Aspects of Coatings on Buckypaper as a Study into the Expected Effects of Coatings on Carbon Nanotube Wires
Abstract
:1. Introduction
2. Materials and Methods
- A solution of 0.0540 g of SWCNTs and 100 mL of N, N-Dimethylformamide obtained from Sigma Aldrich® (St. Louis, MO, USA) is prepared.
- The solution is then sonicated for 45 min with a tip sonicator with a 45% amplitude and a pulse rate of 2 s on and 2 s off.
- As soon as the sonication is over, 50 mL of isopropanol are poured and allowed to sit for 15 min on a filtering setup consisting of a 1000 mL vacuum filtering flask, a rubber stopper, a glass support base, a 300 mL glass funnel, a clamp and a polytetrafluoroethylene membrane filter with a 47 mm diameter and 0.45 µm pore size from Sartorius Stedim Biotech GmbH (Göttingen, Germany).
- The membrane is taken from the setup and the BP is carefully peeled and placed on a Petri dish. It is then placed in the oven at 80 °C for 30 min.
- –
- Glyptal® 1201 Red Enamel (Glyptal®, Chelsea, MA, USA, Xylene solvent, 60% weight of solids).
- –
- Glyptal® 1202 Insulating Varnish (Glyptal®, Chelsea, MA, USA, Xylene solvent, 50% weight of solids).
- –
- 635 Thin Epoxy Resin and 635 Epoxy Hardener from US Composites® (West Palm Beach, FL, USA) in a 2:1 ratio.
- –
- Epolene® wax from Westlake Chemicals (Houston, TX, USA).
3. Results and Discussion
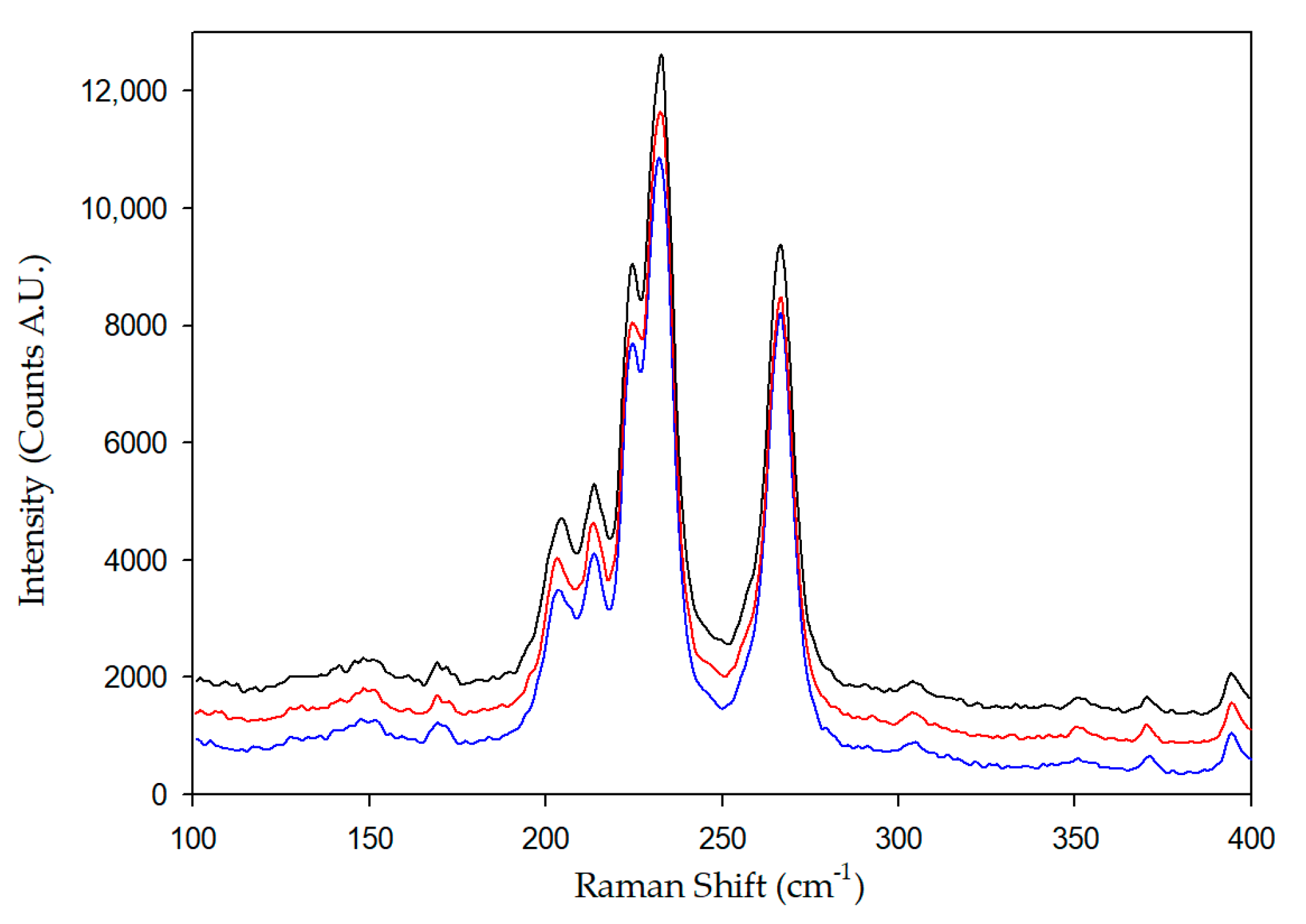
3.1. Raman Spectroscopy
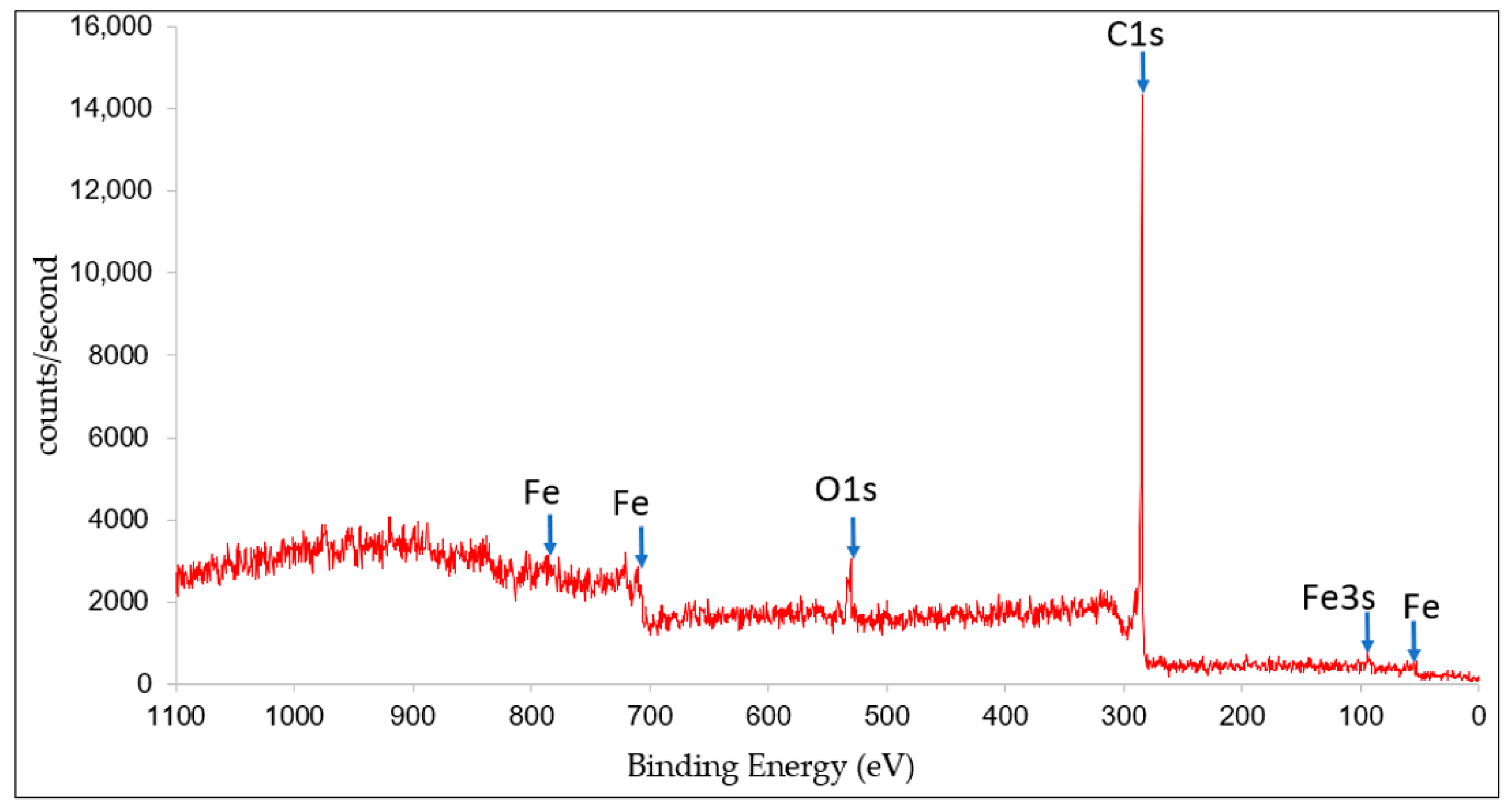
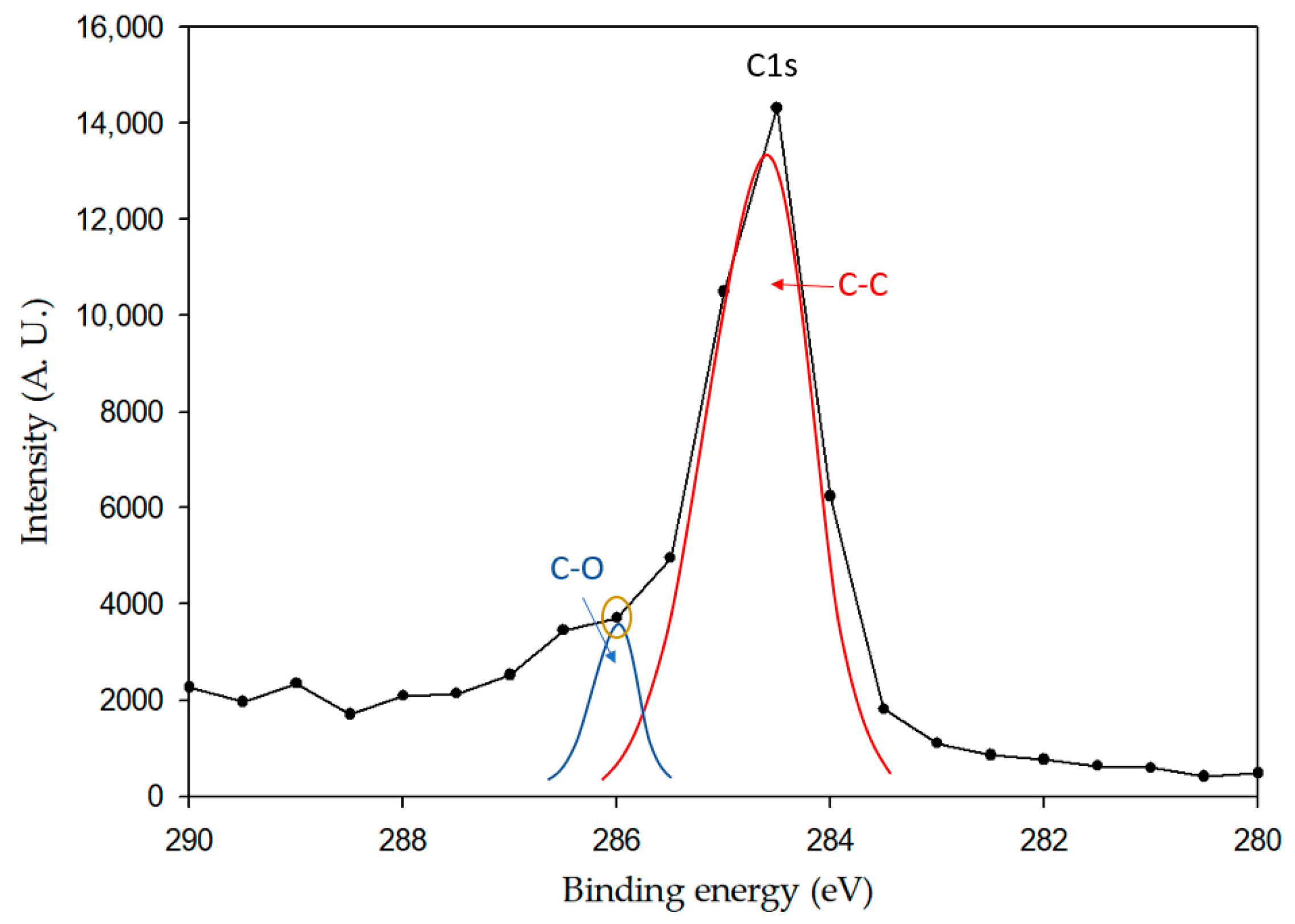
3.2. X-ray Photoelectron Spectroscopy (XPS)
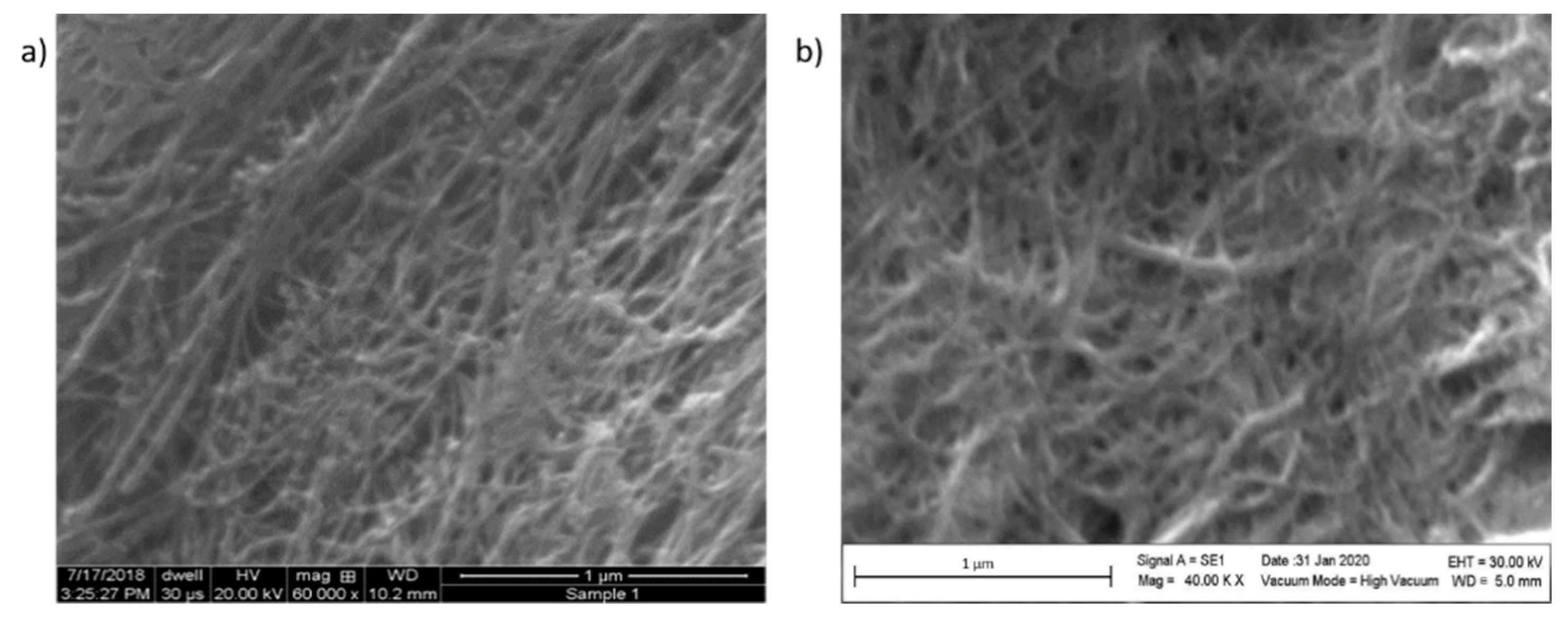
3.3. Surface Comparison
3.4. Nitrogen Physisorption
3.5. Wettability
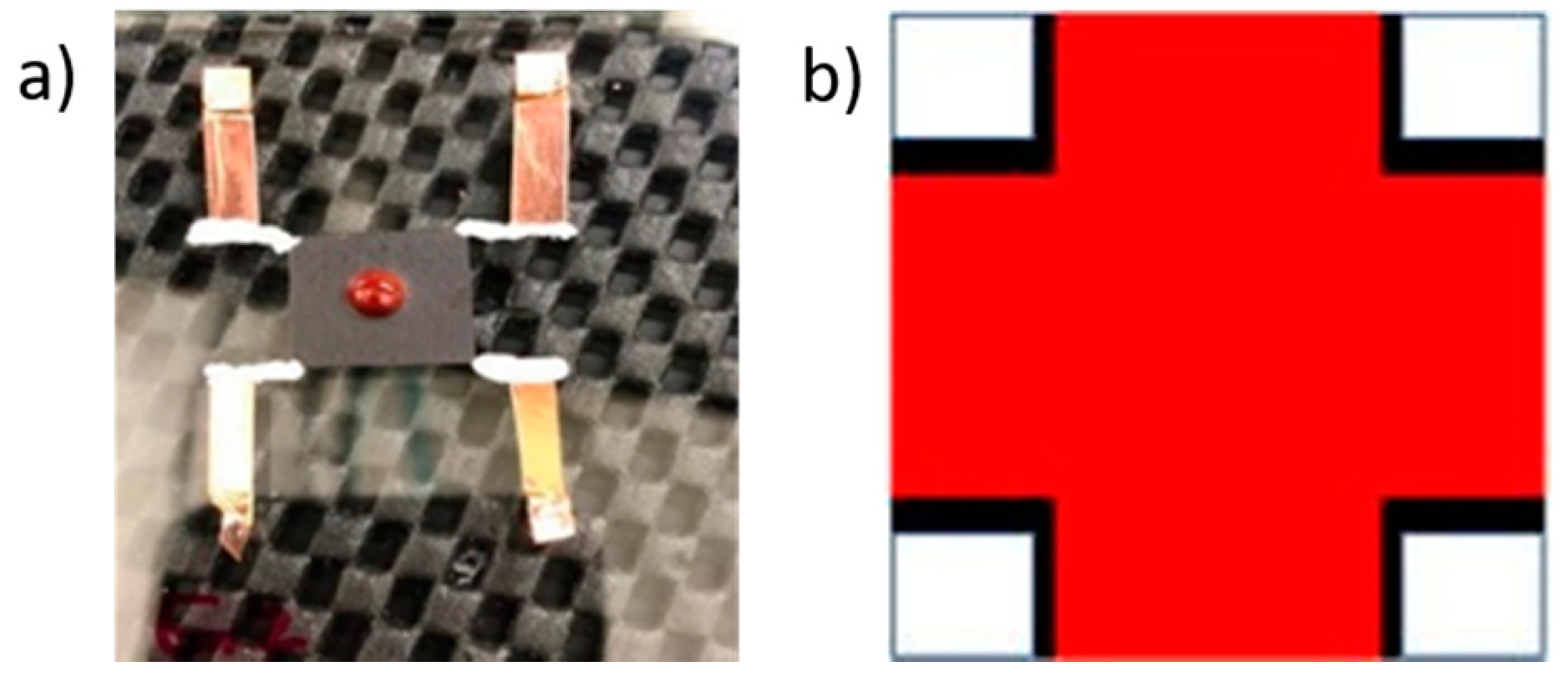
