Recent Developments in Coatings for Orthopedic Metallic Implants
Abstract
:1. Introduction
2. Coating Techniques
3. Coatings for Metallic Implants
3.1. Coatings for Titanium
3.2. Coatings for Stainless Steel
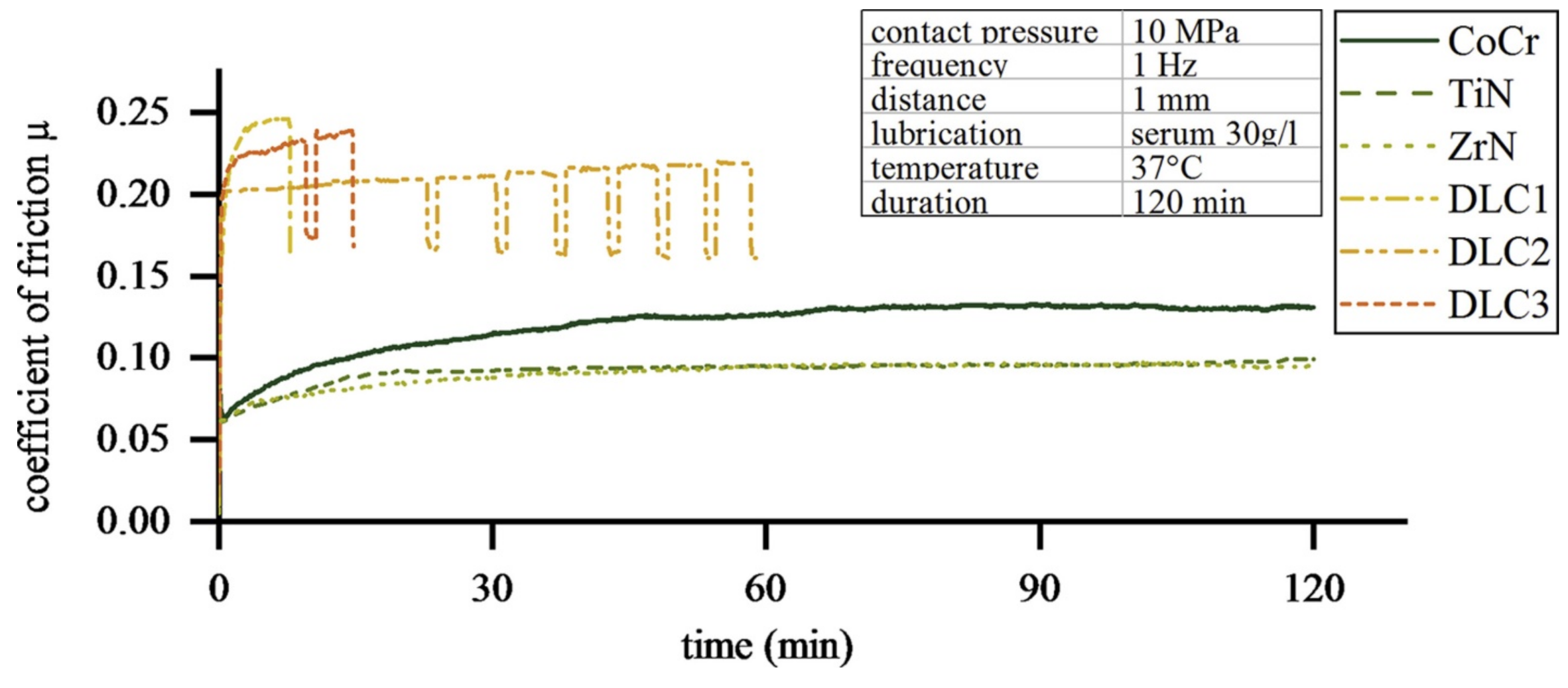
3.3. Coatings for Cobalt-Chrome Molybdenum (CoCrMo) Alloys
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brach del Prever, E.M.; Costa, L.; Piconi, C.; Baricco, M.; Massè, A. Biomaterials for total joint replacements. In Biomechanics and Biomaterials in Orthopedics, 2nd ed.; Springer: London, UK, 2016; pp. 59–70. [Google Scholar]
- Krishnakumar, S.; Senthilvelan, T. Polymer composites in dentistry and orthopedic applications-a review. Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Goswami, C.; Patnaik, A.; Bhat, I.K.; Singh, T. Mechanical physical and wear properties of some oxide ceramics for hip joint application: A short review. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Jones, L.C.; Timmie Topoleski, L.D.; Tsao, A.K. Biomaterials in orthopaedic implants. In Mechanical Testing of Orthopaedic Implants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 17–32. ISBN 9780081002865. [Google Scholar]
- Sharma, A.; Sharma, G. Biomaterials and their applications. In AIP Conference Proceedings; American Institute of Physics Inc.: Melville, NY, USA, 2018; Volume 1953, p. 080041. [Google Scholar]
- Radha, R.; Sreekanth, D. Insight of magnesium alloys and composites for orthopedic implant applications—A review. J. Magnes. Alloy. 2017, 5, 286–312. [Google Scholar] [CrossRef]
- Ali, M.; Hussein, M.A.; Al-Aqeeli, N. Magnesium-based composites and alloys for medical applications: A review of mechanical and corrosion properties. J. Alloy. Compd. 2019, 792, 1162–1190. [Google Scholar] [CrossRef]
- Song, M.S.; Zeng, R.C.; Ding, Y.F.; Li, R.W.; Easton, M.; Cole, I.; Birbilis, N.; Chen, X.B. Recent advances in biodegradation controls over Mg alloys for bone fracture management: A review. J. Mater. Sci. Technol. 2019, 35, 535–544. [Google Scholar] [CrossRef]
- Jaiswal, S.; Dubey, A.; Lahiri, D. In Vitro Biodegradation and Biocompatibility of Mg–HA-Based Composites for Orthopaedic Applications: A Review. J. Indian Inst. Sci. 2019, 99, 303–327. [Google Scholar] [CrossRef]
- Shahin, M.; Munir, K.; Wen, C.; Li, Y. Magnesium matrix nanocomposites for orthopedic applications: A review from mechanical, corrosion, and biological perspectives. Acta Biomater. 2019, 96, 1–19. [Google Scholar] [PubMed]
- Xia, L.; Xie, Y.; Fang, B.; Wang, X.; Lin, K. In situ modulation of crystallinity and nano-structures to enhance the stability and osseointegration of hydroxyapatite coatings on Ti-6Al-4V implants. Chem. Eng. J. 2018, 347, 711–720. [Google Scholar] [CrossRef]
- Gobbi, S. Wear Resistance of Metallic Orthopedic Implants—Mini Review. Biomed. J. Sci. Tech. Res. 2018, 12. [Google Scholar] [CrossRef]
- Xu, W.; Yu, A.; Lu, X.; Tamaddon, M.; Ng, L.; Dilawer Hayat, M.; Wang, M.; Zhang, J.; Qu, X.; Liu, C. Synergistic interactions between wear and corrosion of Ti-16Mo orthopedic alloy. J. Mater. Res. Technol. 2020, 9, 9996–10003. [Google Scholar] [CrossRef]
- Xu, W.; Lu, X.; Tian, J.; Huang, C.; Chen, M.; Yan, Y.; Wang, L.; Qu, X.; Wen, C. Microstructure, wear resistance, and corrosion performance of Ti35Zr28Nb alloy fabricated by powder metallurgy for orthopedic applications. J. Mater. Sci. Technol. 2020, 41, 191–198. [Google Scholar] [CrossRef]
- Priyadarshini, B.; Rama, M.; Chetan; Vijayalakshmi, U. Bioactive coating as a surface modification technique for biocompatible metallic implants: A review. J. Asian Ceram. Soc. 2019, 7, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Saad, M.; Akhtar, S.; Srivastava, S. Composite Polymer in Orthopedic Implants: A Review. In Proceedings of the Materials Today: Proceedings; Elsevier: Amsterdam, The Netherlands, 2018; Volume 5, pp. 20224–20231. [Google Scholar]
- Xia, C.; Ma, X.; Zhang, X.; Li, K.; Tan, J.; Qiao, Y.; Liu, X. Enhanced physicochemical and biological properties of C/Cu dual ions implanted medical titanium. Bioact. Mater. 2020, 5, 377–386. [Google Scholar] [CrossRef]
- Al-Tamimi, A.A.; Peach, C.; Fernandes, P.R.; Cseke, A.; Bartolo, P.J.D.S. Topology Optimization to Reduce the Stress Shielding Effect for Orthopedic Applications. In Procedia CIRP; Elsevier B.V.: Amsterdam, The Netherlands, 2017; Volume 65, pp. 202–206. [Google Scholar]
- Limmahakhun, S.; Oloyede, A.; Sitthiseripratip, K.; Xiao, Y.; Yan, C. Stiffness and strength tailoring of cobalt chromium graded cellular structures for stress-shielding reduction. Mater. Des. 2017, 114, 633–641. [Google Scholar] [CrossRef]
