The Evolution of Intumescent Char in Flame-Retardant Coatings Based on Amino Resin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results
3.1. Flame-Retardant Properties
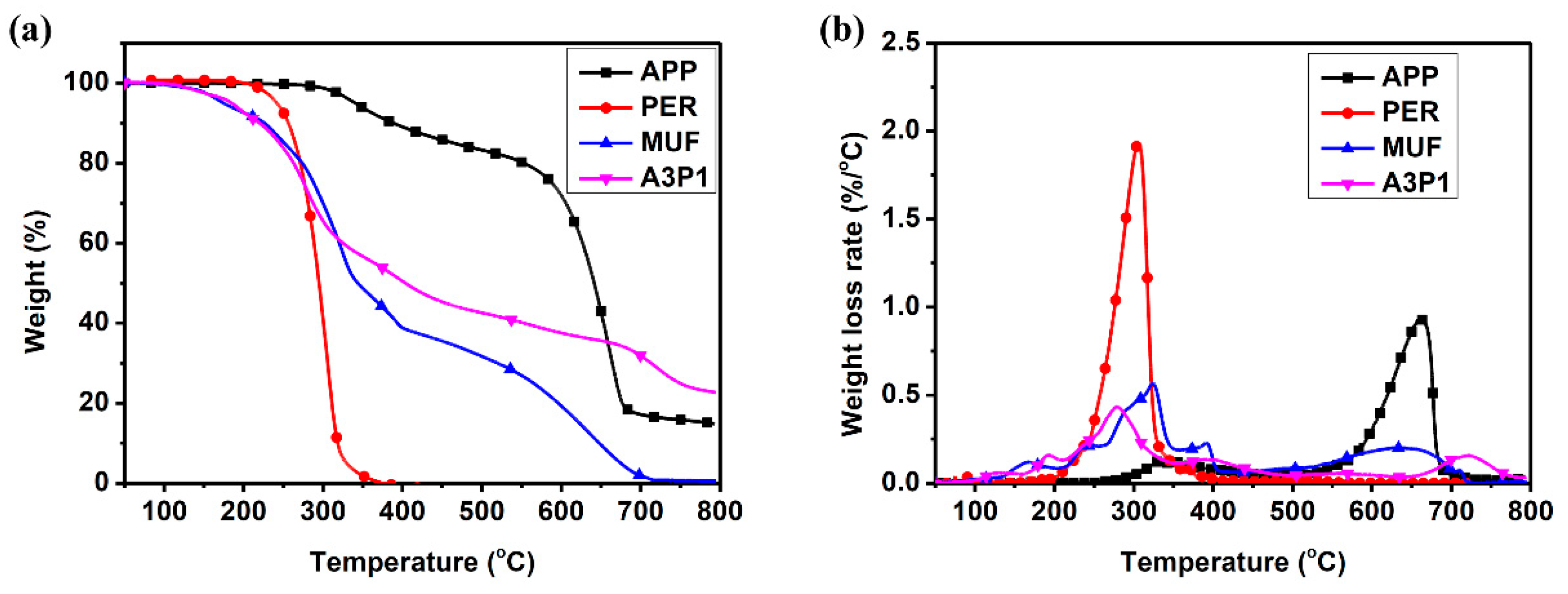
3.2. Thermal Analysis
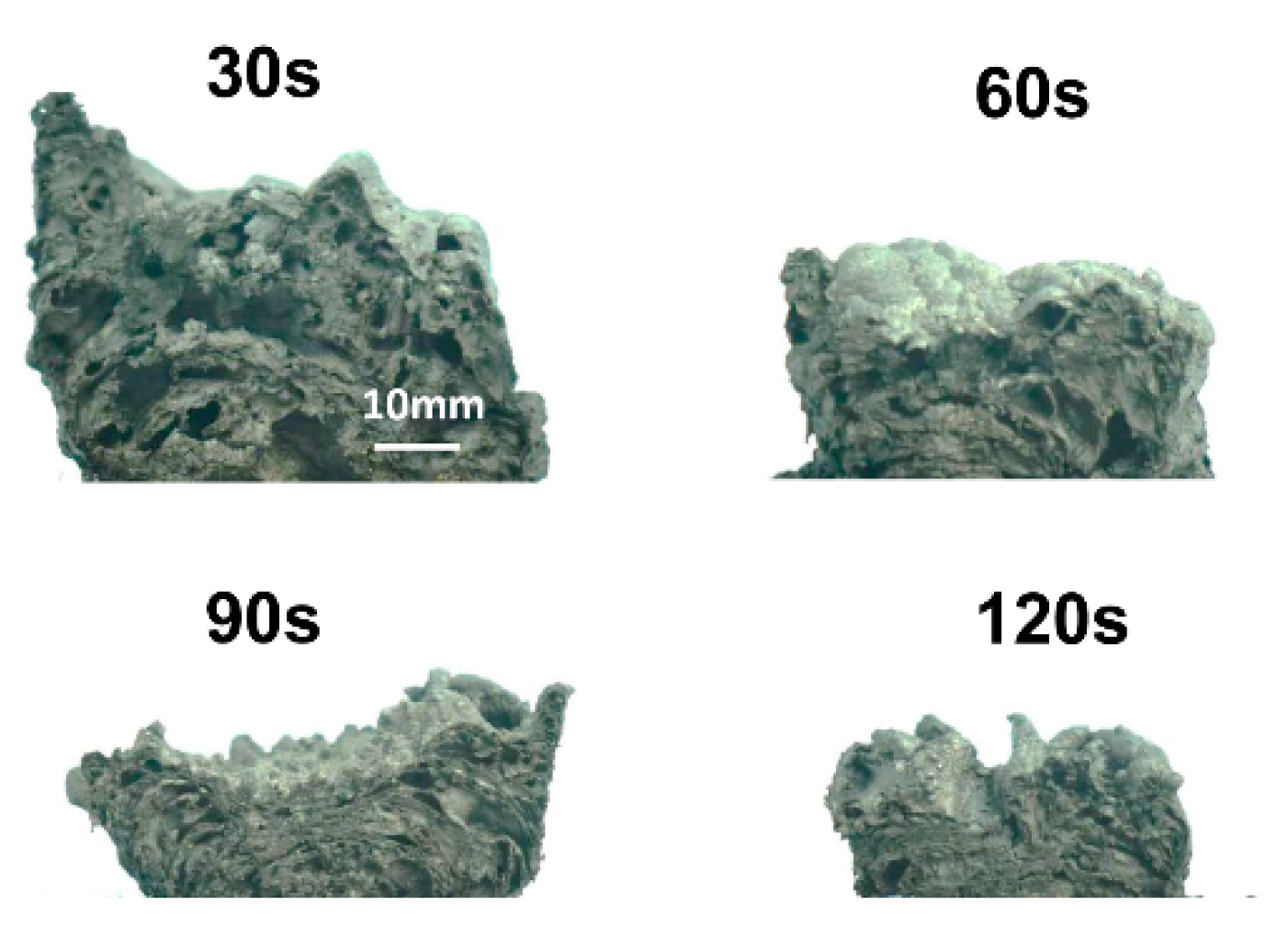
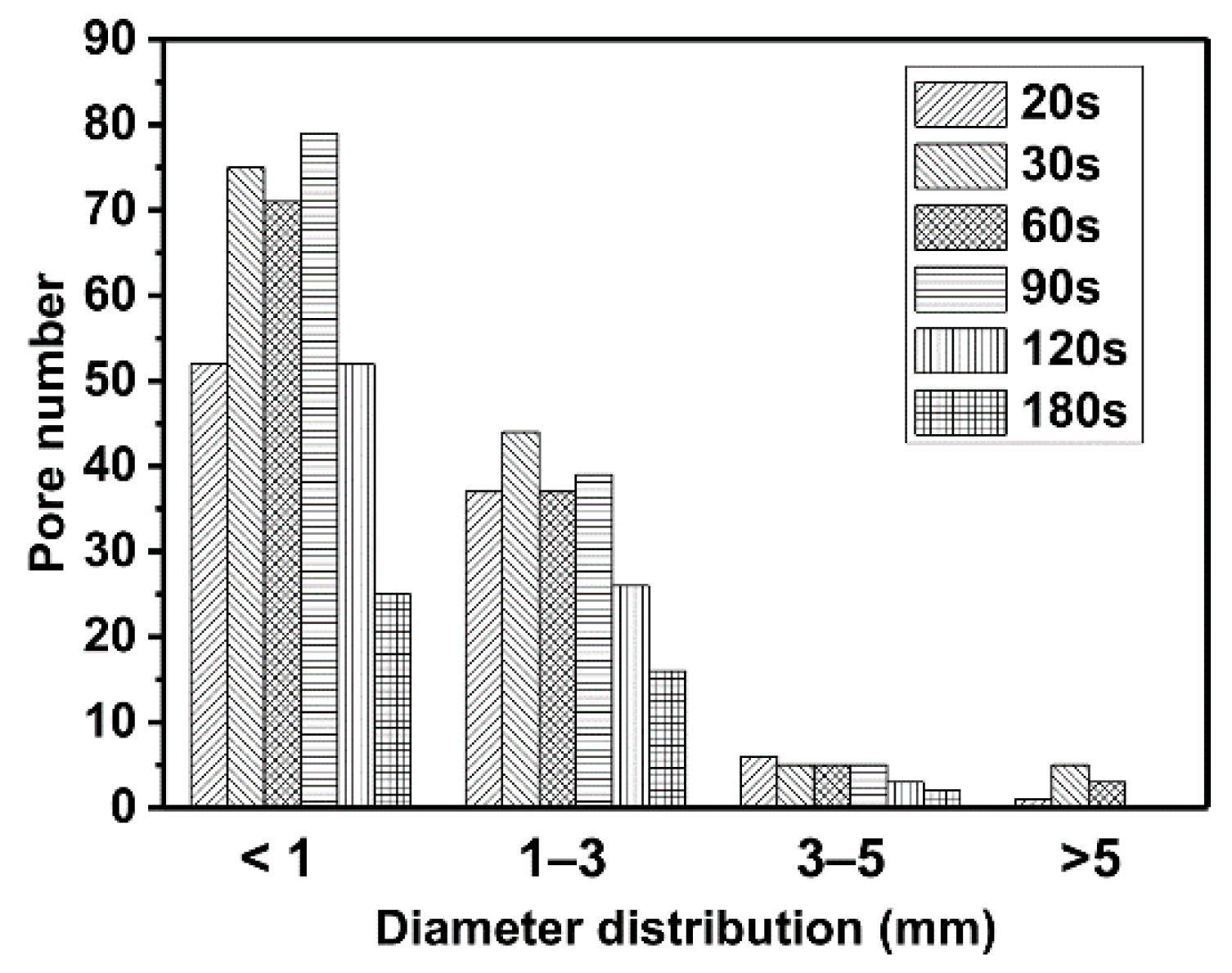
3.3. Volumes and Pore Sizes of Intumescent Chars
- The char was formed before 30 s;
- The volume of char decreased rapidly from 30 s to 90 s;
- The volume of char decreased slowly after 90 s.