3.6. Electric Conductivity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ayyagari, S.; Al-Haik, M.; Rolin, V. Mechanical and Electrical Characterization of Carbon Fiber/Bucky Paper/Zinc Oxide Hybrid Composites. C J. Carbon Res. 2018, 4, 6. [Google Scholar] [CrossRef] [Green Version]
- Chu, K.; Kim, D.; Sohn, Y.; Lee, S.; Moon, C.; Park, S. Electrical and Thermal Properties of Carbon-Nanotube Composite for Flexible Electric Heating-Unit Applications. IEEE Electron Device Lett. 2013, 34, 668–670. [Google Scholar] [CrossRef]
- Zubair, A.; Tsentalovich, D.E.; Young, C.C.; Heimbeck, M.S.; Everitt, H.O.; Pasquali, M.; Kono, J. Carbon nanotube fiber terahertz polarizer. Appl. Phys. Lett. 2016, 108. [Google Scholar] [CrossRef]
- Kim, S.; Haines, C.S.; Li, N.; Kim, K.J.; Mun, T.J.; Choi, C.; Baughman, R.H. Harvesting electrical energy from carbon nanotube yarn twist. Science 2017, 357, 773–778. [Google Scholar] [CrossRef] [Green Version]
- Faraby, H.M.; Rao, A.M.; Bandaru, P.R. Modeling high energy density electrical inductors operating at thz frequencies based on coiled carbon nanotubes. IEEE Electron Device Lett. 2013, 34, 807–809. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, J.; Vajtai, R.; Ajayan, P.M.; Barrera, E.V. Iodine doped carbon nanotube cables exceeding specific electrical conductivity of metals. Sci. Rep. 2011, 1, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wang, J.; Fuentes, C.A.; Zhang, D.; Van Vuure, A.W.; Seo, J.W.; Seveno, D. Wettability of carbon nanotube fibers. Carbon 2017, 122, 128–140. [Google Scholar] [CrossRef]
- Janas, D.; Herman, A.P.; Boncel, S.; Koziol, K.K. Iodine monochloride as a powerful enhancer of electrical conductivity of carbon nanotube wires. Carbon 2014, 73, 225–233. [Google Scholar] [CrossRef]