- Zhang, L.; Song, B.; Choi, S.-K.; Shi, Y. A Topology Strategy to Reduce Stress Shielding of Additively Manufactured Porous Metallic Biomaterials. Int. J. Mech. Sci. 2021, 197, 106331. [Google Scholar] [CrossRef]
- Vijayavenkataraman, S.; Gopinath, A.; Lu, W.F. A new design of 3D-printed orthopedic bone plates with auxetic structures to mitigate stress shielding and improve intra-operative bending. Bio-Des. Manuf. 2020, 3, 98–108. [Google Scholar] [CrossRef]
- Raffa, M.L.; Nguyen, V.; Hernigou, P.; Flouzat-Lachaniette, C.; Haiat, G. Stress shielding at the bone-implant interface: Influence of surface roughness and of the bone-implant contact ratio. J. Orthop. Res. 2020, 39, 1174–1183. [Google Scholar] [CrossRef]
- Ait Moussa, A.; Fischer, J.; Yadav, R.; Khandaker, M. Minimizing Stress Shielding and Cement Damage in Cemented Femoral Component of a Hip Prosthesis through Computational Design Optimization. Adv. Orthop. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Lam, R.R.; Kondaiah, V.V.; Naidubabu, Y.; Dumpala, R.; Ratna Sunil, B. Effect of Crack Angle on Stress Shielding in Bone and Orthopedic Fixing Plate Implant: Design and Simulation. In Lecture Notes in Mechanical Engineering; Springer: Berlin/Heidelberg, Germany, 2021; pp. 785–792. [Google Scholar]
- Hosseinitabatabaei, S.; Ashjaee, N.; Tahani, M. Introduction of Maximum Stress Parameter for the Evaluation of Stress Shielding Around Orthopedic Screws in the Presence of Bone Remodeling Process. J. Med. Biol. Eng. 2017, 37, 703–716. [Google Scholar] [CrossRef]
- Kraus, T.; Fischerauer, S.; Treichler, S.; Martinelli, E.; Eichler, J.; Myrissa, A.; Zötsch, S.; Uggowitzer, P.J.; Löffler, J.F.; Weinberg, A.M. The influence of biodegradable magnesium implants on the growth plate. Acta Biomater. 2018, 66, 109–117. [Google Scholar] [CrossRef]
- Jadhav, P.; Bongale, A.; Kumar, S. Schematic review of plasma arc oxidation process for Mg Alloy Bio Implants. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1017, 012011. [Google Scholar] [CrossRef]
- Kamrani, S.; Fleck, C. Biodegradable magnesium alloys as temporary orthopaedic implants: A review. BioMetals 2019, 32, 185–193. [Google Scholar] [CrossRef]
- Korhonen, L.; Perhomaa, M.; Kyrö, A.; Pokka, T.; Serlo, W.; Merikanto, J.; Sinikumpu, J.J. Intramedullary nailing of forearm shaft fractures by biodegradable compared with titanium nails: Results of a prospective randomized trial in children with at least two years of follow-up. Biomaterials 2018, 185, 383–392. [Google Scholar] [CrossRef]
- Ibrahim, M.Z.; Sarhan, A.A.D.; Yusuf, F.; Hamdi, M. Biomedical materials and techniques to improve the tribological, mechanical and biomedical properties of orthopedic implants—A review article. J. Alloy. Compd. 2017, 714, 636–667. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef] [PubMed]
- Čapek, J.; Stehlíková, K.; Michalcová, A.; Msallamová, Š.; Vojtěch, D. Microstructure, mechanical and corrosion properties of biodegradable powder metallurgical Fe-2 wt% X (X = Pd, Ag and C) alloys. Mater. Chem. Phys. 2016, 181, 501–511. [Google Scholar] [CrossRef]
- Kraus, T.; Moszner, F.; Fischerauer, S.; Fiedler, M.; Martinelli, E.; Eichler, J.; Witte, F.; Willbold, E.; Schinhammer, M.; Meischel, M.; et al. Biodegradable Fe-based alloys for use in osteosynthesis: Outcome of an in vivo study after 52 weeks. Acta Biomater. 2014, 10, 3346–3353. [Google Scholar] [CrossRef] [PubMed]
- Manam, N.S.; Harun, W.S.W.; Shri, D.N.A.; Ghani, S.A.C.; Kurniawan, T.; Ismail, M.H.; Ibrahim, M.H.I. Study of corrosion in biocompatible metals for implants: A review. J. Alloy. Compd. 2017, 701, 698–715. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Naqvi, R.A.; Abbas, N.; Khan, S.M.; Nawaz, S.; Hussain, A.; Zahra, N.; Khalid, M.W. Ultra-High-Molecular-Weight-Polyethylene (UHMWPE) as a Promising Polymer Material for Biomedical Applications: A Concise Review. Polymers 2020, 12, 323. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Sufyan, M.; Abbas, N.; Ahmad, H.; Joyia, F.M.F.M.; Noman, M.; Ahsan, M.M.M.M.; Raza, M.N.M.N.; Razaq, A.; Zulqernain, M.; et al. Influence of laser processing conditions for texturing on ultra-high-molecular-weight-polyethylene (UHMWPE) surface. Case Stud. Therm. Eng. 2019, 14, 100491. [Google Scholar] [CrossRef]
- Sufyan, M.; Hussain, M.; Ahmad, H.; Abbas, N.; Ashraf, J.; Zahra, N. Bulge micro-textures influence on tribological performance of ultra-high-molecular-weight-polyethylene (UHMWPE) under phosphatidylcholine (Lipid) and bovine serum albumin (BSA) solutions. Biomed. Phys. Eng. Express 2019, 5, 035021. [Google Scholar] [CrossRef]