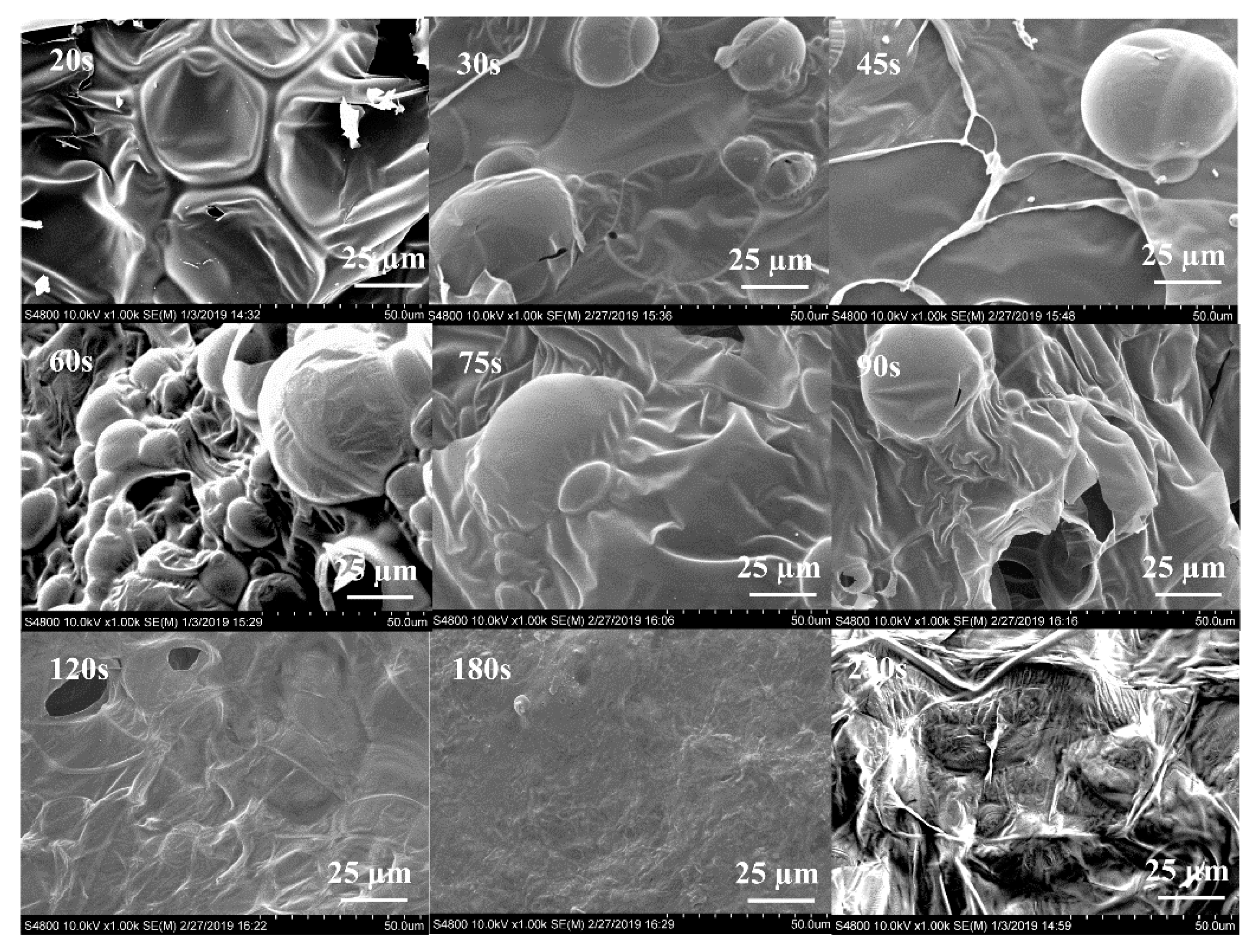
3.4. Morphological Structures of Intumescent Char
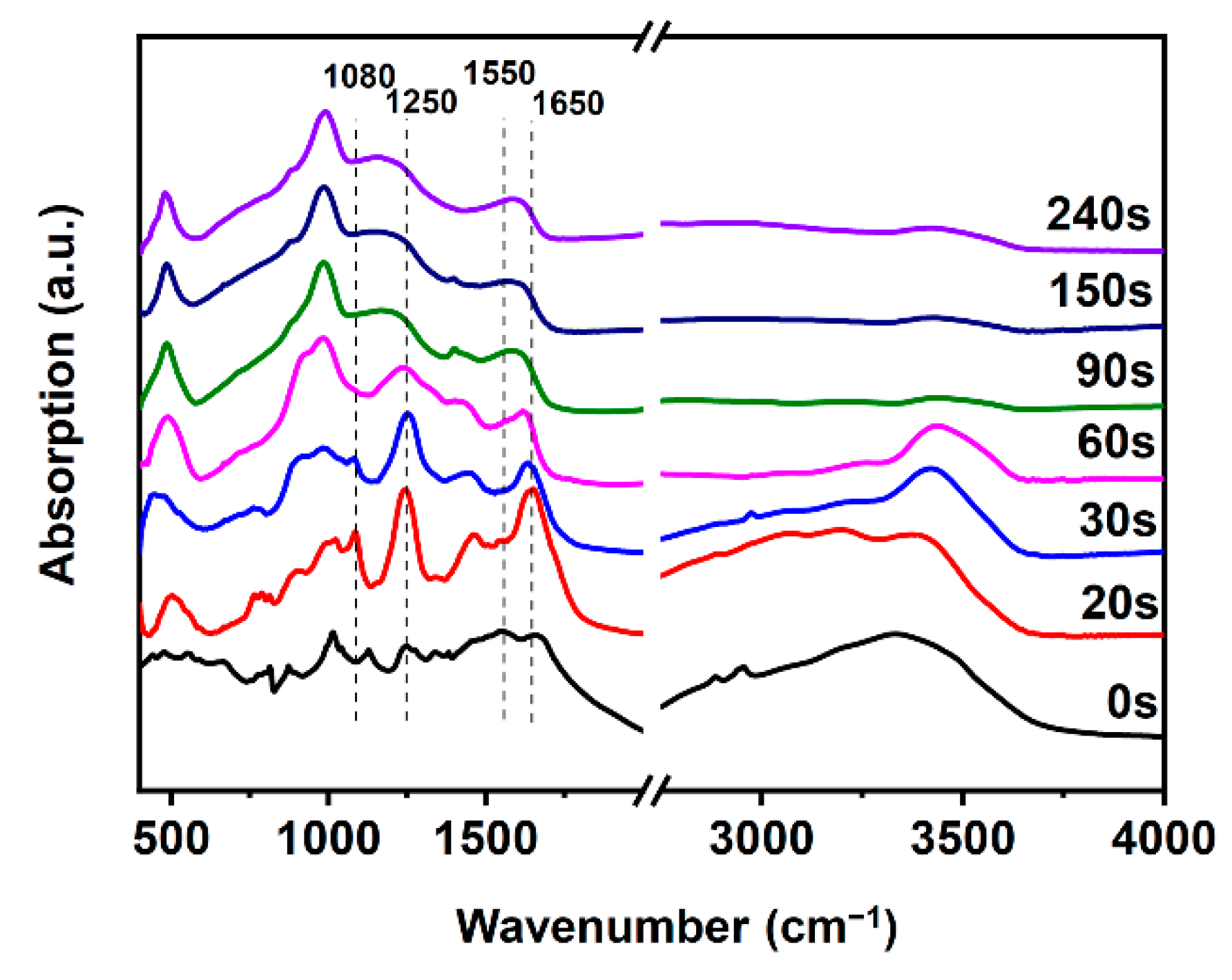
3.5. FTIR Analysis
4. Conclusions
- The char was formed before 30 s;
- The volume of char decreased rapidly from 30 s to 90 s;
- The volume of char decreased slowly after 90 s. More than 50% shrinkage of char was developed in the second stage due to the collapse of big pores (D > 5 mm).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weil, E.D. Fire-Protective and Flame-Retardant Coatings—A State-of-the-Art Review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Duquesne, S.; Magnet, S.; Jama, C.; Delobel, R. Thermoplastic resins for thin film intumescent coatings—towards a better understanding of their effect on intumescence efficiency. Polym. Degrad. Stab. 2005, 88, 63–69. [Google Scholar] [CrossRef]
- Gu, J.-W.; Zhang, G.-C.; Dong, S.-L.; Zhang, Q.-Y.; Kong, J. Study on preparation and fire-retardant mechanism analysis of intumescent flame-retardant coatings. Surf. Coat. Technol. 2007, 201, 7835–7841. [Google Scholar] [CrossRef]
- Jiao, C.; Chen, X. Flammability and thermal degradation of intumescent flame-retardant polypropylene composites. Polym. Eng. Sci. 2010, 50, 767–772. [Google Scholar] [CrossRef]
- Lu, H.; Song, L.; Hu, Y. A review on flame retardant technology in China. Part II: Flame retardant polymeric nanocomposites and coatings. Polym. Adv. Technol. 2011, 22, 379–394. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A.S. Intumescent coatings: A review on recent progress. J. Coat. Technol. Res. 2017, 14, 1–20. [Google Scholar] [CrossRef]
- Battegazzore, D.; Alongi, J.; Fontaine, G.; Frache, A.; Bourbigot, S.; Malucelli, G. Bulk vs. surface flame retardancy of fully bio-based polyamide 10,10. RSC Adv. 2015, 5, 39424–39432. [Google Scholar] [CrossRef]