- Qiu, J.; Terrones, J.; Vilatela, J.J.; Vickers, M.E.; Elliott, J.A.; Windle, A.H. Liquid infiltration into carbon nanotube fibers: Effect on structure and electrical properties. ACS Nano 2013, 7, 8412–8422. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Borowiak-Palen, E.; Kruszynska, M.; Bachmatiuk, A.; Kalenczuk, R.J. Characterization of carbon nanotubes by raman spectroscopy. Mater. Sci. Pol. 2008, 26, 433–441. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Pore Size Analysis by Gas Adsorption and the Density Functional Theory. Available online: https://www.azom.com/article.aspx?ArticleID=5189 (accessed on 15 January 2020).
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87. [Google Scholar] [CrossRef] [Green Version]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Dujardin, E.; Ebbesen, T.W.; Krishnan, A.; Treacy, M.M. Wetting of single shell carbon nanotubes. Adv. Mater. 1998, 10, 1472–1475. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- McHale, G. Cassie and Wenzel: Were they really so wrong? Langmuir 2007, 23, 8200–8205. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Resistivity and Hall Measurements. Available online: https://www.nist.gov/pml/engineering-physics-division/popular-links/hall-effect/resistivity-and-hall-measurements (accessed on 30 September 2019).

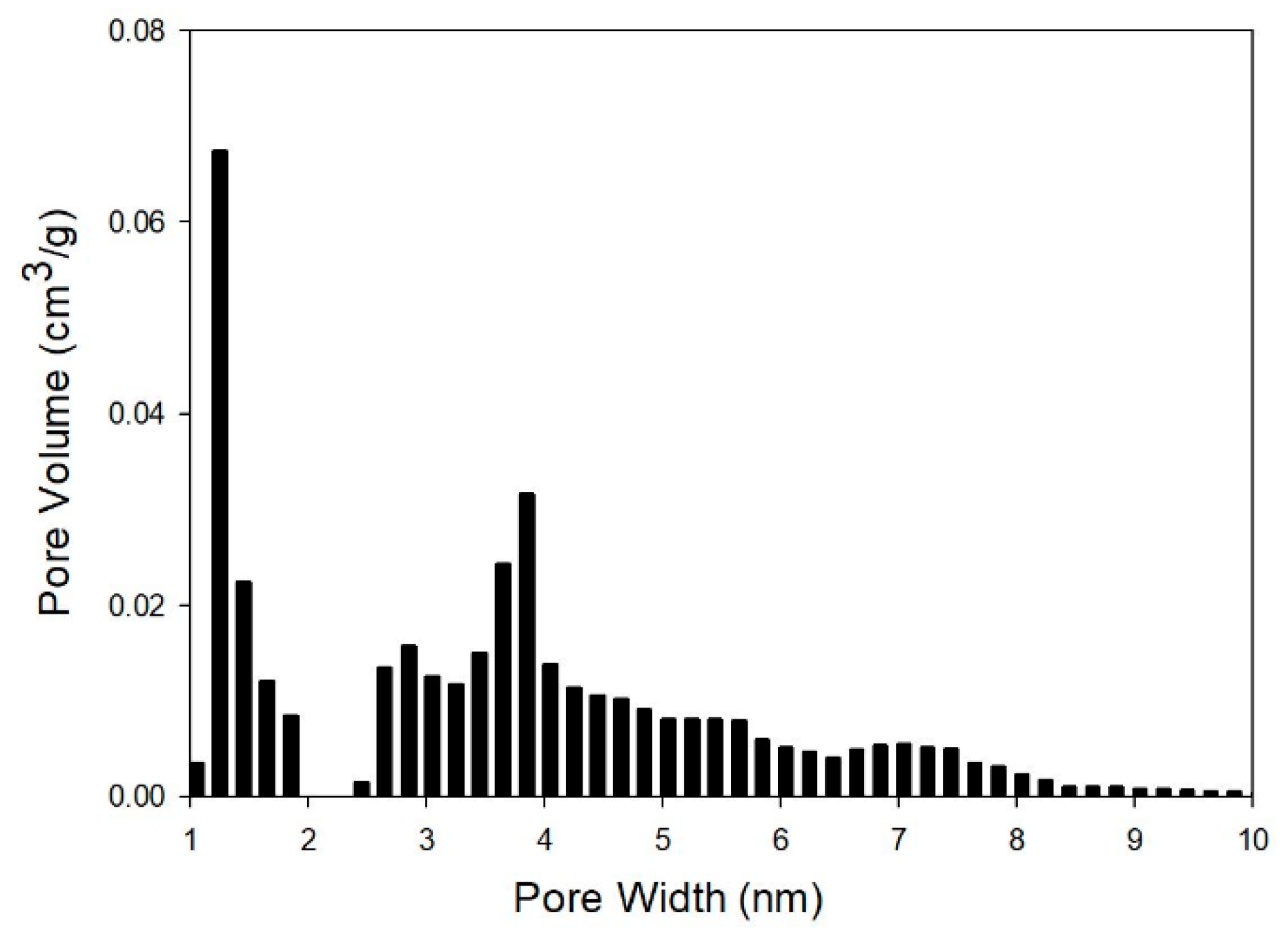
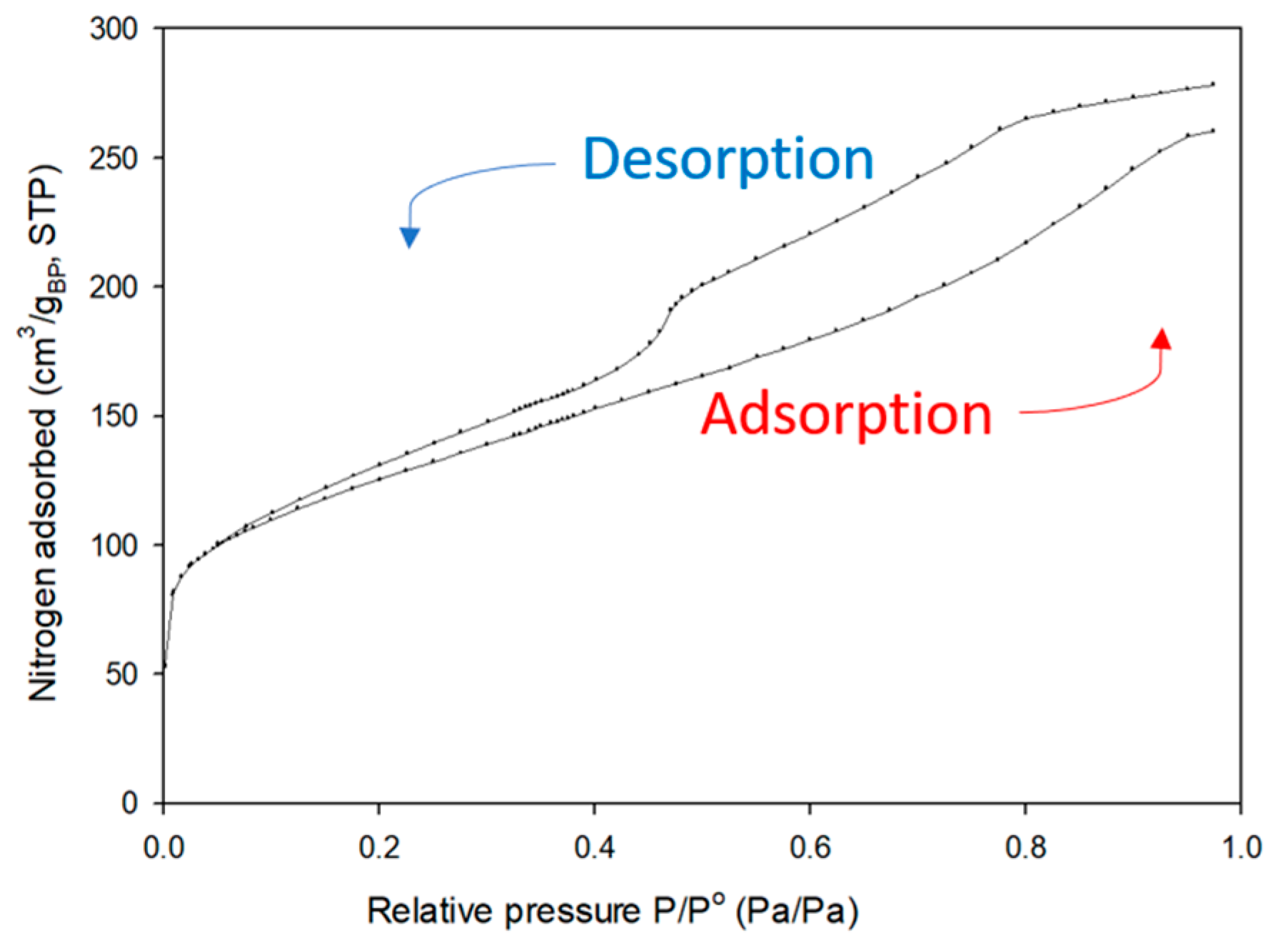

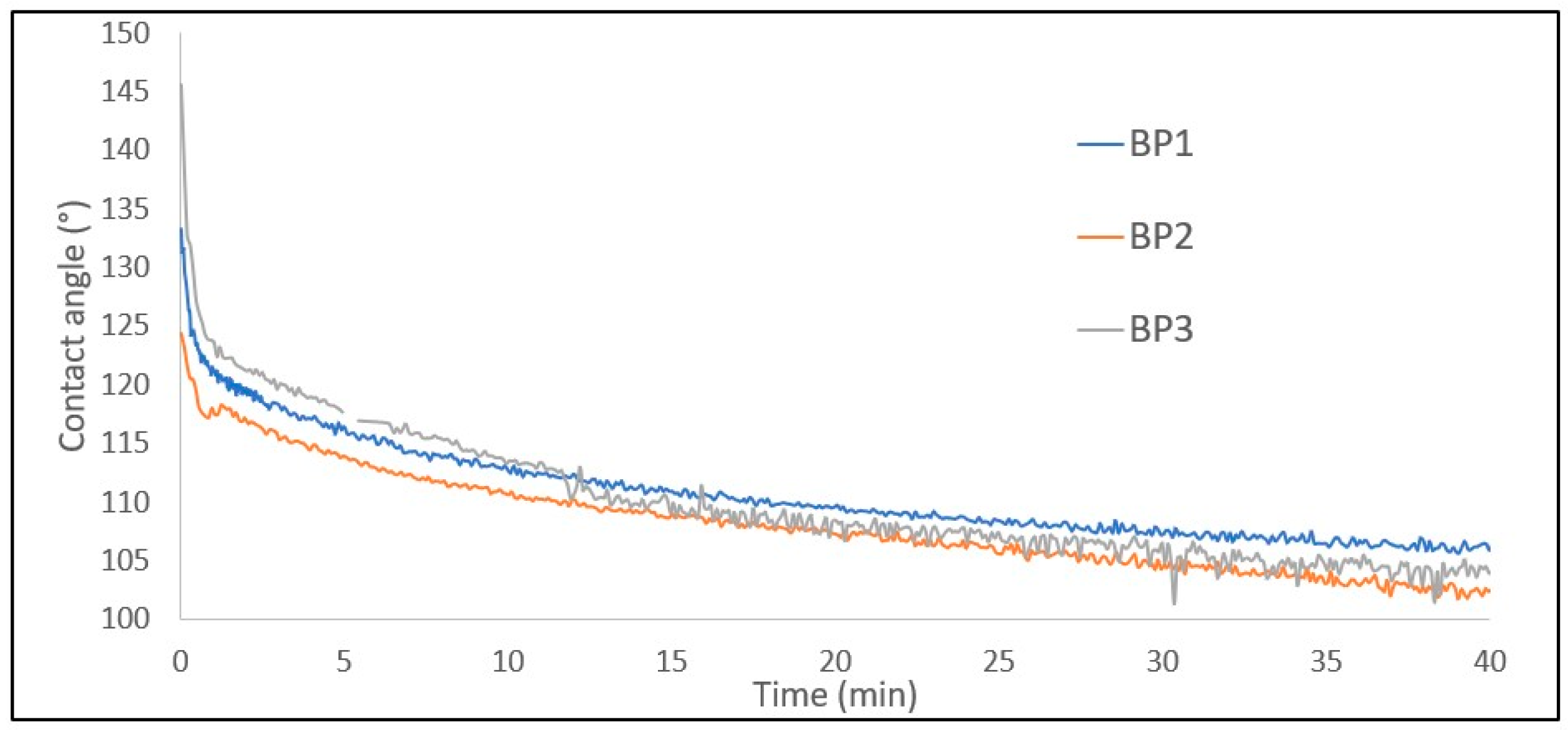
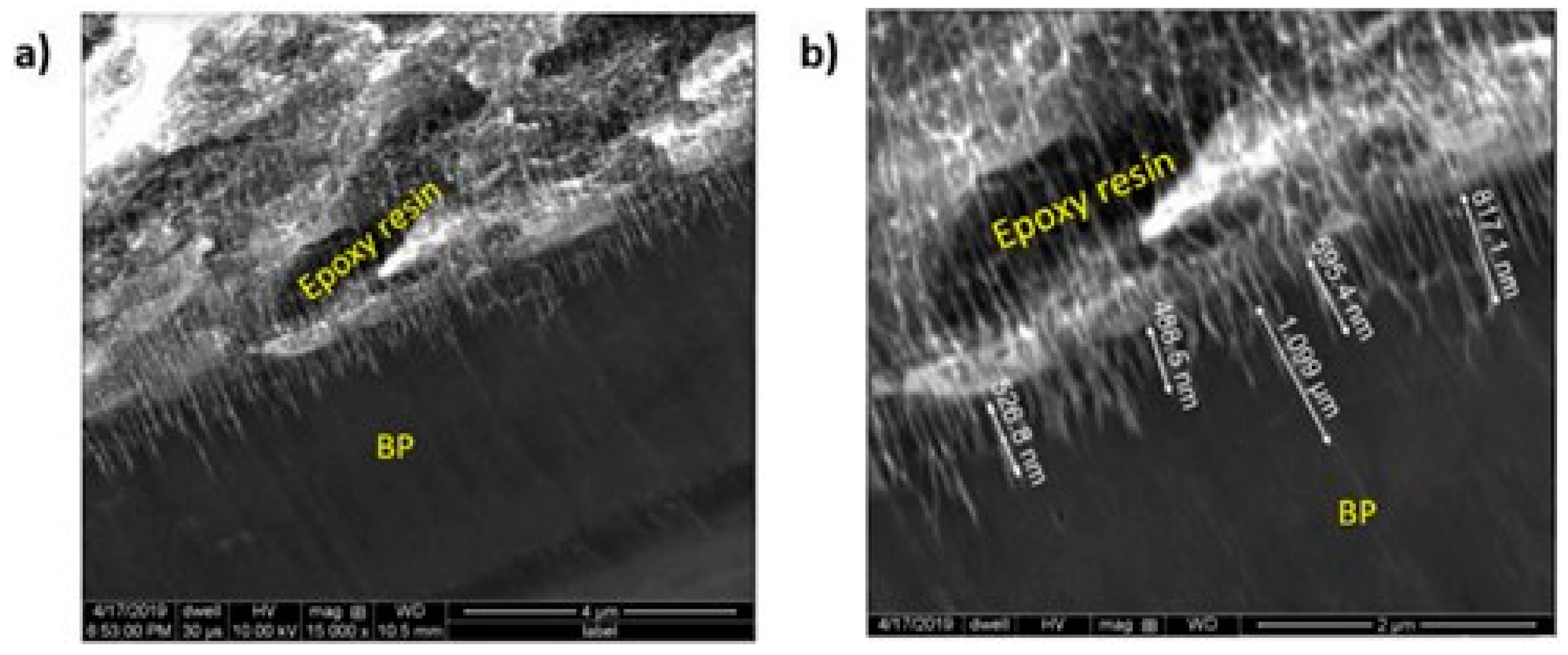
| Method | Surface Area (m2/gBP) | Pore Volume (cm3/gBP) | Pore width (nm) | Porosity (%) |
|---|---|---|---|---|
| BET | 447 | - | - | - |
| DFT | 312 | 0.4 | 1.18 | 30 |
| BJH | 247 | 0.3 | 3.74 | 23 |
| Coating | Surface Tension (mN/m) |
|---|---|
| Insulating Varnish | 29.75 |
| Red Enamel | 36.39 |
| Epoxy Resin with hardener | 34.47 |
| Coating | CA0(°) | CA5(°) | CAAvg(°) |
