Preparation and Application of Efficient Biobased Carbon Adsorbents Prepared from Spruce Bark Residues for Efficient Removal of Reactive Dyes and Colors from Synthetic Effluents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of BBCs
2.2. BBC Characterization
2.2.1. Textural Properties
2.2.2. Elemental Analysis, Yield (%), Raman Spectroscopy, and Zeta Potential
2.2.3. Water Vapor Sorption and Hydrophobicity/Hydrophilicity
2.3. Dye Adsorption Analysis
2.3.1. Batch Adsorption Studies
2.3.2. Adsorption Kinetics and Equilibrium Analysis
2.3.3. Preparation of the Dyeing Synthetic Effluents
2.3.4. Analytical Control of the Measurements and Statistical Evaluation of Nonlinear Models
3. Results and Discussion
3.1. BBC Characteristics
3.1.1. Textural Properties and Porosity
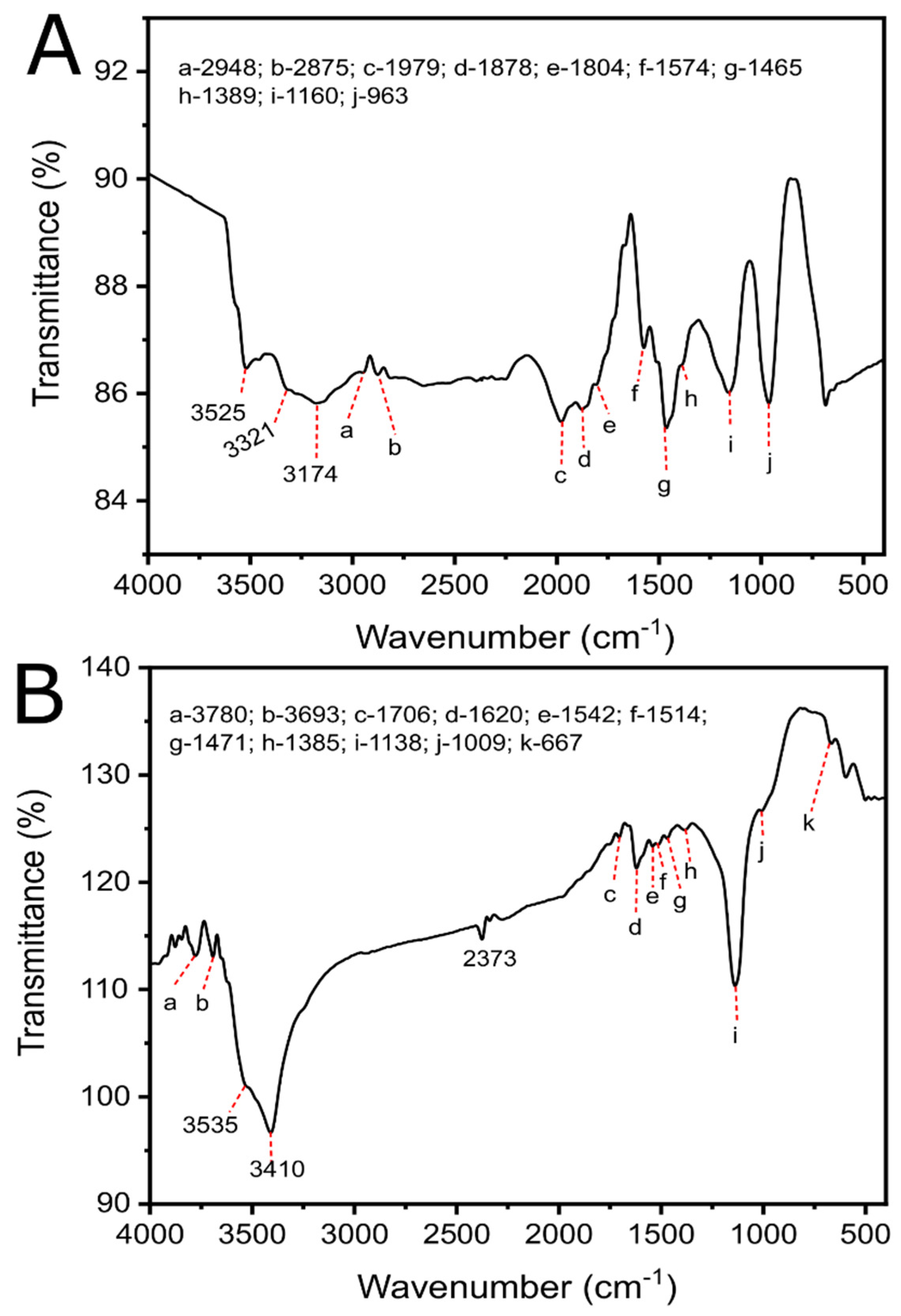
3.1.2. Elemental Analysis, Carbon Yield, Raman Spectroscopy, Zeta-Potential, and FTIR
3.1.3. Water Vapor Adsorption Isotherms, Hydrophilicity Index (HI)
3.2. Dye Adsorption Analysis
Adsorption Kinetics
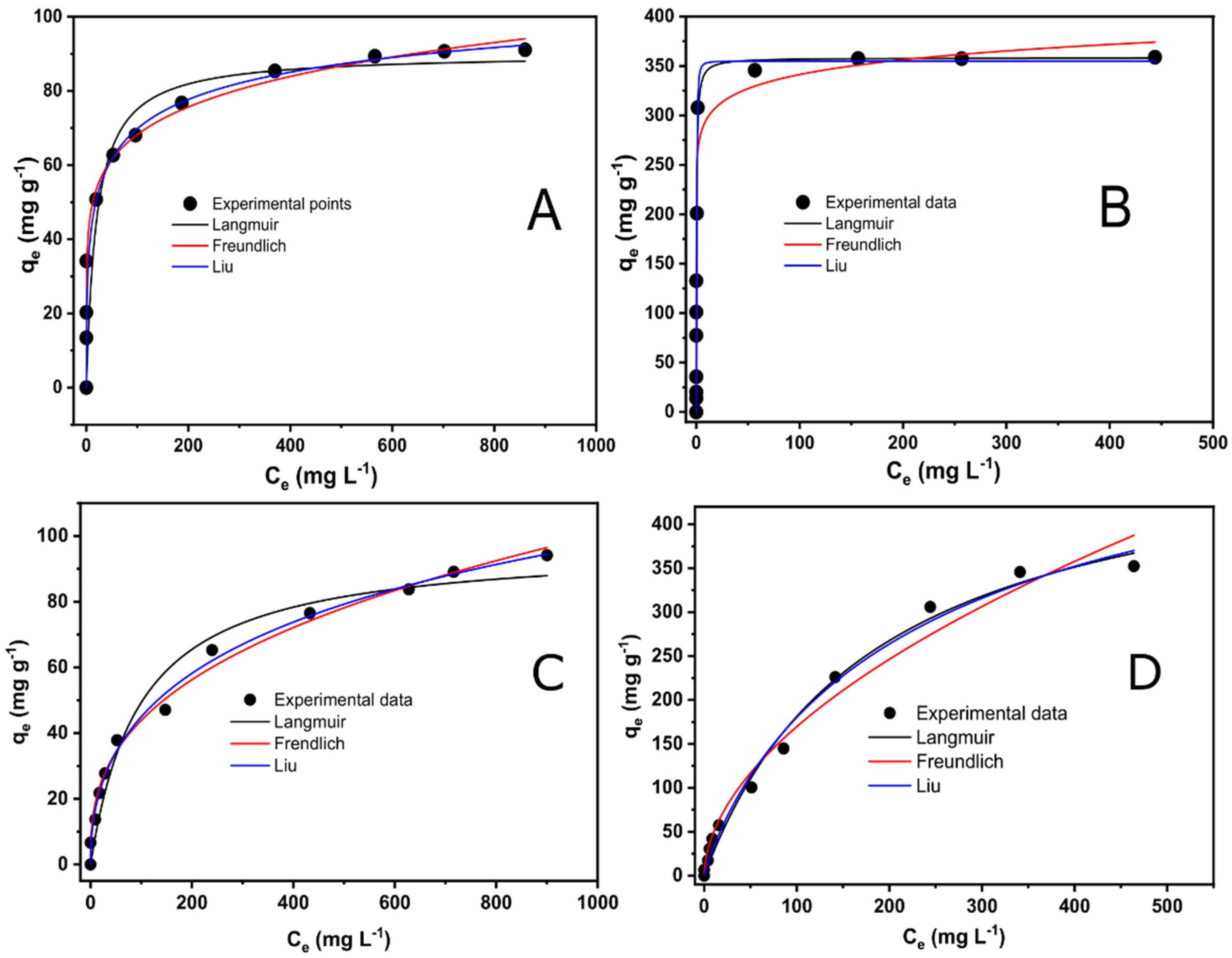
3.3. Equilibrium of Adsorption
3.4. Mechanism of Adsorption
3.5. Adsorbent Performance: Comparison with Literature
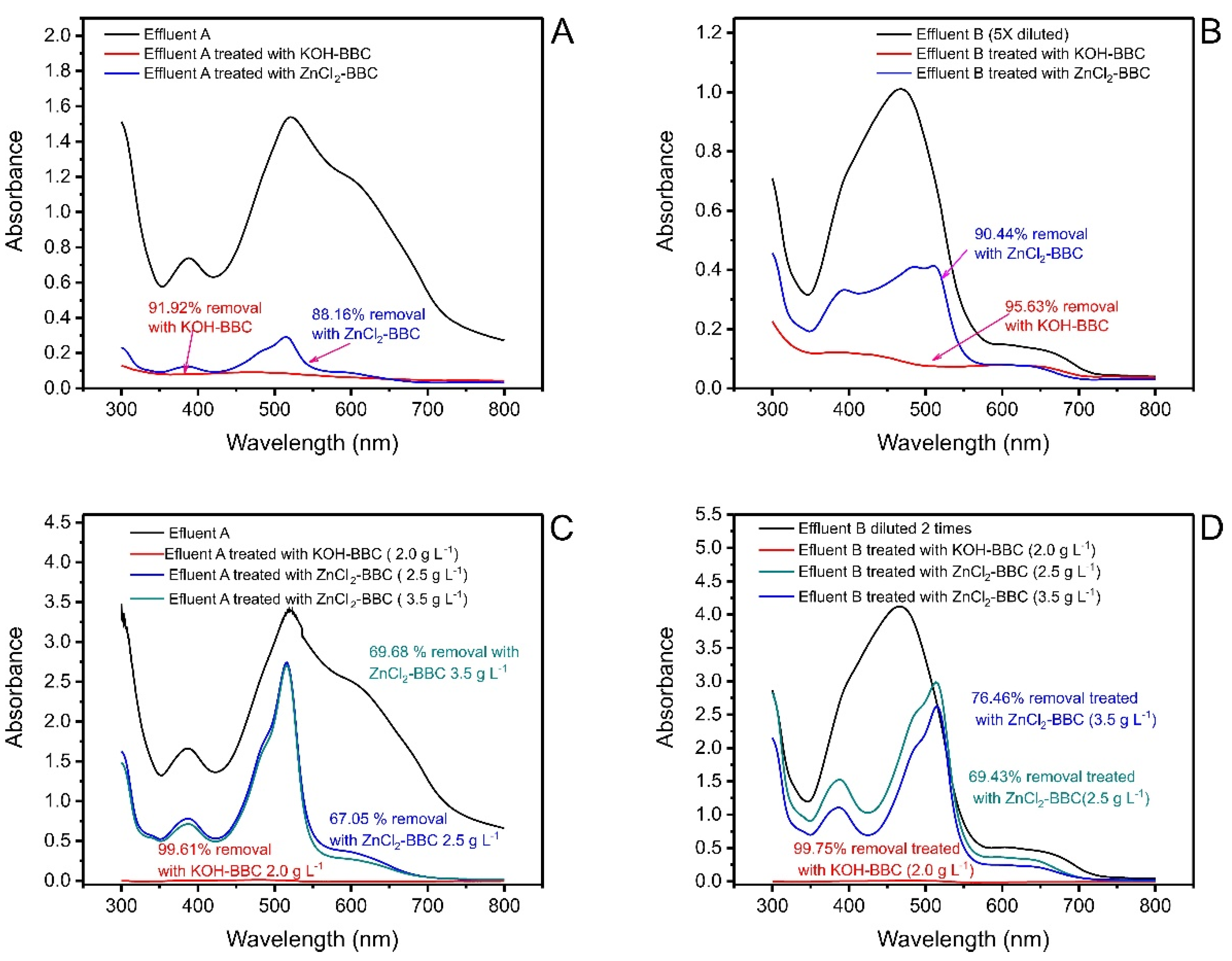
3.6. Treatment of Synthetic Dye Effluents
4. Possible Application of Used BBC after Adsorption of Dyes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Woerden, V.F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; International Bank for Reconstruction and Development; The World Bank: Washington, DC, USA, 2018. [Google Scholar]
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316. [Google Scholar] [CrossRef]
- Hira, S.A.; Yusuf, M.; Annas, D.; Hui, H.S.; Park, K.H. Biomass-Derived Activated Carbon as a Catalyst for the Effective Degradation of Rhodamine B dye. Processes 2020, 8, 926. [Google Scholar] [CrossRef]
- Aldea, J.; Ruiz-Peinado, R.; del Río, M.; Pretzsch, H.; Heym, M.; Brazaitis, G.; Jansons, A.; Metslaid, M.; Barbeito, I.; Bielak, K.; et al. Species stratification and weather conditions drive tree growth in Scots pine and Norway spruce mixed stands along with Europe. Ecol. Manag. 2021, 481, 118697. [Google Scholar] [CrossRef]
- Anerud, E.; Routa, J.; Bergström, D.; Eliasson, L. Fuel quality of stored spruce bark—Influence of semi-permeable covering material. Fuel 2020, 279, 118467. [Google Scholar] [CrossRef]
- Corsi, I.; Winther-Nielsenb, M.; Sethic, R.; Punta, C.; Della Torre, D.; Libralato, G.; Lofrano, G.; Sabatini, L.; Aiello, M.; Fiordi, L.; et al. Ecofriendly nanotechnologies and nanomaterials for environmental applications: Key issue and consensus recommendations for sustainable and eco-safe nano remediation. Ecotoxicol. Environ. Saf. 2018, 154, 237–244. [Google Scholar] [CrossRef]
- Marsh, H.; Reinoso, F.R. Activated Carbon; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Siipola, V.; Pflugmacher, S.; Romar, H.; Wendling, L.; Koukkari, P. Low-Cost Biochar Adsorbents for Water Purification Including Microplastics Removal. Appl. Sci. 2020, 10, 788. [Google Scholar] [CrossRef] [Green Version]
- Varila, T.; Brännström, H.; Kilpeläinen, P.; Hellström, J.; Romar, H.; Nurmi, J.; Lassi, U. From Norway spruce bark to carbon foams: Characterization and applications. BioResources 2020, 15, 3651–3666. [Google Scholar]
