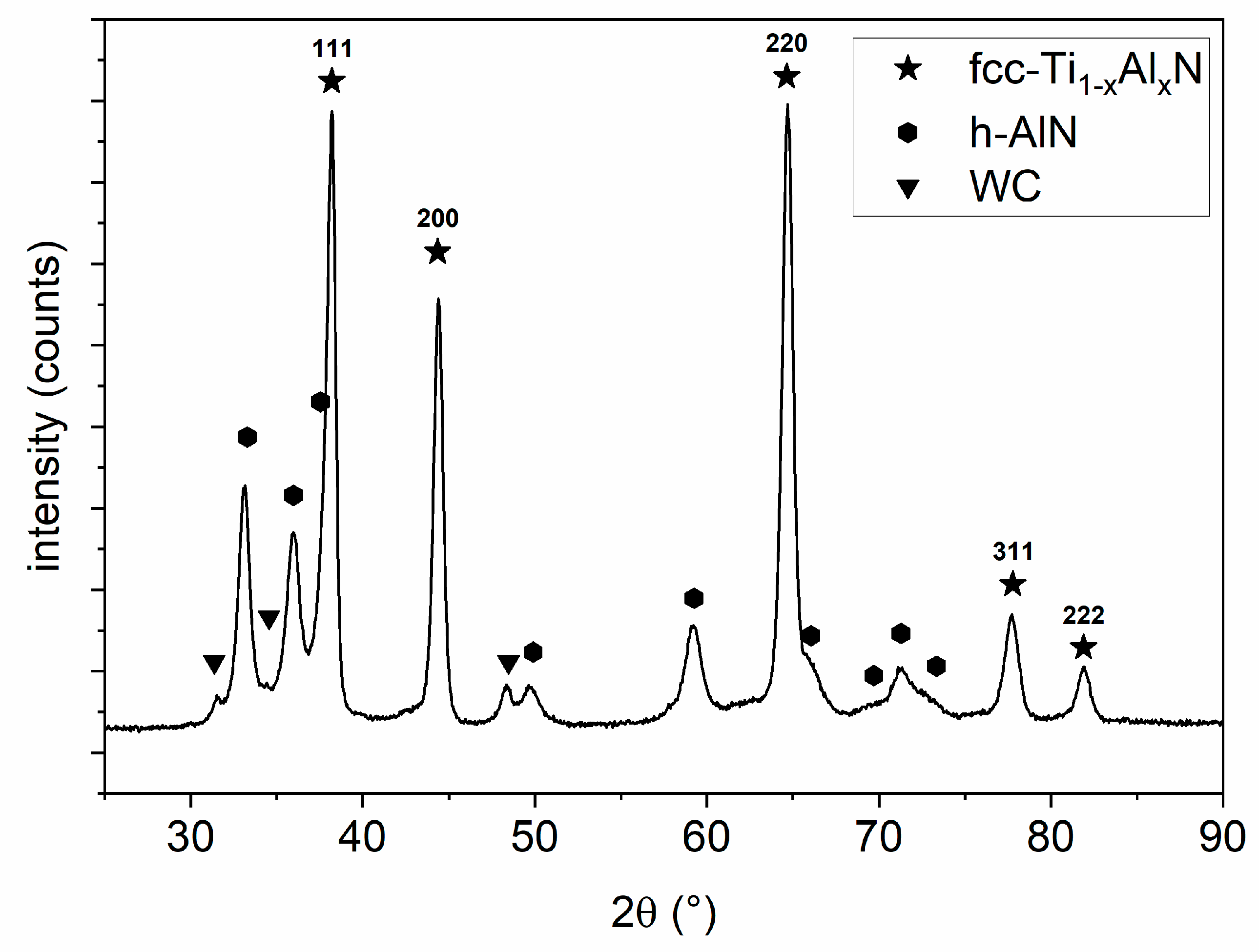
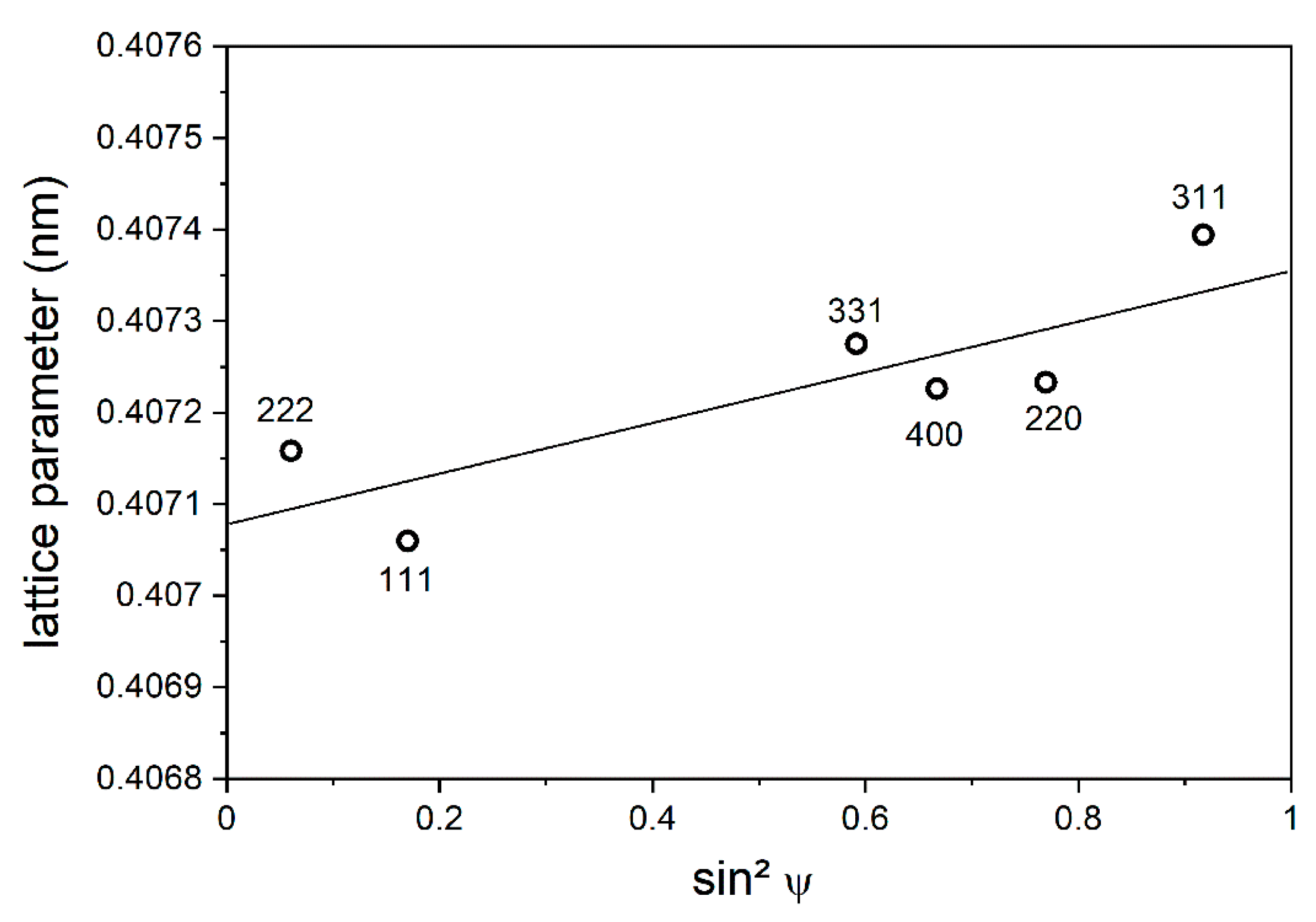
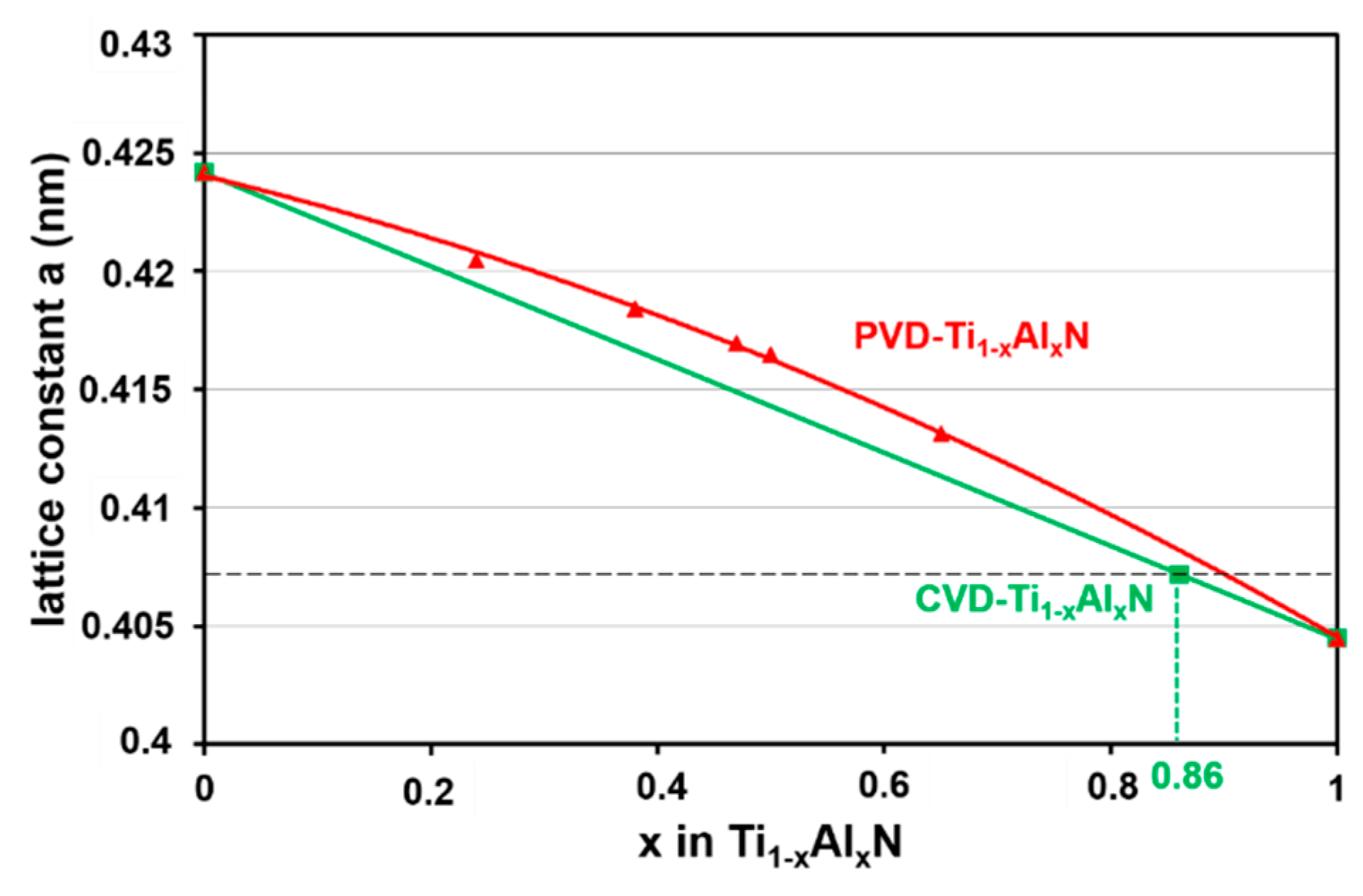
3.1.2. X-ray Analysis of the Ti1−xAlxN Coating
3.2. Microstructure, Composition and X-ray Analysis of the Ti1−xAlxN Powder
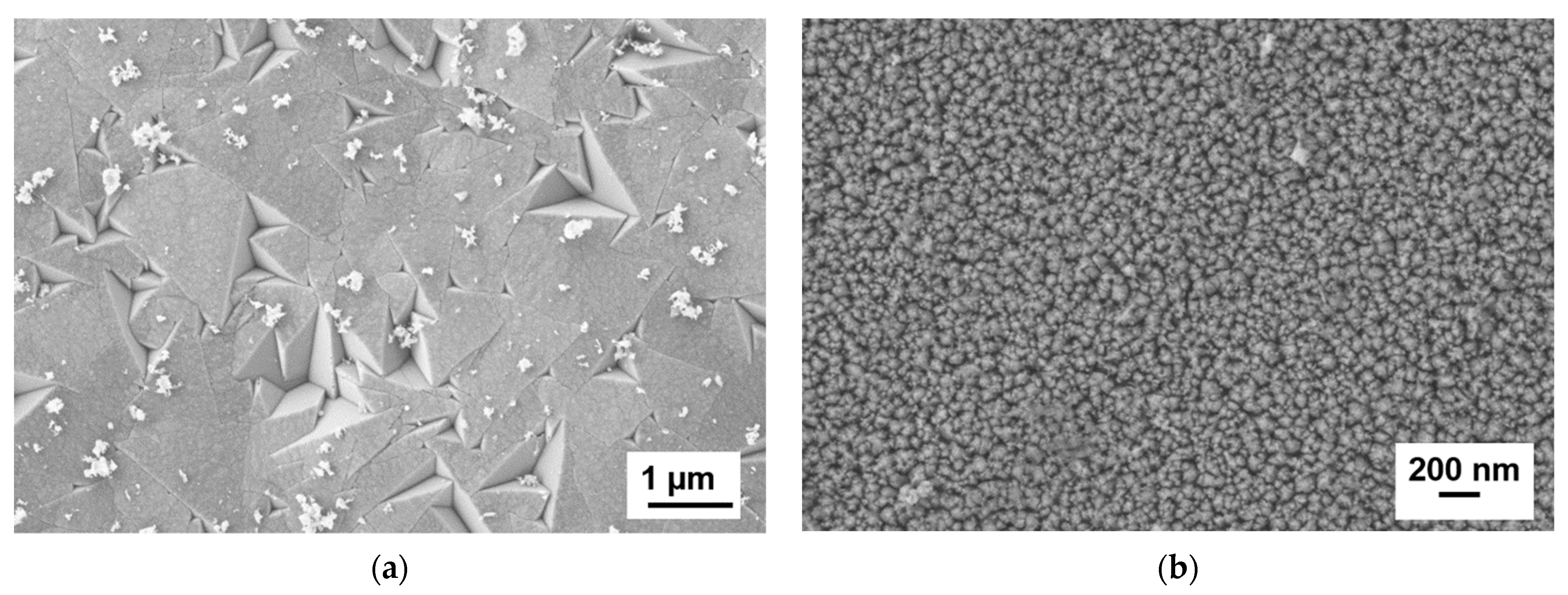
3.2.1. Microstructure of the Ti1−xAlxN Powder
3.2.2. Composition of the Ti1−xAlxN Powder
3.2.3. X-ray Analysis of the Ti1−xAlxN Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knotek, O.; Böhmer, M.; Leyendecker, T. On structure and properties of