- Sequeira, S.; Fernandes, M.H.; Neves, N.; Almeida, M.M. Development and characterization of zirconia–alumina composites for orthopedic implants. Ceram. Int. 2017, 43, 693–703. [Google Scholar] [CrossRef]
- Gopal, V.; Manivasagam, G. Zirconia-alumina composite for orthopedic implant application. In Applications of Nanocomposite Materials in Orthopedics; Elsevier: Amsterdam, The Netherlands, 2018; pp. 201–219. ISBN 9780128137574. [Google Scholar]
- Baptista, A.; Silva, F.; Porteiro, J.; Míguez, J.; Pinto, G. Sputtering physical vapour deposition (PVD) coatings: A critical review on process improvement andmarket trend demands. Coatings 2018, 8, 402. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Yang, F.C.; Chen, W.C.; Lee, J.W.; Chang, C.L. Effect of nitrogen-argon flow ratio on the microstructural and mechanical properties of AlSiN thin films prepared by high power impulse magnetron sputtering. Surf. Coat. Technol. 2017, 320, 138–145. [Google Scholar] [CrossRef]
- Tang, J.F.; Lin, C.Y.; Yang, F.C.; Tsai, Y.J.; Chang, C.L. Effects of nitrogen-argon flow ratio on the microstructural and mechanical properties of AlCrN coatings prepared using high power impulse magnetron sputtering. Surf. Coat. Technol. 2020, 386, 125484. [Google Scholar] [CrossRef]
- Geyao, L.; Yang, D.; Wanglin, C.; Chengyong, W. Development and application of physical vapor deposited coatings for medical devices: A review. In Procedia CIRP.; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 89, pp. 250–262. [Google Scholar]
- Hussein, M.A.; Adesina, A.Y.; Kumar, A.M.; Sorour, A.A.; Ankah, N.; Al-Aqeeli, N. Mechanical, in-vitro corrosion, and tribological characteristics of TiN coating produced by cathodic arc physical vapor deposition on Ti20Nb13Zr alloy for biomedical applications. Thin Solid Film. 2020, 709, 138183. [Google Scholar] [CrossRef]
- Esmaeili, M.M.; Mahmoodi, M.; Imani, R. Tantalum carbide coating on Ti-6Al-4V by electron beam physical vapor deposition method: Study of corrosion and biocompatibility behavior. Int. J. Appl. Ceram. Technol. 2017, 14, 374–382. [Google Scholar] [CrossRef]
- Bobzin, K.; Brögelmann, T.; Kalscheuer, C.; Liang, T. Post-annealing of (Ti,Al,Si)N coatings deposited by high speed physical vapor deposition (HS-PVD). Surf. Coat. Technol. 2019, 375, 752–762. [Google Scholar] [CrossRef]
- Azar, G.T.P.; Yelkarasi, C.; Ürgen, M. The role of droplets on the cavitation erosion damage of TiN coatings produced with cathodic arc physical vapor deposition. Surf. Coat. Technol. 2017, 322, 211–217. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Kamalan, K.K.; Alagarsamy, K.; Vishwakarma, V. Silver-ceria stabilized zirconia composite coatings on titanium for potential implant applications. Surf. Coat. Technol. 2019, 368, 224–231. [Google Scholar] [CrossRef]
- Qadir, M.; Li, Y.; Wen, C. Ion-substituted calcium phosphate coatings by physical vapor deposition magnetron sputtering for biomedical applications: A review. Acta Biomater. 2019, 89, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Hussain, M.; Zahra, N.; Ahmad, H.; Mehdi, S.M.Z.; Amer, U.S.; Zain Mehdi, S.M.; Sajjad, U.; Amer, M. Optimization of Cr Seed Layer Effect for Surface Roughness of As-Deposited Silver Film using Electron Beam Deposition Method. J. Chem. Soc. Pak. 2020, 42, 23. [Google Scholar]
- Abbas, N.; Shad, M.R.; Hussain, M.; Mehdi, S.M.Z.; Sajjad, U. Fabrication and characterization of silver thin films using physical vapor deposition, and the investigation of annealing effects on their structures. Mater. Res. Express 2019, 6, 116437. [Google Scholar] [CrossRef]
- Jalil, S.A.; Ahmed, Q.S.; Akram, M.; Abbas, N.; Khalid, A.; Khalil, A.; Khalid, M.L.; Mehar, M.M.; Riaz, K.; Mehmood, M.Q. Fabrication of high refractive index TiO2 films using electron beam evaporator for all dielectric metasurfaces. Mater. Res. Express 2018, 5, 016410. [Google Scholar] [CrossRef]
- Joshi, P.; Haque, A.; Gupta, S.; Narayan, R.J.; Narayan, J. Synthesis of multifunctional microdiamonds on stainless steel substrates by chemical vapor deposition. Carbon N. Y. 2021, 171, 739–749. [Google Scholar] [CrossRef]
- Ullah, S.; Hasan, M.; Ta, H.Q.; Zhao, L.; Shi, Q.; Fu, L.; Choi, J.; Yang, R.; Liu, Z.; Rümmeli, M.H. Synthesis of Doped Porous 3D Graphene Structures by Chemical Vapor Deposition and Its Applications. Adv. Funct. Mater. 2019, 29, 1904457. [Google Scholar] [CrossRef]
- Nowak, D.; Januszewicz, B.; Niedzielski, P. Morphology, mechanical and tribological properties of hybrid carbon layer fabricated by Radio Frequency Plasma Assisted Chemical Vapor Deposition. Surf. Coat. Technol. 2017, 329, 1–10. [Google Scholar] [CrossRef]