- Thue, P.S.; Umpierres, C.S.; Lima, E.C.; Lima, D.R.; Machado, F.M.; Reis, G.S.d.; Silva, R.S.; Pavan, F.A.; Tran, H.N. Single-step pyrolysis for producing magnetic activated carbon from tucumã (Astrocaryum aculeatum) seed and nickel(II) chloride and zinc(II) chloride. Application for removal of nicotinamide and propranolol. J. Hazard. Mater. 2020, 398, 122903. [Google Scholar] [CrossRef]
- He, X.; Zhu, J.; Wang, H.; Zhou, M.; Zhang, S. Surface Functionalization of Activated Carbon with Phosphonium Ionic Liquid for CO2 Adsorption. Coatings 2019, 9, 590. [Google Scholar] [CrossRef] [Green Version]
- Fröhlich, A.C.; dos Reis, G.S.; Pavan, F.A.; Lima, E.C.; Foletto, E.L.; Dotto, G.L. Improvement of activated carbon characteristics by sonication and its application for pharmaceutical contaminant adsorption. Environ. Sci. Pollut. Res. 2018, 25, 24713–24725. [Google Scholar] [CrossRef]
- Reis, G.S.d.; Adebayo, M.A.; Lima, E.C.; Sampaio, C.H.; Prola, L.D.T. Activated carbon from sewage sludge for preconcentration of copper. Anal. Lett. 2016, 49, 541–555. [Google Scholar] [CrossRef]
- Reis, G.S.D.; Wilhelm, M.; Silva, T.C.A.; Rezwan, K.; Sampaio, C.H.; Lima, E.C.; Souza, S.M.A.G.U. The use of the design of experiments for the evaluation of the production of surface-rich activated carbon from sewage sludge via microwave and conventional pyrolysis. Appl. Therm. Eng. 2016, 93, 590. [Google Scholar] [CrossRef]
- Kasperiski, F.M.; Lima, E.C.; Umpierres, C.S.; Reis, G.S.d.; Thue, P.S.; Lima, D.R.; Dias, S.L.P.; Saucier, C.; da Costa, J.B. Production of porous activated carbons from Caesalpinia ferrea seed pod wastes: Highly efficient removal of captopril from aqueous solutions. J. Clean. Prod. 2018, 197, 919–929. [Google Scholar] [CrossRef]
- Cunha, M.R.; Lima, E.C.; Cimirro, N.F.G.M.; Thue, P.S.; Dias, S.L.P.; Gelesky, M.A.; Dotto, G.L.; Reis, G.S.d.; Pavan, F.A. Conversion of Eragrostis plana Nees leaves to activated carbon by microwave-assisted pyrolysis for the removal of organic emerging contaminants from aqueous solutions. Environ. Sci. Pollut. Res. 2018, 25, 23315–23327. [Google Scholar] [CrossRef] [Green Version]
- Leite, A.B.; Saucier, C.; Lima, E.C.; dos Reis, G.S.; Umpierres, C.S.; Mello, B.L.; Shirmardi, M.; Dias, S.L.P.; Sampaio, C.H. Activated carbons from avocado seed: Optimisation and application for removal several emerging organic compounds. Environ. Sci. Pollut. Res. 2018, 25, 7647–7661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chequer, F.M.D.; de Oliveira, G.A.R.; Anastacio Ferraz, E.R.; Carvalho, J.; Zanoni, M.V.B.; Oliveira, D.P. Textile dyes: Dyeing process and environmental impact. In Eco-Friendly Textile Dyeing and Finishing; Gunay, M., Ed.; InTech: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, P.A.; Umbuzeiro, G.A.; Oliveira, D.P.; Zanoni, M.V.B. Assessment of water contamination caused by a mutagenic textile effluent/dyehouse effluent bearing disperse dyes. J. Hazard. Mater. 2010, 174, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Lima, V.V.C.; Dalla Nora, F.B.; Peres, E.C.; Reis, G.S.; Lima, É.C.; Oliveira, M.L.S.; Dotto, G.L. Synthesis and characterization of biopolymers functionalized with APTES (3–aminopropyltriethoxysilane) for the adsorption of sunset yellow dye. J. Environ. Chem. Eng. 2019, 7, 103410. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Adebayo, M.A.; Sampaio, C.H.; Lima, E.C.; Thue, P.S.; de Brum, I.A.S.; Dias, S.l.P.; Pavan, F.A. Removal of Phenolic Compounds from Aqueous Solutions Using Sludge-Based Activated Carbons Prepared by Conventional Heating and Microwave-Assisted Pyrolysis. Water Air Soil Pollut 2017, 228, 33. [Google Scholar] [CrossRef]