- Xu, Z.; Chu, Z.; Yan, L.; Chen, H.; Jia, H.; Tang, W. Effect of chicken eggshell on the flame-retardant and smoke suppression properties of an epoxy-based traditional APP-PER-MEL system. Polym. Compos. 2019, 40, 2712–2723. [Google Scholar] [CrossRef]
- Wang, Z.; Han, E.; Ke, W. Effect of acrylic polymer and nanocomposite with nano-SiO2 on thermal degradation and fire resistance of APP–DPER–MEL coating. Polym. Degrad. Stab. 2006, 91, 1937–1947. [Google Scholar] [CrossRef]
- Jimenez, M.; Bellayer, S.; Revel, B.; Duquesne, S.; Bourbigot, S. Comprehensive Study of the Influence of Different Aging Scenarios on the Fire Protective Behavior of an Epoxy Based Intumescent Coating. Ind. Eng. Chem. Res. 2013, 52, 729–743. [Google Scholar] [CrossRef]
- Gravit, M.; Gumenyuk, V.; Sychov, M.; Nedryshkin, O. Estimation of the Pores Dimensions of Intumescent Coatings for Increase the Fire Resistance of Building Structures. Procedia Eng. 2015, 117, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Pan, D.X.; Sun, C.Y. Study on the thermal degradation of wood treated with amino resin and amino resin modified with phosphoric acid. J. Fire Sci. 2003, 21, 189–201. [Google Scholar] [CrossRef]
- Ma, X.-X.; Wu, Y.-Z.; Zhu, H.-L. The fire-retardant properties of the melamine-modified urea–formaldehyde resins mixed with ammonium polyphosphate. J. Wood Sci. 2013, 59, 419–425. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Kandola, B.K.; Davies, P.J.; Zhang, S.; Padbury, S.A. Developments in flame retardant textiles—A review. Polym. Degrad. Stab. 2005, 88, 3–12. [Google Scholar] [CrossRef]
- Vroman, I.; Giraud, S.; Salaun, F.; Bourbigot, S. Polypropylene fabrics padded with microencapsulated ammonium phosphate: Effect of the shell structure on the thermal stability and fire performance. Polym. Degrad. Stab. 2010, 95, 1716–1720. [Google Scholar] [CrossRef]