sputtered Ti and Al based hard compound films. J. Vac. Sci. Technol. A 1986, 4, 2695–2700. [Google Scholar] [CrossRef]
- PalDey, S.; Deevi, S.C. Single layer and multilayer wear resistant coatings of (Ti,Al)N: A review. Mat. Sci. Eng. A 2003, 342, 58–79. [Google Scholar] [CrossRef]
- Cremer, R.; Witthaut, M.; von Richthofen, A.; Neuschütz, D. Determination of the cubic to hexagonal structure transition in the metastable system TiN-AlN, Fresenius. J. Anal. Chem. 1998, 361, 642–645. [Google Scholar]
- Rafaja, D.; Šıma, M.; Klemm, V.; Schreiber, G.; Heger, D.; Havela, L.; Kužel, R. X-ray diffraction on nanocrystalline Ti1−xAlxN thin films. J. Alloys Comp. 2004, 378, 107–111. [Google Scholar] [CrossRef]
- Endler, I.; Höhn, M.; Herrmann, M.; Pitonak, R.; Ruppi, S.; Schneider, M.; van den Berg, H.; Westphal, H. Novel aluminum-rich Ti1−xAlxN coatings by LPCVD. Surf. Coat. Technol. 2008, 203, 530–533. [Google Scholar] [CrossRef]
- Pitonak, R.; Köpf, A.; Weißenbacher, R.; Keckes, J.; Stefenelli, M.; Todt, J.; Endler, I.; Höhn, M. Novel TiAlN coating by Medium Temperature Low Pressure CVD. In Proceedings of the 18th Plansee Seminar, Reutte, Austria, 3–7 June 2013. HM 37/1-37/12. [Google Scholar]
- Czettl, C.; Schleinkofer, U.; Schedle, F.; Wolf, C.; Lechleitner, M.; Holzschuh, H.; Bürgin, W. CVD TiAlN – Development and challenges for use in mass production History and development of CVD-TiAlN. In Proceedings of the 19th Plansee Seminar, Reutte, Austria, 29 May–2 June 2017. HM 45/1-45/13. [Google Scholar]
- PDF Database; The International Centre for Diffraction Data (ICDD): Newton Square, PA, USA.