- Morejón-Alonso, L.; Mochales, C.; Nascimento, L.; Müller, W.D. Electrochemical deposition of Sr and Sr/Mg-co-substituted hydroxyapatite on Ti-40Nb alloy. Mater. Lett. 2019, 248, 65–68. [Google Scholar] [CrossRef]
- Chakraborty, R.; Seesala, V.S.; Manna, J.S.; Saha, P.; Dhara, S. Synthesis, characterization and cytocompatibility assessment of hydroxyapatite-polypyrrole composite coating synthesized through pulsed reverse electrochemical deposition. Mater. Sci. Eng. C 2019, 94, 597–607. [Google Scholar] [CrossRef]
- Li, T.T.; Ling, L.; Lin, M.C.; Peng, H.K.; Ren, H.T.; Lou, C.W.; Lin, J.H. Recent advances in multifunctional hydroxyapatite coating by electrochemical deposition. J. Mater. Sci. 2020, 55, 6352–6374. [Google Scholar] [CrossRef]
- Cotrut, C.M.; Vladescu, A.; Dinu, M.; Vranceanu, D.M. Influence of deposition temperature on the properties of hydroxyapatite obtained by electrochemical assisted deposition. Ceram. Int. 2018, 44, 669–677. [Google Scholar] [CrossRef]
- Lopes, N.I.A.; Henrique Jardim Freire, N.; Resende, P.D.; Santos, L.A.; Buono, V.T.L. Electrochemical deposition and characterization of ZrO2 ceramic nanocoatings on superelastic NiTi alloy. Appl. Surf. Sci. 2018, 450, 21–30. [Google Scholar] [CrossRef]
- Wang, X.; Yan, L.; Ye, T.; Cheng, R.; Tian, J.; Ma, C.; Wang, Y.; Cui, W. Osteogenic and antiseptic nanocoating by in situ chitosan regulated electrochemical deposition for promoting osseointegration. Mater. Sci. Eng. C 2019, 102, 415–426. [Google Scholar] [CrossRef]
- Fathyunes, L.; Khalil-Allafi, J. The effect of graphene oxide on surface features, biological performance and bio-stability of calcium phosphate coating applied by pulse electrochemical deposition. Appl. Surf. Sci. 2018, 437, 122–135. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef]
- Yu, S.; Li, Z.; Han, L.; Zhao, Y.; Fu, T. Biocompatible MgO Film on Titanium Substrate Prepared by Sol-gel Method. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2018, 47, 2663–2667. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Giovanardi, R.; Veronesi, P. Modification of Ti6Al4V implant surfaces by biocompatible TiO2/PCL hybrid layers prepared via sol-gel dip coating: Structural characterization, mechanical and corrosion behavior. Mater. Sci. Eng. C 2017, 74, 501–507. [Google Scholar] [CrossRef]
- Choi, G.; Choi, A.H.; Evans, L.A.; Akyol, S.; Ben-Nissan, B. A review: Recent advances in sol-gel-derived hydroxyapatite nanocoatings for clinical applications. J. Am. Ceram. Soc. 2020, 103, 5442–5453. [Google Scholar] [CrossRef]
- Kaur, S.; Bala, N.; Khosla, C. Characterization of Hydroxyapatite Coating on 316L Stainless Steel by Sol–Gel Technique. Surf. Eng. Appl. Electrochem. 2019, 55, 357–366. [Google Scholar] [CrossRef]
- Jaafar, A.; Hecker, C.; Árki, P.; Joseph, Y. Sol-gel derived hydroxyapatite coatings for titanium implants: A review. Bioengineering 2020, 7, 127. [Google Scholar] [CrossRef]
- Shahali, H.; Jaggessar, A.; Yarlagadda, P.K.D.V. Recent Advances in Manufacturing and Surface Modification of Titanium Orthopaedic Applications. In Procedia Engineering; Elsevier: Amsterdam, The Netherlands, 2017; Volume 174, pp. 1067–1076. [Google Scholar]
- Sun, L. Thermal Spray Coatings on Orthopedic Devices: When and How the FDA Reviews Your Coatings. J. Therm. Spray Technol. 2018, 27, 1280–1290. [Google Scholar] [CrossRef] [Green Version]
- Fox, K.E.; Tran, N.L.; Nguyen, T.A.; Nguyen, T.T.; Tran, P.A. Surface modification of medical devices at nanoscale-recent development and translational perspectives. In Biomaterials in Translational Medicine: A Biomaterials Approach; Elsevier: Amsterdam, The Netherlands, 2018; pp. 163–189. ISBN 9780128134771. [Google Scholar]
- Ullah, I.; Siddiqui, M.A.; Liu, H.; Kolawole, S.K.; Zhang, J.; Zhang, S.; Ren, L.; Yang, K. Mechanical, Biological, and Antibacterial Characteristics of Plasma-Sprayed (Sr,Zn) Substituted Hydroxyapatite Coating. ACS Biomater. Sci. Eng. 2020, 6, 1355–1366. [Google Scholar] [CrossRef]
- Badshah, M.A.; Koh, N.Y.; Zia, A.W.; Abbas, N.; Zahra, Z.; Saleem, M.W. Recent developments in plasmonic nanostructures for metal enhanced fluorescence-based biosensing. Nanomaterials 2020, 10, 1749. [Google Scholar] [CrossRef]