- Umpierres, C.S.; Thue, P.S.; Lima, E.; dos Reis, G.S.; de Brum, I.A.S.; de Alencar, W.A.; Dias, S.L.P.; Dotto, G.L. Microwave-activated carbons from tucumã (Astrocaryum aculeatum) seed for efficient removal of 2-nitrophenol from aqueous solutions. Environ. Technol. 2018, 39, 1173–1187. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Lima, E.C.; Sampaio, C.H.; Rodembusch, F.S.; Petter, C.O.; Cazacliu, B.G.; Dotto, G.L.; Hidalgo, G.E.N. Novel kaolin/ polysiloxane based organic-inorganic hybrid materials: Sol−gel synthesis, characterization, and photocatalytic properties. J. Solid State Chem. 2018, 260, 106–116. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Thue, P.S.; Cazacliu, B.G.; Lima, E.C.; Sampaio, C.H.; Quattrone, M.; Ovsyannikova, E.; Kruse, A.; Dotto, G.L. Effect of concrete carbonation on phosphate removal through adsorption process and its potential application as fertilizer. J. Clean Prod. 2020, 256, 120416. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Cazacliu, B.G.; Correa, C.R.; Ovsyannikova, E.; Kruse, A.; Sampaio, C.H.; Lima, E.C.; Dotto, G.L. Adsorption and recovery of phosphate from aqueous solution by the construction and demolition wastes sludge and its potential use as phosphate-based fertilizer. J. Environ. Chem. Eng. 2020, 8, 103605. [Google Scholar] [CrossRef]
- Ma, Y. Comparison of Activated Carbons Prepared from Wheat Straw via ZnCl2 and KOH Activation. Waste Biomass Valor. 2017, 8, 549–559. [Google Scholar] [CrossRef]
- Ma, J.; Yang, H.; Li, L.; Xie, X.; Liu, B.; Zhang, L.; Zhang, L. Synthesis of aligned ZnO submicron rod arrays by heating zinc foil covered with ZnCl2 solution. Acta. Chimi. Sin. 2009, 67, 1515–1522. [Google Scholar]
- Song, X.; Ma, X.; Li, Y.; Ding, L.; Jiang, R. Tea waste-derived microporous active carbon with enhanced double-layer supercapacitor behaviors. Appl. Surf. Sci. 2019, 487, 189–197. [Google Scholar] [CrossRef]
- Efeovbokhan, V.E.; Alagbe, E.E.; Odika, B.; Babalola, R.; Oladimeji, T.E.; Abatan, O.G.; Yusuf, E.O. Preparation and characterization of activated carbon from plantain peel and coconut shell using biological activators. J. Phys. Conf. Ser. 2019, 1378, 032035. [Google Scholar] [CrossRef]
- Huang, G.G.; Liu, Y.F.; Wu, X.-X.; Cai, J.-J. Activated carbons prepared by the KOH activation of a hydrochar from garlic peel and their CO2 adsorption performance. New Carbon Mater. 2019, 34, 247–257. [Google Scholar] [CrossRef]
- Cuong, D.V.; Liu, N.-L.; Nguyen, V.A.; Hou, C.-H. Meso/micropore-controlled hierarchical porous carbon derived from activated biochar as a high-performance adsorbent for copper removal. Sci. Total Environ. 2019, 692, 844–853. [Google Scholar] [CrossRef]