- Gates-Rector, S.D.; Blanton, T.N. The powder diffraction file: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Powder Diffraction File, Int. Centre for Diffraction Data; Newton Square, PN, USA, 2011; file no. 00-025-1495.
- Powder Diffraction File, Int. Centre for Diffraction Data; Newton Square, PN, USA, 2019; file no. 00-046-1200.
- Vollstädt, H.; Ito, E.; Akaishi, M.; Akimoto, S.; Fukunaga, O. High pressure synthesis of rocksalt type of AlN. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 1990, 66, 7–9. [Google Scholar] [CrossRef]
- Powder Diffraction File, Int. Centre for Diffraction Data; Newton Square, PN, USA, 2019; file no. 00-038-1420.
- Madan, A.; Kim, I.W.; Cheng, S.C.; Yashar, P.; Dravid, V.P.; Barn, S.A. Stabilization of cubic AlN in epitaxial AlN/TiN superlattices. Phys. Rev. Lett. 1997, 78, 1743–1746. [Google Scholar] [CrossRef]
- Schwarz, M.R.; Antlauf, M.; Schmerler, S.; Keller, K.; Schlothauer, T.; Kortus, J.; Heide, G.; Kroke, E. Formation and properties of rocksalt-type AlN and implications for high pressure phase relations in the system Si-Al-O-N. High. Press. Res. 2014, 34, 22–38. [Google Scholar] [CrossRef]
- Wüstefeld, C.; Rafaja, D.; Dopita, M.; Motylenko, M.; Baehtz, C.; Michotte, C.; Kathrein, M. Decomposition kinetics in Ti1−xAlxN coatings as studied by in-situ X-ray diffraction during annealing. Surf. Coat. Technol. 2011, 206, 1727–1734. [Google Scholar] [CrossRef]
- Rafaja, D.; Wüstefeld, C.; Baehtz, C.; Klemm, V.; Dopita, M.; Motylenko, M.; Michotte, C.; Kathrein, M. Effect of internal interfaces on hardness and thermal stability of nanocrystalline Ti0.5Al0.5N coatings. Metall. Mater. Trans. A 2011, 42A, 559–569. [Google Scholar] [CrossRef]
- Rogström, L.; Ullbrand, J.; Almer, J.; Hultman, L.; Jansson, B.; Odén, M. Strain evolution during spinodal decomposition of TiAlN thin films. Thin Solid Films 2012, 520, 5542–5549. [Google Scholar] [CrossRef]