- He, X.; Zhang, X.; Wang, X.; Qin, L. Review of Antibacterial Activity of Titanium-Based Implants’ Surfaces Fabricated by Micro-Arc Oxidation. Coatings 2017, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Qadir, M.; Li, Y.; Munir, K.; Wen, C. Calcium Phosphate-Based Composite Coating by Micro-Arc Oxidation (MAO) for Biomedical Application: A Review. Crit. Rev. Solid State Mater. Sci. 2018, 43, 392–416. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, Z.; Lu, X.; Lv, Y.; Cai, G.; Yang, L.; Dong, Z. Microstructural evolution and biological performance of Cu-incorporated TiO2 coating fabricated through one-step micro-arc oxidation. Appl. Surf. Sci. 2020, 508, 144766. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Hashemi, M.; Moghaddam, N.S.; Elahinia, M. Improving corrosion resistance of additively manufactured nickel–titanium biomedical devices by micro-arc oxidation process. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- Tang, H.; Han, Y.; Wu, T.; Tao, W.; Jian, X.; Wu, Y.; Xu, F. Synthesis and properties of hydroxyapatite-containing coating on AZ31 magnesium alloy by micro-arc oxidation. Appl. Surf. Sci. 2017, 400, 391–404. [Google Scholar] [CrossRef]
- Vernardou, D.; Parkin, I.P.; Drosos, C. Chemical vapor deposition of oxide materials at atmospheric pressure. In Handbook of Modern Coating Technologies; Elsevier: Amsterdam, The Netherlands, 2021; pp. 101–119. [Google Scholar]
- Sun, L.; Yuan, G.; Gao, L.; Yang, J.; Chhowalla, M.; Gharahcheshmeh, M.H.; Gleason, K.K.; Choi, Y.S.; Hong, B.H.; Liu, Z. Chemical vapour deposition. Nat. Rev. Methods Prim. 2021, 1, 5. [Google Scholar] [CrossRef]
- Prodan, A.M.; Iconaru, S.L.; Predoi, M.V.; Predoi, D.; Motelica-Heino, M.; Turculet, C.S.; Beuran, M. Silver-doped hydroxyapatite thin layers obtained by sol-gel spin coating procedure. Coatings 2020, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Tejero-Martin, D.; Rezvani Rad, M.; McDonald, A.; Hussain, T. Beyond Traditional Coatings: A Review on Thermal-Sprayed Functional and Smart Coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef] [Green Version]
- Aruna, S.T.; Kulkarni, S.; Chakraborty, M.; Kumar, S.S.; Balaji, N.; Mandal, C. A comparative study on the synthesis and properties of suspension and solution precursor plasma sprayed hydroxyapatite coatings. Ceram. Int. 2017, 43, 9715–9722. [Google Scholar] [CrossRef]
- Behera, R.R.; Das, A.; Pamu, D.; Pandey, L.M.; Sankar, M.R. Mechano-tribological properties and in vitro bioactivity of biphasic calcium phosphate coating on Ti-6Al-4V. J. Mech. Behav. Biomed. Mater. 2018, 86, 143–157. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Li, R.; Tang, X.; Guo, D.; Qing, Y.; Qin, Y. Enhanced antibacterial properties of orthopedic implants by titanium nanotube surface modification: A review of current techniques. Int. J. Nanomed. 2019, 14, 7217–7236. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.G.X.; Myers, D.E.; Wallace, G.G.; Brandt, M.; Choong, P.F.M. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int. J. Mol. Sci. 2014, 15, 11878–11921. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Ying, B.; Wei, Y.; Xing, H.; Qin, Y.; Li, D. Comparative evaluation of Sr-incorporated calcium phosphate and calcium silicate as bioactive osteogenesis coating orthopedics applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 600, 124834. [Google Scholar] [CrossRef]
- Li, B.; Xia, X.; Guo, M.; Jiang, Y.; Li, Y.; Zhang, Z.; Liu, S.; Li, H.; Liang, C.; Wang, H. Biological and antibacterial properties of the micro-nanostructured hydroxyapatite/chitosan coating on titanium. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hussein, M.A.; Ankah, N.K.; Kumar, A.M.; Azeem, M.A.; Saravanan, S.; Sorour, A.A.; Al Aqeeli, N. Mechanical, biocorrosion, and antibacterial properties of nanocrystalline TiN coating for orthopedic applications. Ceram. Int. 2020, 46, 18573–18583. [Google Scholar] [CrossRef]
- Sun, Y.S.; Huang, H.H. Biphasic calcium phosphates/tantalum pentoxide hybrid layer and its effects on corrosion resistance and biocompatibility of titanium surface for orthopedic implant applications. J. Alloy. Compd. 2018, 743, 99–107. [Google Scholar] [CrossRef]
- Cui, W.; Qin, G.; Duan, J.; Wang, H. A graded nano-TiN coating on biomedical Ti alloy: Low friction coefficient, good bonding and biocompatibility. Mater. Sci. Eng. C 2017, 71, 520–528. [Google Scholar] [CrossRef]
- Cao, X.-Y.; Tian, N.; Dong, X.; Cheng, C.-K. Implant Coating Manufactured by Micro-Arc Oxidation and Dip Coating in Resorbable Polylactide for Antimicrobial Applications in Orthopedics. Coatings 2019, 9, 284. [Google Scholar] [CrossRef] [Green Version]
- Moriche, R.; Beltrán, A.M.; Begines, B.; Rodríguez-Ortiz, J.A.; Alcudia, A.; Torres, Y. Influence of the porosity and type of bioglass on the micro-mechanical and bioactive behavior of coated porous titanium substrates. J. Non. Cryst. Solids 2021, 551, 120436. [Google Scholar] [CrossRef]