- Lima, D.R.; Bandegharaei, A.H.; Thue, P.S.; Lima, E.C.; de Albuquerque, Y.R.T.; dos Reis, G.S.; Umpierres, C.S.; Dias, S.L.P.; Tran, H.N. Efficient acetaminophen removal from water and hospital effluents treatment by activated carbons derived from Brazil nutshells. Colloids Surface A 2019, 583, 123966. [Google Scholar] [CrossRef]
- Dos Reis, G.S.; Mahbub, M.K.B.; Wilhelm, M.; Lima, E.C.; Sampaio, C.H.; Saucier, C.; Dias, S.L.P. Activated carbon from sewage sludge for removal of sodium diclofenac and nimesulide from aqueous solutions. Korean J. Chem. Eng. 2016, 33, 3149–3161. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From coconut shell to porous graphene-like nanosheets for high-power supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Correa, C.R.; Otto, T.; Kruse, A. Influence of the biomass components on the pore formation of activated carbon. Biomass Bioenergy 2017, 97, 53–64. [Google Scholar] [CrossRef]
- Duan, X.-H.; Srinivasakannan, C.; Yang, K.-B.; Peng, J.-H.; Zhang, L.-B. Effects of Heating Method and Activating Agent on the Porous Structure of Activated Carbons from Coconut Shells. Waste Biomass Valor. 2012, 3, 131–139. [Google Scholar] [CrossRef]
- Ravichandran, P.; Sugumaran, P.; Seshadri, S.; Basta, A.H. Optimizing the route for the production of activated carbon from Casuarina equisetifolia fruit waste. R. Soc. Open Sci. 2018, 5, 171578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caturla, F.; Molina-Sabio, M.; Rodriguez-Reinoso, F. Preparation of activated carbon by chemical activation with ZnCl2. Carbon 1991, 29, 999–1007. [Google Scholar] [CrossRef]
- Ucar, S.; Erdem, M.; Tay, T.; Karago, S. Preparation and characterization of activated carbon produced from pomegranate seeds by ZnCl2 activation. Appl. Surf. Sci. 2009, 255, 8890–8896. [Google Scholar] [CrossRef]
- Xu, D.; Cao, J.; Li, L.; Howard, A.; Yu, K. Effect of pyrolysis temperature on characteristics of biochars derived from different feedstocks: A case study on ammonium adsorption capacity. Waste Manag. 2019, 87, 652–660. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPBBC technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Machado, F.M.; Bergmann, C.P.; Lima, E.C.; Royer, B.; de Souza, F.E.; Jauris, I.M.; Calvete, T.; Fagan, S.B. Adsorption of Reactive Blue 4 dye from water solutions by carbon nanotubes: Experiment and theory. Phys. Chem. Chem. Phys. 2012, 14, 11139–11153. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Ibrahim, M.H.; Adullah, A.Z.; Salamatinia, B.; Gholami, Z. Chitosan hydrogel beads impregnated with hexadecyl amine for improved reactive blue-4 adsorption. Carbo. Polym. 2016, 137, 139–146. [Google Scholar] [CrossRef]
- Dison, S.P.F.; Tanabe, E.H.; Bertuol, D.A.; dos Reis, G.S.; Lima, E.C.; Dotto, G.L. Alternative treatments to improve the potential of rice husk as adsorbent for methylene blue. Water Sci. Technol. 2017, 75, 296–305. [Google Scholar]
- Teixeira, R.A.; Lima, E.C.; Benetti, A.D.; Thue, P.S.; Cunha, M.R.; Cimirro, N.F.G.M.; Sher, F.; Dehghani, M.H.; dos Reis, G.S.; Dotto, G.L. Preparation of hybrids of wood sawdust with 3-aminopropyl-triethoxysilane. Application as an adsorbent to remove Reactive Blue 4 dye from wastewater effluents. J. Taiwan Inst. Chem. Eng. 2021. [Google Scholar] [CrossRef]