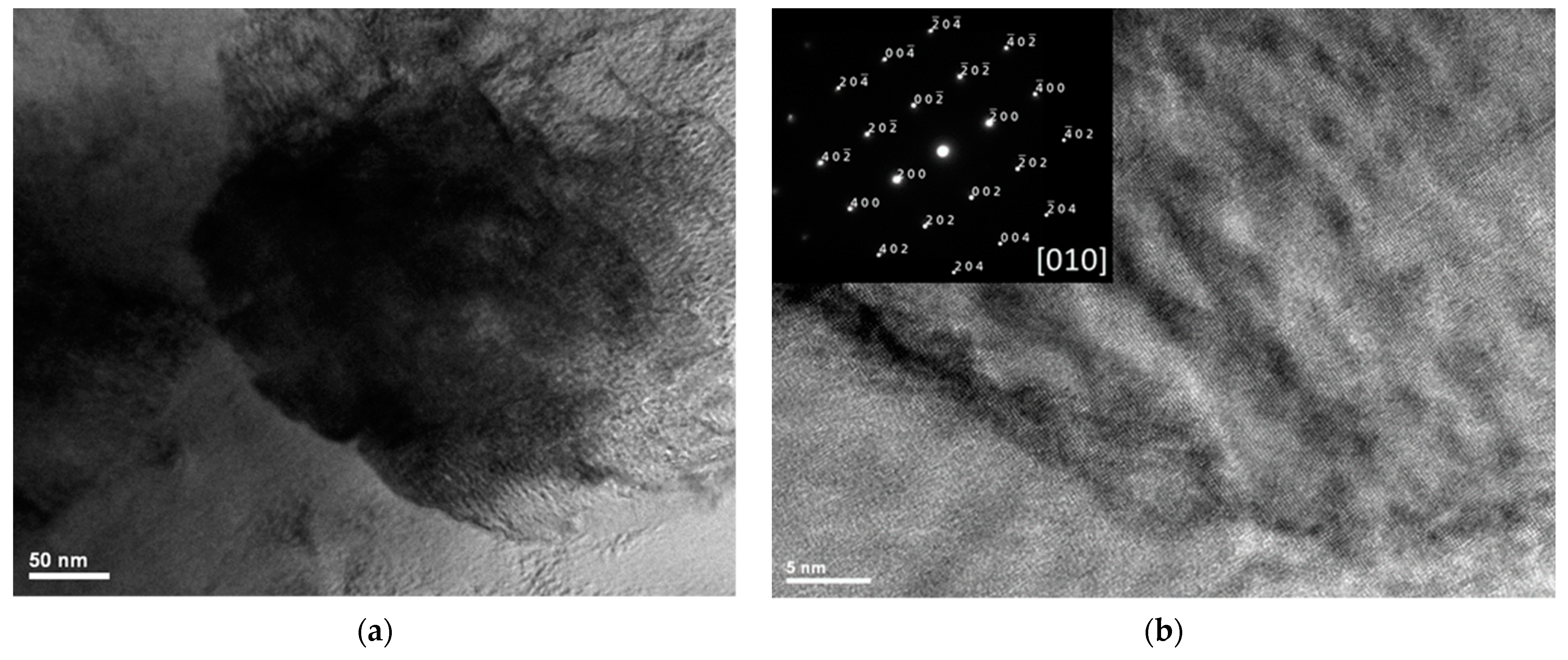

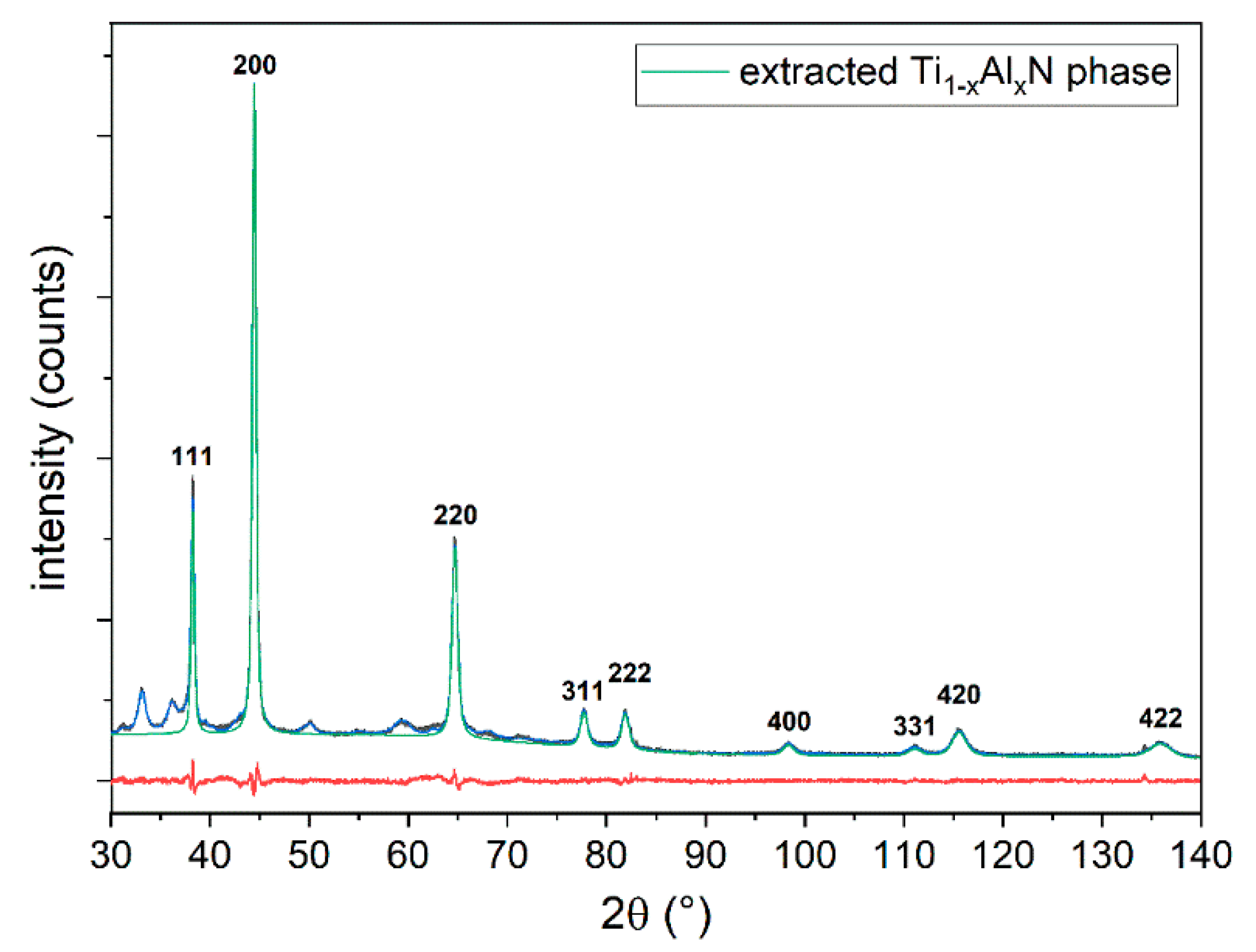
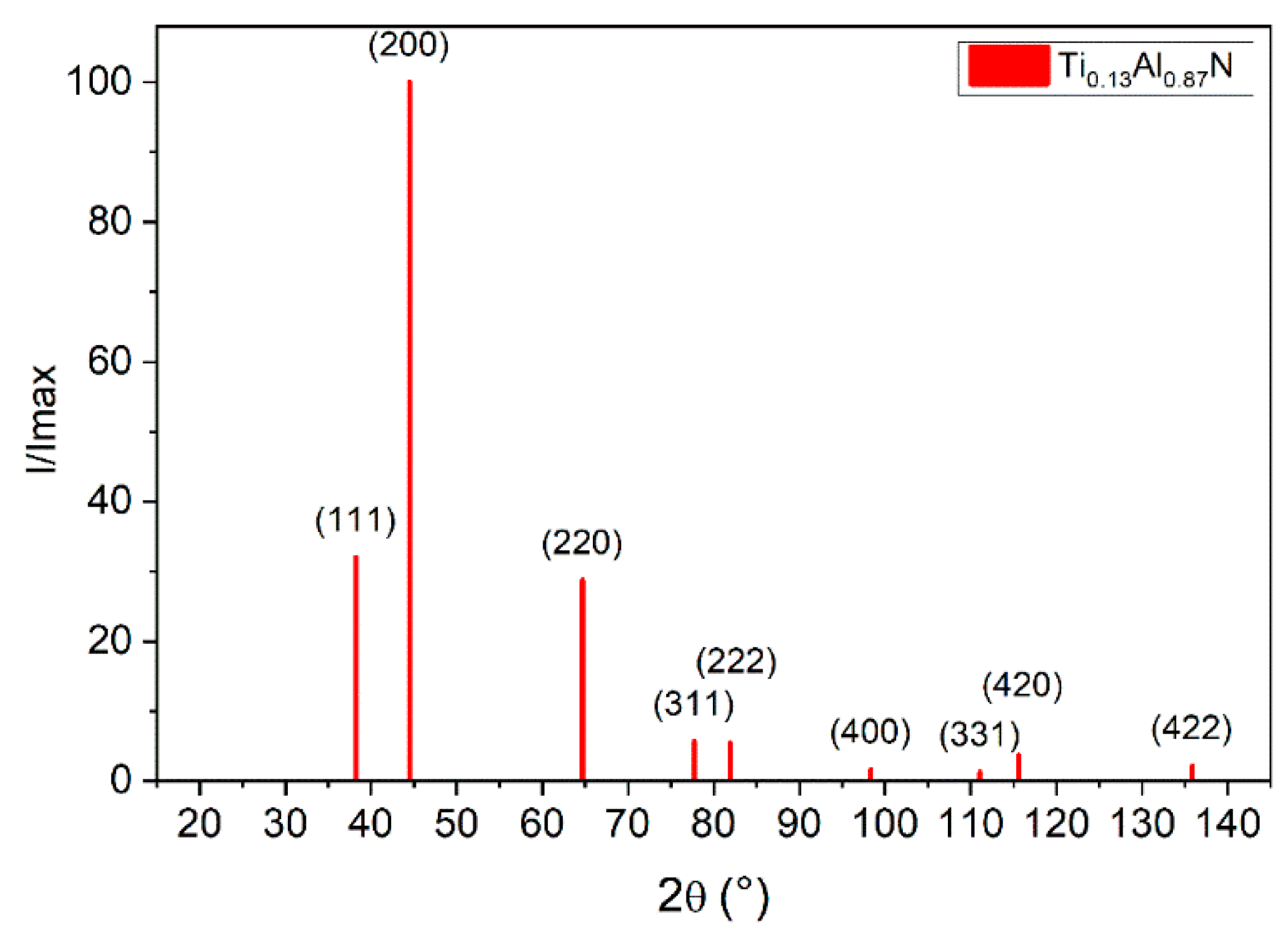
| Sample | Al (WDS) at.% | Ti (WDS) at.% | N (EDS) at.% | O (WDS) at.% | x |
|---|---|---|---|---|---|
| Ti1−xAlxN flake top side | 43.7 | 5.8 | 49.9 | 0.6 | 0.88 |
| Ti1−xAlxN flake bottom side | 41.0 | 6.1 | 52.1 | 0.8 | 0.87 |
| h k l | D (Å) | 2θ (°, Cu Kα) | I/Imax (%) | M Multiplicity | N (h2 + k2 + l2) |
|---|---|---|---|---|---|
| 1 1 1 | 2.35079 | 38.256 | 32.0 | 8 | 3 |
| 2 0 0 | 2.03584 | 44.465 | 100.0 | 6 | 4 |
| 2 2 0 | 1.43956 | 64.701 | 28.7 | 12 | 8 |
| 3 1 1 | 1.22766 | 77.725 | 5.7 | 24 | 11 |
| 2 2 2 | 1.17539 | 81.893 | 5.5 | 8 | 12 |
| 4 0 0 | 1.01792 | 98.355 | 1.7 | 6 | 16 |
| 3 3 1 | 0.93411 | 111.103 | 1.2 | 24 | 19 |
| 4 2 0 | 0.91046 | 115.571 | 3.7 | 24 | 20 |