- Torres, Y.; Begines, B.; Beltrán, A.M.; Boccaccini, A.R. Deposition of bioactive gelatin coatings on porous titanium: Influence of processing parameters, size and pore morphology. Surf. Coat. Technol. 2021, 421, 127366. [Google Scholar] [CrossRef]
- Gaviria, J.; Alcudia, A.; Begines, B.; Beltrán, A.M.; Villarraga, J.; Moriche, R.; Rodríguez-Ortiz, J.A.; Torres, Y. Synthesis and deposition of silver nanoparticles on porous titanium substrates for biomedical applications. Surf. Coat. Technol. 2021, 406, 126667. [Google Scholar] [CrossRef]
- Gaviria, J.; Alcudia, A.; Begines, B.; Beltrán, A.M.; Rodríguez-ortiz, J.A.; Trueba, P.; Villarraga, J.; Torres, Y. Biofunctionalization of porous ti substrates coated with ag nanoparticles for potential antibacterial behavior. Metals 2021, 11, 692. [Google Scholar] [CrossRef]
- Beltrán, A.M.; Alcudia, A.; Begines, B.; Rodríguez-Ortiz, J.A.; Torres, Y. Porous titanium substrates coated with a bilayer of bioactive glasses. J. Non. Cryst. Solids 2020, 544. [Google Scholar] [CrossRef]
- Beltrán, A.M.; Begines, B.; Alcudia, A.; Rodríguez-Ortiz, J.A.; Torres, Y. Biofunctional and Tribomechanical Behavior of Porous Titanium Substrates Coated with a Bioactive Glass Bilayer (45S5-1393). ACS Appl. Mater. Interfaces 2020, 12, 30170–30180. [Google Scholar] [CrossRef]
- Singh, A.; Singh, G.; Chawla, V. Characterization of Vacuum Plasma Sprayed Reinforced Hydroxyapatite Coatings on Ti–6Al–4V alloy. Trans. Indian Inst. Met. 2017, 70, 2609–2628. [Google Scholar] [CrossRef]
- Liu, X.; Li, M.; Zhu, Y.; Yeung, K.W.K.; Chu, P.K.; Wu, S. The modulation of stem cell behaviors by functionalized nanoceramic coatings on Ti-based implants. Bioact. Mater. 2016, 1, 65–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Aindow, M.; Wei, M. Focused ion beam sectioning studies of biomimetic hydroxyapatite coatings on Ti-6Al-4V substrates. Surf. Coat. Technol. 2017, 313, 255–262. [Google Scholar] [CrossRef]
- Bucur, A.I.; Linul, E.; Taranu, B.O. Hydroxyapatite coatings on Ti substrates by simultaneous precipitation and electrodeposition. Appl. Surf. Sci. 2020, 527, 146820. [Google Scholar] [CrossRef]
- Pillai, R.S.; Frasnelli, M.; Sglavo, V.M. HA/β-TCP plasma sprayed coatings on Ti substrate for biomedical applications. Ceram. Int. 2018, 44, 1328–1333. [Google Scholar] [CrossRef]
- Behera, R.R.; Das, A.; Hasan, A.; Pamu, D.; Pandey, L.M.; Sankar, M.R. Deposition of biphasic calcium phosphate film on laser surface textured Ti–6Al–4V and its effect on different biological properties for orthopedic applications. J. Alloy. Compd. 2020, 842, 155683. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Li, M.; Liu, Q.; Jia, Z.J.; Xu, X.C.; Cheng, Y.; Zheng, Y.F. Electrophoretic deposition of graphene oxide reinforced chitosan–hydroxyapatite nanocomposite coatings on Ti substrate. J. Mater. Sci. Mater. Med. 2016, 27, 1–13. [Google Scholar] [CrossRef]
- Göncü, Y.; Geçgin, M.; Bakan, F.; Ay, N. Electrophoretic deposition of hydroxyapatite-hexagonal boron nitride composite coatings on Ti substrate. Mater. Sci. Eng. C 2017, 79, 343–353. [Google Scholar] [CrossRef]
- Yu, J.M.; Choe, H.C. Mg-containing hydroxyapatite coatings on Ti-6Al-4V alloy for dental materials. Appl. Surf. Sci. 2018, 432, 294–299. [Google Scholar] [CrossRef]
- Hu, C.; Yu, L.; Wei, M. Sectioning studies of biomimetic collagen-hydroxyapatite coatings on Ti-6Al-4V substrates using focused ion beam. Appl. Surf. Sci. 2018, 444, 590–597. [Google Scholar] [CrossRef]
- Singh, S.; Singh, G.; Bala, N. Electrophoretic deposition of hydroxyapatite-iron oxide-chitosan composite coatings on Ti–13Nb–13Zr alloy for biomedical applications. Thin Solid Film. 2020, 697, 137801. [Google Scholar] [CrossRef]
- Lu, R.J.; Wang, X.; He, H.X.; Ling-Ling, E.; Li, Y.; Zhang, G.L.; Li, C.J.; Ning, C.Y.; Liu, H.C. Tantalum-incorporated hydroxyapatite coating on titanium implants: Its mechanical and in vitro osteogenic properties. J. Mater. Sci. Mater. Med. 2019, 30, 1–14. [Google Scholar] [CrossRef]
- Dikici, B.; Niinomi, M.; Topuz, M.; Koc, S.G.; Nakai, M. Synthesis of biphasic calcium phosphate (BCP) coatings on β‒type titanium alloys reinforced with rutile-TiO2 compounds: Adhesion resistance and in-vitro corrosion. J. Sol-Gel Sci. Technol. 2018, 87, 713–724. [Google Scholar] [CrossRef]
- Singh, G.; Singh, H.; Sidhu, B.S. Characterization and corrosion resistance of plasma sprayed HA and HA-SiO2 coatings on Ti-6Al-4V. Surf. Coat. Technol. 2013, 228, 242–247. [Google Scholar] [CrossRef]