- Alongi, J.; Han, Z.; Bourbigot, S. Intumescence: Tradition versus novelty. A comprehensive review. Prog. Polym. Sci. 2015, 51, 28–73. [Google Scholar] [CrossRef]
- Wang, Z.; Han, E.; Ke, W. Influence of expandable graphite on fire resistance and water resistance of flame-retardant coatings. Corros. Sci. 2007, 49, 2237–2253. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.C.; Bailey, C.G.; Taylor, A.P. Global modelling of fire protection performance of intumescent coating under different cone calorimeter heating conditions. Fire Saf. J. 2012, 50, 51–62. [Google Scholar] [CrossRef]
- Wang, Z.; Han, E.; Ke, W. Influence of nano-LDHs on char formation and fire-resistant properties of flame-retardant coating. Prog. Polym. Sci. 2005, 53, 29–37. [Google Scholar] [CrossRef]
- Gu, H.; Guo, J.; He, Q.; Tadakamalla, S.; Zhang, X.; Yan, X.; Huang, Y.; Colorado, H.A.; Wei, S.; Guo, Z. Flame-Retardant Epoxy Resin Nanocomposites Reinforced with Polyaniline-Stabilized Silica Nanoparticles. Ind. Eng. Chem. Res. 2013, 52, 7718–7728. [Google Scholar] [CrossRef]
- Amir, N.; Ahmad, F.; Megat Yusoff, P.S.M. Char Strength of Wool Fibre Reinforced Epoxy-Based Intumescent Coatings (FRIC). Adv. Mater. Res. 2013, 626, 504–508. [Google Scholar] [CrossRef]
- Toldy, A.; Szolnoki, B.; Marosi, G. Flame retardancy of fibre-reinforced epoxy resin composites for aerospace applications. Polym. Degrad. Stab. 2011, 96, 371–376. [Google Scholar] [CrossRef]
- ISO 5660-1, Reaction-to-Fire Tests‒Heat Release, Smoke Production and Mass Loss Rate‒Part. 1: Heat Release Rate (Cone Calorimeter Method); Internationan Organization for Standardization: Geneva, Switzerland, 2002.