| 4 2 2 | 0.83113 | 135.886 | 2.2 | 24 | 36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endler, I.; Höhn, M.; Matthey, B.; Zálešák, J.; Keckes, J.; Pitonak, R. Powder Diffraction Data of Aluminum-Rich FCC-Ti1−xAlxN Prepared by CVD. Coatings 2021, 11, 683. https://doi.org/10.3390/coatings11060683
Endler I, Höhn M, Matthey B, Zálešák J, Keckes J, Pitonak R. Powder Diffraction Data of Aluminum-Rich FCC-Ti1−xAlxN Prepared by CVD. Coatings. 2021; 11(6):683. https://doi.org/10.3390/coatings11060683
Chicago/Turabian StyleEndler, Ingolf, Mandy Höhn, Björn Matthey, Jakub Zálešák, Jozef Keckes, and Reinhard Pitonak. 2021. "Powder Diffraction Data of Aluminum-Rich FCC-Ti1−xAlxN Prepared by CVD" Coatings 11, no. 6: 683. https://doi.org/10.3390/coatings11060683
APA StyleEndler, I., Höhn, M., Matthey, B., Zálešák, J., Keckes, J., & Pitonak, R. (2021). Powder Diffraction Data of Aluminum-Rich FCC-Ti1−xAlxN Prepared by CVD. Coatings, 11(6), 683. https://doi.org/10.3390/coatings11060683