- Marrakchi, F.; Khandaya, W.A.; Asif, M.; Hameed, B.H. Cross-linked chitosan/sepiolite composite for the adsorption of methylene blue and reactive orange 16. Inter. J. Biol. Macro. 2016, 93, 1231–1239. [Google Scholar] [CrossRef]
- Marrakchi, F.; Ahmed, M.J.; Khanday, W.A.; Asif, M.; Hameed, B.H. Mesoporous carbonaceous material from fish scales as a low-cost adsorbent for reactive orange 16 adsorption. J. Taiwan Inst. Chem. Eng. 2017, 71, 47–54. [Google Scholar] [CrossRef]
- Calvete, T.; Lima, E.C.; Cardoso, N.F.; Vaghetti, J.C.P.; Dias, S.L.P.; Pavan, F.A. Application of carbon adsorbents prepared from Brazilian-pine fruit shell for the removal of reactive orange 16 from aqueous solution: Kinetic, equilibrium, and thermodynamic studies. J. Environ. Manag. 2010, 91, 1695–1706. [Google Scholar] [CrossRef]
- Akbar, A.A.M.; Karthikeyan, R.K.; Sentamil, S.M.; Mithilesh, K.R.; Madhangi, P.; Maheswari, N.; Janani, S.G.; Padmanaban, V.C.; Singh, R.S. Removal of Reactive Orange 16 by adsorption onto activated carbon prepared from rice husk ash: Statistical modelling and adsorption kinetics. Sep. Sci. Technol. 2020, 55, 26–34. [Google Scholar]
- Shah, J.A.; Butt, T.A.; Mirza, C.R.; Shaikh, A.J.; Khan, M.S.; Arshad, M.; Riaz, N.; Haroon, H.; Gardazi, S.M.H.; Yaqoo, K.; et al. Phosphoric Acid Activated Carbon from Melia azedarach Waste Sawdust for Adsorptive Removal of Reactive Orange 16: Equilibrium Modelling and Thermodynamic Analysis. Molecules 2020, 25, 2118. [Google Scholar] [CrossRef] [PubMed]
- Malakootian, M.; Heidari, M.R. Reactive orange 16 dye adsorption from aqueous solutions by psyllium seed powder as a low-cost biosorbent: Kinetic and equilibrium studies. Appl. Water Sci. 2018, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Auta, M.; Hameed, B.H. Optimized and functionalized paper sludge activated with potassium fluoride for single and binary adsorption of reactive dyes. J. Ind. Eng. Chem. 2014, 20, 830–840. [Google Scholar] [CrossRef]
- Ramachandran, P.; Vairamuthu, R.; Ponnusamy, S. Adsorption isotherms, kinetics, thermodynamics and desorption studies of reactive orange 16 on activated carbon derived from Ananas comosus (L.) carbon. J. Eng. Appl. Sci. 2011, 6, 15–22. [Google Scholar]
- Won, S.W.; Choi, S.B.; Yun, Y.S. Performance and mechanism in the binding of reactive orange 16 to various types of sludge. Biochem. Eng. J. 2006, 28, 208–214. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Rahman, N.K. Equilibrium, kinetics and thermodynamic of Remazol Brilliant Orange 3R dye adsorption on coffee husk-based activated carbon. Chem. Eng. J. 2011, 170, 154–161. [Google Scholar] [CrossRef]
- Deshuai, S.; Zhongyi, Z.; Mengling, W.; Yude, W. Adsorption of reactive dyes on activated carbon developed from Enteromorpha prolifera. American. J. Anal. Chem. 2013, 4, 17–26. [Google Scholar]
- Aguiar, J.E.; Bezerra, B.T.C.; Braga, B.M.; Lima, P.D.S.; Nogueira, R.E.F.Q.; de Lucena, S.M.P. Adsorption of anionic and cationic dyes from aqueous solution on non-calcined Mg-Al layered double hydroxide: Experimental and theoretical study. Sep. Sci. Technol. 2013, 48, 2307–2316. [Google Scholar] [CrossRef]