- Wang, X.; Li, B.; Zhou, L.; Ma, J.; Zhang, X.; Li, H.; Liang, C.; Liu, S.; Wang, H. Influence of surface structures on biocompatibility of TiO2/HA coatings prepared by MAO. Mater. Chem. Phys. 2018, 215, 339–345. [Google Scholar] [CrossRef]
- Quirama, A.; Echavarría, A.M.; Meza, J.M.; Osorio, J.; Bejarano G., G. Improvement of the mechanical behavior of the calcium phosphate coatings deposited onto Ti6Al4V alloy using an intermediate TiN/TiO2 bilayer. Vacuum 2017, 146, 22–30. [Google Scholar] [CrossRef]
- Robertson, S.F.; Bandyopadhyay, A.; Bose, S. Titania nanotube interface to increase adhesion strength of hydroxyapatite sol-gel coatings on Ti-6Al-4V for orthopedic applications. Surf. Coat. Technol. 2019, 372, 140–147. [Google Scholar] [CrossRef]
- Yi, P.; Peng, L.; Huang, J. Multilayered TiAlN films on Ti6Al4V alloy for biomedical applications by closed field unbalanced magnetron sputter ion plating process. Mater. Sci. Eng. C 2016, 59, 669–676. [Google Scholar] [CrossRef]
- Marques, D.M.; Oliveira, V.d.C.; Souza, M.T.; Zanotto, E.D.; Issa, J.P.M.; Watanabe, E. Biomaterials for orthopedics: Anti-biofilm activity of a new bioactive glass coating on titanium implants. Biofouling 2020, 36, 234–244. [Google Scholar] [CrossRef]
- Tüten, N.; Canadinc, D.; Motallebzadeh, A.; Bal, B. Microstructure and tribological properties of TiTaHfNbZr high entropy alloy coatings deposited on Ti–6Al–4V substrates. Intermetallics 2019, 105, 99–106. [Google Scholar] [CrossRef]
- Wang, J.; Ma, J.; Huang, W.; Wang, L.; He, H.; Liu, C. The investigation of the structures and tribological properties of F-DLC coatings deposited on Ti-6Al-4V alloys. Surf. Coat. Technol. 2017, 316, 22–29. [Google Scholar] [CrossRef]
- Heise, S.; Wirth, T.; Höhlinger, M.; Hernández, Y.T.; Ortiz, J.A.R.; Wagener, V.; Virtanen, S.; Boccaccini, A.R. Electrophoretic deposition of chitosan/bioactive glass/silica coatings on stainless steel and WE43 Mg alloy substrates. Surf. Coat. Technol. 2018, 344, 553–563. [Google Scholar] [CrossRef]
- Rehman, M.A.U.; Munawar, M.A.; Schubert, D.W.; Boccaccini, A.R. Electrophoretic deposition of chitosan/gelatin/bioactive glass composite coatings on 316L stainless steel: A design of experiment study. Surf. Coat. Technol. 2019, 358, 976–986. [Google Scholar] [CrossRef]
- Zarghami, V.; Ghorbani, M.; Pooshang Bagheri, K.; Shokrgozar, M.A. Prolongation of bactericidal efficiency of chitosan—Bioactive glass coating by drug controlled release. Prog. Org. Coat. 2019, 139, 105440. [Google Scholar] [CrossRef]
- Ur Rehman, M.A.; Bastan, F.E.; Nawaz, A.; Nawaz, Q.; Wadood, A. Electrophoretic deposition of PEEK/bioactive glass composite coatings on stainless steel for orthopedic applications: An optimization for in vitro bioactivity and adhesion strength. Int. J. Adv. Manuf. Technol. 2020, 108, 1849–1862. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Iijima, M.; Endo, K.; Mizoguchi, I. Electrophoretic Deposition as a New Bioactive Glass Coating Process for Orthodontic Stainless Steel. Coatings 2017, 7, 199. [Google Scholar] [CrossRef] [Green Version]
- Rivera, L.R.; Cochis, A.; Biser, S.; Canciani, E.; Ferraris, S.; Rimondini, L.; Boccaccini, A.R. Antibacterial, pro-angiogenic and pro-osteointegrative zein-bioactive glass/copper based coatings for implantable stainless steel aimed at bone healing. Bioact. Mater. 2021, 6, 1479–1490. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Iijima, M.; Muguruma, T.; Endo, K.; Mizoguchi, I. Effects of bioactive glass coating by electrophoretic deposition on esthetical, bending, and frictional performance of orthodontic stainless steel wire. Dent. Mater. J. 2020, 39, 593–600. [Google Scholar] [CrossRef]
- Oliver, J.; Anne, N.; Su, Y.; Lu, X.; Kuo, P.H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- López-Cuevas, J.; Rendón-Angeles, J.C.; Méndez-Nonell, J.; Barrientos-Rodríguez, H. In Vitro Bioactivity of AISI 316L Stainless Steel Coated with Hydroxyapatite-Seeded 58S Bioglass. MRS Adv. 2019, 4, 3133–3142. [Google Scholar] [CrossRef]
- Al-Rashidy, Z.M.; Farag, M.M.; Abdel Ghany, N.A.; Ibrahim, A.M.; Abdel-Fattah, W.I. Orthopaedic bioactive glass/chitosan composites coated 316L stainless steel by green electrophoretic co-deposition. Surf. Coat. Technol. 2018, 334, 479–490. [Google Scholar] [CrossRef]
- Rezaei, A.; Golenji, R.B.; Alipour, F.; Hadavi, M.M.; Mobasherpour, I. Hydroxyapatite/hydroxyapatite-magnesium double-layer coatings as potential candidates for surface modification of 316 LVM stainless steel implants. Ceram. Int. 2020, 46, 25374–25381. [Google Scholar] [CrossRef]