- Park, S.B.; Lee, M.; Son, D.W.; Lee, S.M.; Kim, J.I. Fire performance of carbonized medium density fiberboard manufactured at different temperatures. J. Wood Sci. 2014, 60, 74–79. [Google Scholar] [CrossRef]
- Mariappan, T. Recent developments of intumescent fire protection coatings for structural steel: A review. J. Fire Sci. 2016, 34, 120–163. [Google Scholar] [CrossRef]
- JIS A 1321:1994, Testing Method for Incombustibility of Internal Finish Material and Procedure of Buildings; Japanese Standards Association: Tokyo, Japan, 1994.
- Wang, L.L.; Wang, Y.C.; Yuan, J.F.; Li, G.Q. Thermal conductivity of intumescent coating char after accelerated aging. Fire Mater. 2013, 37, 440–456. [Google Scholar] [CrossRef]
- Li, G.X.; Yang, J.F.; He, T.S.; Wu, Y.H.; Liang, G.Z. An Investigation of the thermal degradation of the intumescent coating containing MoO3 and Fe2O3. Surf. Coat. Technol. 2008, 202, 3121–3128. [Google Scholar] [CrossRef]
- Davies, P.J.; Horrocks, A.R.; Miraftab, M. Scanning electron microscopic studies of wool/intumescent char formation. Polym. Inter. 2000, 49, 1125–1132. [Google Scholar] [CrossRef]
- Zhang, R.; Xiao, X.; Tai, Q.; Huang, H.; Hu, Y. Modification of lignin and its application as char agent in intumescent flame-retardant poly(lactic acid). Polym. Eng. Sci. 2012, 52, 2620–2626. [Google Scholar] [CrossRef]
- Pan, H.; Wang, W.; Pan, Y.; Song, L.; Hu, Y.; Liew, K.M. Formation of self-extinguishing flame retardant biobased coating on cotton fabrics via Layer-by-Layer assembly of chitin derivatives. Carbohyd. Polym. 2015, 115, 516–524. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, X.; Meng, Y.; Gu, Z.; Bao, C.; Ding, X.; Li, S.; Chen, X.; Tian, X. Intumescent flame retardant coatings on cotton fabric of chitosan and ammonium polyphosphate via layer-by-layer assembly. Surf. Coat. Tech. 2015, 262, 9–14. [Google Scholar] [CrossRef]
- Lai, X.; Qiu, J.; Li, H.; Zhou, R.; Xie, H.; Zeng, X. Thermal degradation and combustion behavior of novel intumescent flame retardant polypropylene with N-alkoxy hindered amine. J. Anal. Appl. Pyrol. 2016, 120, 361–370. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Wu, M.; He, Y.; Wu, Y.; Qu, W. The Evolution of Intumescent Char in Flame-Retardant Coatings Based on Amino Resin. Coatings 2021, 11, 709. https://doi.org/10.3390/coatings11060709
Song W, Wu M, He Y, Wu Y, Qu W. The Evolution of Intumescent Char in Flame-Retardant Coatings Based on Amino Resin. Coatings. 2021; 11(6):709. https://doi.org/10.3390/coatings11060709
Chicago/Turabian StyleSong, Wei, Muting Wu, Yanrong He, Yuzhang Wu, and Wei Qu. 2021. "The Evolution of Intumescent Char in Flame-Retardant Coatings Based on Amino Resin" Coatings 11, no. 6: 709. https://doi.org/10.3390/coatings11060709
APA StyleSong, W., Wu, M., He, Y., Wu, Y., & Qu, W. (2021). The Evolution of Intumescent Char in Flame-Retardant Coatings Based on Amino Resin. Coatings, 11(6), 709. https://doi.org/10.3390/coatings11060709