- Periolatto, M.; Ferrero, F. Cotton filter fabrics functionalization by chitosan UV-grafting for removal of dyes. Chem. Eng. Trans. 2013, 32, 1–6. [Google Scholar]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wu, D.; Wang, H.; Gao, Z.; Xu, F.; Jiang, K. A green and scalable route to yield porous carbon sheets from biomass for supercapacitors with high capacity. J. Mater. Chem. A 2018, 6, 1244. [Google Scholar] [CrossRef]







| Compounds | Concentration (mg L−1) | λmax (nm) | |
|---|---|---|---|
| Effluent | A | B | |
| RO-16 | 50 | 50 | 494 |
| RB- 4 | 50 | 50 | 595 |
| Methylene Blue | 50 | 50 | 668 |
| Bismarck Brown | 50 | - | 468 |
| Crystal Violet | 50 | - | 590 |
| Methyl Red | - | 50 | 507 |
| Methyl Orange | - | 50 | 522 |
| Phenol Red | - | 50 | 550 |
| Sodium Dodecyl | 25 | 25 | - |
| Sodium sulfate | 25 | 25 | - |
| Ammonium chloride | 20 | 25 | - |
| Sodium acetate | 20 | 25 | - |
| pH | 5.1 | 4.9 | - |
| Samples | ZnCl2-BBC | KOH-BBC |
|---|---|---|
| Parameters | ||
| SBET (m2 g−1) | 754 | 1067 |
| External surface area (m2 g−1) | 328 | 526 |
| % of mesopore area (%) | 43.51 | 49.29 |
| t-plot Micropore area (m2 g−1) | 425.8 | 541.2 |
| % of micropore area (%) | 56.49 | 50.71 |
| Total pore volume (cm3 g−1) | 0.4205 | 0.5585 |
| t-plot micropore volume (cm3 g−1) | 0.2172 | 0.2776 |
| % of micropore volume (%) | 51.65 | 49.70 |
| Volume of mesopores (cm3 g−1) | 0.2033 | 0.2809 |
| Average pore size (nm) | 2.231 | 2.093 |
| Adsorbent | Activation Reagent | Preparation Conditions | SBET (m2 g−1) | Ref. |
|---|---|---|---|---|
| Scots pine bark | Steam + N2 | Firstly, the biomass was carbonized using slow pyrolysis at 475 °C for 3 h. Afterward, heated at 800 °C for 3.5 h under steam activation [steam (30 and 40%) + N2 (66 and 300 L/h)]. | 539–603 | [8] |
| Norway spruce bark | Steam + N2 | Firstly, the biomass was carbonized using slow pyrolysis at 475 °C for 3 h. Afterward, heated at 800 °C for 3.5 h under steam activation [steam (30 and 40%) + N2 (66 and 300 L/h)]. | 187– 369 | [8] |
| Norway spruce bark | Steam + N2 | The biomass was heated at 600 °C for 2 h under steam activation (steam + N2). | 351 | [9] |
| Norway spruce bark | ZnCl2 | A mixture of ZnCl2 and biomass powder at ratio 2.0:1.0 (ZnCl2:biomass) and pyrolyzed at 600 °C for 2 h. Afterward, it was washed with HCl to remove the inorganic compounds. | 1495 | [9] |
| Tea leave residue | KOH | A mixture of KOH and tea powder (2:1) and pyrolyzed at 900 °C for 60 min. Afterward, it was washed with HCl to remove the potassium compounds and further pyrolyzed at 1200 °C. | 912 | [28] |
| Palm shell | KOH+ ZnCl2 | Pre-carbonization of biomass at 400 °C for 2 h. Afterward, a mixture of biomass and both KOH (75%) and ZnCl2 (25%) at the final ratio of biomass: chemical activator 1:4. The mixture was then pyrolyzed at 850 °C for 1 h and washed with HCl. | 1295 | [29] |
| Garlic peel | KOH | First, it was hydro-carbonized and then chemically activated by KOH (ratio 2:1, KOH: biomass) and pyrolyzed at 600 °C at 4 °C/min under N2 flow for 2 h. | 947 | [30] |
| Rice plants | KOH | The biomass was Pre-carbonized at 500 °C for 1 h, followed by NaOH washing. Afterward, the pyrolyzed BBC was mixed with KOH at ratio 1:4. The mixture was then pyrolyzed at 800 °C for 30 min and then washed with HCl. | 2330 | [31] |
| Brazil nutshells | ZnCl2 | A mixture of ZnCl2 and biomass powder at ratio 1.5:1.0 (ZnCl2:biomass) and pyrolyzed at 600 °C for 30 min. Afterward, it was washed with 6.0 M HCl to remove the inorganic compounds. | 1457 | [32] |