|---|---|---|---|
| Insulating Varnish | 118.59 | 100.05 | 109.32 |
| Red Enamel | 131.84 | 114.82 | 123.33 |
| Epoxy Resin | 42.95 | 31.01 | 36.98 |
| Coating | γsl | Wa | S | ∆F |
|---|---|---|---|---|
| Red Enamel | 72.43 | 23.59 | −49.19 | −12.80 |
| Insulating Varnish | 60.73 | 28.65 | −30.85 | −1.10 |
| Epoxy resin | 32.09 | 62.01 | −6.93 | 27.54 |
| Coating | Temperature | Time |
|---|---|---|
| Insulating Varnish | 100 °C | 3 h |
| Red Enamel | 100 °C | 3 h |
| Epoxy Resin | 40 °C | 5 h |
| Wax | 25 °C | 8 h |
| Sample | σ Day 0 (S/m) | σ Day 7 (S/m) |
|---|---|---|
| Control | 2.27 × 104 | 2.82 × 104 |
| Varnish | 2.51 × 104 | 2.27 × 104 |
| Epoxy | 2.73 × 104 | 0.96 × 104 |
| Enamel | 2.78 × 104 | 2.50 × 104 |
| Wax | 2.54 × 104 | 1.53 × 104 |
| Parameter | Red Enamel | Varnish | Epoxy Resin |
|---|---|---|---|
| γsl | 72.43 | 60.73 | 32.09 |
| Wa | 23.59 | 28.65 | 62.01 |
| S | −49.19 | −30.85 | −6.93 |
| ∆F | −12.80 | −1.10 | 27.54 |
| ∆σ | −10.20% | −9.31% | −64.99% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arano, J.; Bonilla-Rios, J.; Barrera, E.; Isenhart, L. Aspects of Coatings on Buckypaper as a Study into the Expected Effects of Coatings on Carbon Nanotube Wires. Coatings 2021, 11, 743. https://doi.org/10.3390/coatings11070743
Arano J, Bonilla-Rios J, Barrera E, Isenhart L. Aspects of Coatings on Buckypaper as a Study into the Expected Effects of Coatings on Carbon Nanotube Wires. Coatings. 2021; 11(7):743. https://doi.org/10.3390/coatings11070743
Chicago/Turabian StyleArano, Jafet, Jaime Bonilla-Rios, Enrique Barrera, and Lucas Isenhart. 2021. "Aspects of Coatings on Buckypaper as a Study into the Expected Effects of Coatings on Carbon Nanotube Wires" Coatings 11, no. 7: 743. https://doi.org/10.3390/coatings11070743