| Sewage sludge | ZnCl2 | A mixture of ZnCl2 and biomass powder at ratio 0.5:1.0 (ZnCl2:biomass) and pyrolyzed at 500 °C for 15 min. Afterward, it was washed with HCl to remove the inorganic compounds. | 679 | [33] |
| Coconut shell | ZnCl2 | Blending coconut shell powder and ZnCl2 at ratio 1:3 in 50 mL of 3 M FeCl3 solution. Afterward, heated at 900 °C for 1 h under an inert atmosphere. Afterward, it was washed with HCl to remove the inorganic compounds. | 1874 | [34] |
| Norway spruce bark | ZnCl2 | ZnCl2 and biomass powder mixture at ratio 1.0:1.0 (ZnCl2:biomass) and pyrolyzed at 800 °C for 60 min. Afterward, it was washed with 6.0 M HCl to remove the inorganic compounds. | 754 | This work |
| Norway spruce bark | KOH | A mixture of KOH and biomass powder at ratio 1.0:1.0 (KOH: biomass) and pyrolyzed at 800 °C for 60 min. Afterward, it was washed with 1.0 M HCl to remove the inorganic compounds. | 1067 | This work |
| Samples | ZnCl2-BBC | KOH-BBC |
|---|---|---|
| Parameters | ||
| HI (H2O/n-heptane) | 1.19 | 1.29 |
| Zeta potential (mV) | −19.4 | −20.5 |
| pH | 5.1 | 6.0 |
| Carbon content (%) | 94.8 | 91.6 |
| Nitrogen content (%) | 0.51 | 0.29 |
| Hydrogen (%) | 1.2 | 1.6 |
| Oxygen (%) | 2.5 | 5.3 |
| Ash (%) | 0.99 | 1.21 |
| BBC yield (%) | 38.1 | 14.2 |
| Model | RO-16 Initial Concentration (1000 mg L−1) | RB-4 Initial Concentration (1000 mg L−1) | ||
|---|---|---|---|---|
| ZnCl2-BBC | KOH-BBC | ZnCl2-BBC | KOH-BBC | |
| Pseudo-first order | ||||
| q1 (mg g−1) | 74.71 | 307.5 | 84.01 | 301.7 |
| k1 (min−1) | 0.4529 | 3.502 | 0.7381 | 2.489 |
| R2 | 0.9639 | 0.9606 | 0.8502 | 0.8182 |
| R2adj | 0.9614 | 0.9573 | 0.8408 | 0.8068 |
| SD (mg g−1) | 5.107 | 20.22 | 11.79 | 46.03 |
| Pseudo-second order | ||||
| q2 (mg g−1) | 89.96 | 332.7 | 92.62 | 329.1 |
| k2 (g mg−1 min−1) | 0.00566 | 0.01539 | 0.01277 | 0.009700 |
| R2 | 0.9783 | 0.9963 | 0.9102 | 0.9019 |
| R2adj | 0.9768 | 0.9960 | 0.9046 | 0.8958 |
| SD (mg g−1) | 3.957 | 6.217 | 9.126 | 33.81 |
| General order | ||||
| qn (mg g−1) | 78.98 | 356.3 | 136.6 | 355.2 |
| kn (min−1 (g mg−1)n−1) | 1.114 × 10−6 | 4.140 × 10−4 | 4.964 × 10−7 | 2.595 × 10−5 |
| n (-) | 22.69 | 2.6270 | 33.08 | 40.08 |
| R2 | 0.9852 | 0.9985 | 0.9629 | 0.9799 |
| R2adj | 0.9838 | 0.9983 | 0.9580 | 0.9831 |
| t0.5 (hour) | 1.46 | 0.24 | 1.57 | 0.43 |
| T0.95 (hour) | 6.00 | 2.98 | 6.95 | 3.21 |
| SD (mg g−1) | 3.311 | 4.049 | 8.861 | 10.22 |
| Model | Samples | |||
|---|---|---|---|---|
| ZnCl2-BBC | KOH-BBC | ZnCl2-BBC | KOH-BBC | |
| Langmuir | RO-16 | RB-4 | ||
| Qmax (mg g−1) | 90.04 | 358.2 | 59.00 | 339.15 |
| kL (L mg−1) | 0.05004 | 2.579 | 0.02698 | 0.005491 |
| R2 | 0.8386 | 0.8614 | 0.9534 | 0.9905 |
| R2adj | 0.8225 | 0.8488 | 0.9488 | 0.9896 |
| SD (mg g−1)2 | 13.77 | 57.72 | 4.794 | 13.93 |
| Freundlich | ||||
| kF ((mg g−1) (mg L−1)−1/nF) | 34.37 | 257.1 | 11.10 | 14.25 |
| nF (dimensionless) | 6.7123 | 16.24 | 3.906 | 1.859 |
| R2 | 0.8480 | 0.8467 | 0.9889 | 0.9818 |
| R2adj | 0.8328 | 0.8328 | 0.9878 | 0.9799 |
| SD (mg g−1)2 | 13.36 | 60.71 | 2.399 | 19.31 |
| Liu | ||||
| Qmax (mg g−1) | 123.1 | 354.9 | 332.9 | 582.5 |
| kS (mg L−1) | 0.02017 | 1.943 | 0.007468 | 0.004040 |
| nL (dimensionless) | 0.3850 | 1.780 | 0.2913 | 0.8848 |
| R2 | 0.8498 | 0.8646 | 0.9891 | 0.9911 |
| R2adj | 0.8164 | 0.8375 | 0.9899 | 0.9891 |
| SD (mg g−1)2 | 13.99 | 59.84 | 2.344 | 14.21 |
| Adsorbent | pH | Dosage (g L−1) | T (°C) | Qmax (mg g–1) | Ref. |
|---|---|---|---|---|---|
| RO-16 | |||||
| BBC-KOH-800 | 5.5 | 1.5 | 22 | 354.8 | This study |
| BBC-ZnCl2-800 | 5.5 | 1.5 | 22 | 90.1 | This study |
| Chitosan/sepiolite composite | 6.5 | 2.0 | 30 | 190.96 | [46] |
| Fish scales Mesoporous BBC | 6.0 | 1.0 | 50 | 114.2 | [47] |
| BBC Brazilian-pine fruit shell | 2.5 | 2.5 | 50 | 314.0 | [48] |
| BBC Brazilian-pine fruit shell | 2.5 | 2.5 | 50 | 470.0 | [48] |
| BBC from rice husk ash | 11 | 2.5 | 30 | 13.32 | [49] |
| Phosphoric BBC from biomass | 6.2 | 0.4 | 30 | 58.54 | [50] |
| Psyllium seed powder biosorbent | 4.0 | 2.0 | 30 | 206.6 | [51] |
| Paper sludge activated carbon | 2.0 | 1.0 | 30 | 178.0 | [52] |
| Ananas Comosus leaves BBC | 2–3 | 1.0 | 30 | 147.05 | [53] |
| Sewage sludge BBC | 2.0 | 10.0 | 25 | 114.7 | [54] |
| Coffee husk-based BBC | 4.0 | 2.0 | 30 | 66.76 | [55] |
| Coffee husk-based BBC | 4.0 | 2.0 | 50 | 76.57 | [55] |
| RB-4 | |||||
| BBC-KOH-800 | 4.0 | 1.5 | 22 | 582.5 | This study |
| BBC-ZnCl2-800 | 4.0 | 1.5 | 22 | 332.9 | This study |
| Multi-walled carbon nanotubes | 2.0 | 1.5 | 25 | 502.5 | [42] |
| Single-walled carbon nanotubes | 2.0 | 1.5 | 25 | 567.7 | [42] |
| Chitosan hydrogel beads (CHB) | 4.0 | 1.0 | 30 | 317 | [43] |
| CHB modified with hexadecylamine | 4.0 | 1.0 | 30 | 454 | [43] |
| Enteromorpha prolifera BBC | 6.0 | - | 27 | 131 | [56] |
| Mg–Al layered double hydroxide | 2.0 | 0.75 | 22 | 328 | [57] |
| Cotton grafted with chitosan | 4.0 | 10 | 25 | 180 | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Reis, G.S.; Larsson, S.H.; Thyrel, M.; Pham, T.N.; Claudio Lima, E.; de Oliveira, H.P.; Dotto, G.L. Preparation and Application of Efficient Biobased Carbon Adsorbents Prepared from Spruce Bark Residues for Efficient Removal of Reactive Dyes and Colors from Synthetic Effluents. Coatings 2021, 11, 772. https://doi.org/10.3390/coatings11070772
dos Reis GS, Larsson SH, Thyrel M, Pham TN, Claudio Lima E, de Oliveira HP, Dotto GL. Preparation and Application of Efficient Biobased Carbon Adsorbents Prepared from Spruce Bark Residues for Efficient Removal of Reactive Dyes and Colors from Synthetic Effluents. Coatings. 2021; 11(7):772. https://doi.org/10.3390/coatings11070772
Chicago/Turabian Styledos Reis, Glaydson Simões, Sylvia H. Larsson, Mikael Thyrel, Tung Ngoc Pham, Eder Claudio Lima, Helinando Pequeno de Oliveira, and Guilherme L. Dotto. 2021. "Preparation and Application of Efficient Biobased Carbon Adsorbents Prepared from Spruce Bark Residues for Efficient Removal of Reactive Dyes and Colors from Synthetic Effluents" Coatings 11, no. 7: 772. https://doi.org/10.3390/coatings11070772
APA Styledos Reis, G. S., Larsson, S. H., Thyrel, M., Pham, T. N., Claudio Lima, E., de Oliveira, H. P., & Dotto, G. L. (2021). Preparation and Application of Efficient Biobased Carbon Adsorbents Prepared from Spruce Bark Residues for Efficient Removal of Reactive Dyes and Colors from Synthetic Effluents. Coatings, 11(7), 772. https://doi.org/10.3390/coatings11070772