- Balestriere, M.A.; Schuhladen, K.; Herrera Seitz, K.; Boccaccini, A.R.; Cere, S.M.; Ballarre, J. Sol-gel coatings incorporating borosilicate bioactive glass enhance anti corrosive and surface performance of stainless steel implants. J. Electroanal. Chem. 2020, 876, 114735. [Google Scholar] [CrossRef]
- Hosseini, M.R.; Ahangari, M.; Johar, M.H.; Allahkaram, S.R. Optimization of nano HA-SiC coating on AISI 316L medical grade stainless steel via electrophoretic deposition. Mater. Lett. 2021, 285, 129097. [Google Scholar] [CrossRef]
- Omar, S.A.; Ballarre, J.; Ceré, S.M. Protection and functionalization of AISI 316 L stainless steel for orthopedic implants: Hybrid coating and sol gel glasses by spray to promote bioactivity. Electrochim. Acta 2016, 203, 309–315. [Google Scholar] [CrossRef]
- Tumer, D.; Gungorurler, M.; Havitcioglu, H.; Arman, Y. Investigation of effective coating of the Tie6Ale4V alloy and 316L stainless steel with graphene or carbon nanotubes with finite element methods. J. Mater. Res. Technol. 2020, 9, 15880–15893. [Google Scholar] [CrossRef]
- Vafa, E.; Bazargan-Lari, R.; Bahrololoom, M.E. Electrophoretic deposition of polyvinyl alcohol/natural chitosan/bioactive glass composite coatings on 316L stainless steel for biomedical application. Prog. Org. Coat. 2021, 151, 106059. [Google Scholar] [CrossRef]
- Basiaga, M.; Walke, W.; Kajzer, W.; Sambok-Kiełbowicz, A.; Szewczenko, J.; Simka, W.; Szindler, M.; Ziębowicz, B.; Lubenets, V. Atomic layer deposited ZnO films on stainless steel for biomedical applications. Arch. Civ. Mech. Eng. 2021, 21, 1. [Google Scholar] [CrossRef]
- Khamseh, S.; Alibakhshi, E.; Ramezanzadeh, B.; Ganjaee Sari, M. A tailored pulsed substrate bias voltage deposited (a-C: Nb) thin-film coating on GTD-450 stainless steel: Enhancing mechanical and corrosion protection characteristics. Chem. Eng. J. 2021, 404, 126490. [Google Scholar] [CrossRef]
- Aqib, R.; Kiani, S.; Bano, S.; Wadood, A.; Ur Rehman, M.A. Ag–Sr doped mesoporous bioactive glass nanoparticles loaded chitosan/gelatin coating for orthopedic implants. Int. J. Appl. Ceram. Technol. 2021, 18, 544–562. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Thukkaram, S.; Alagarsamy, K.; Kirubaharan, A.M.K.; Paul, L.K.; Abraham, L.; Vishwakarma, V.; Sagadevan, S. Silver-calcia stabilized zirconia nanocomposite coated medical grade stainless steel as potential bioimplants. Surf. Interfaces 2021, 24, 101086. [Google Scholar] [CrossRef]
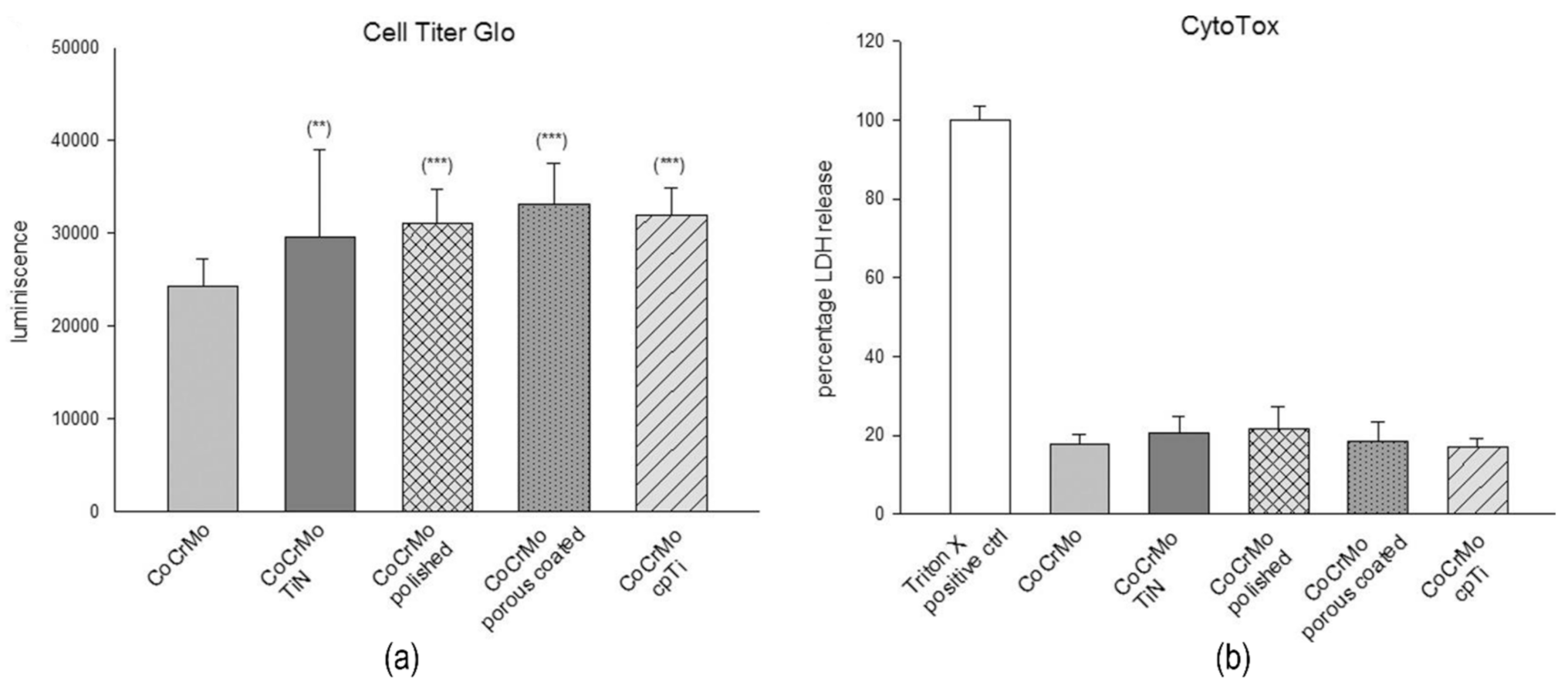
- Lohberger, B.; Stuendl, N.; Glaenzer, D.; Rinner, B.; Donohue, N.; Lichtenegger, H.C.; Ploszczanski, L.; Leithner, A. CoCrMo surface modifications affect biocompatibility, adhesion, and inflammation in human osteoblasts. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Döring, J.; Crackau, M.; Nestler, C.; Welzel, F.; Bertrand, J.; Lohmann, C.H. Characteristics of different cathodic arc deposition coatings on CoCrMo for biomedical applications. J. Mech. Behav. Biomed. Mater. 2019, 97, 212–221. [Google Scholar] [CrossRef]
- Ayu, H.M.; Izman, S.; Daud, R.; Krishnamurithy, G.; Shah, A.; Tomadi, S.H.; Salwani, M.S. Surface Modification on CoCrMo Alloy to Improve the Adhesion Strength of Hydroxyapatite Coating. In Procedia Engineering; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 184, pp. 399–408. [Google Scholar]
- Romonţi, D.C.; Iskra, J.; Bele, M.; Demetrescu, I.; Milošev, I. Elaboration and characterization of fluorohydroxyapatite and fluoroapatite sol-gel coatings on CoCrMo alloy. J. Alloy. Compd. 2016, 665, 355–364. [Google Scholar] [CrossRef]
- Shiri, S.; Zhang, C.; Odeshi, A.; Yang, Q. Growth and characterization of tantalum multilayer thin films on CoCrMo alloy for orthopedic implant applications. Thin Solid Film. 2018, 645, 405–408. [Google Scholar] [CrossRef]
- Tsai, C.E.; Hung, J.; Hu, Y.; Wang, D.Y.; Pilliar, R.M.; Wang, R. Improving fretting corrosion resistance of CoCrMo alloy with TiSiN and ZrN coatings for orthopedic applications. J. Mech. Behav. Biomed. Mater. 2021, 114, 104233. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, K.; Yan, J.; Wang, Z.; Wu, Q.; Bi, L.; Yang, M.; Han, Y. Graphene coating on the surface of CoCrMo alloy enhances the adhesion and proliferation of bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018, 497, 1011–1017. [Google Scholar] [CrossRef]
- Ragone, V.; Canciani, E.; Biffi, C.A.; D’Ambrosi, R.; Sanvito, R.; Dellavia, C.; Galliera, E. CoCrMo alloys ions release behavior by TiNbN coating: An in vitro study. Biomed. Microdevices 2019, 21, 1–9. [Google Scholar] [CrossRef]
- Jakovljević, S.; Alar, V.; Ivanković, A. Electrochemical Behaviour of PACVD TiN-Coated CoCrMo Medical Alloy. Metals 2017, 7, 231. [Google Scholar] [CrossRef] [Green Version]
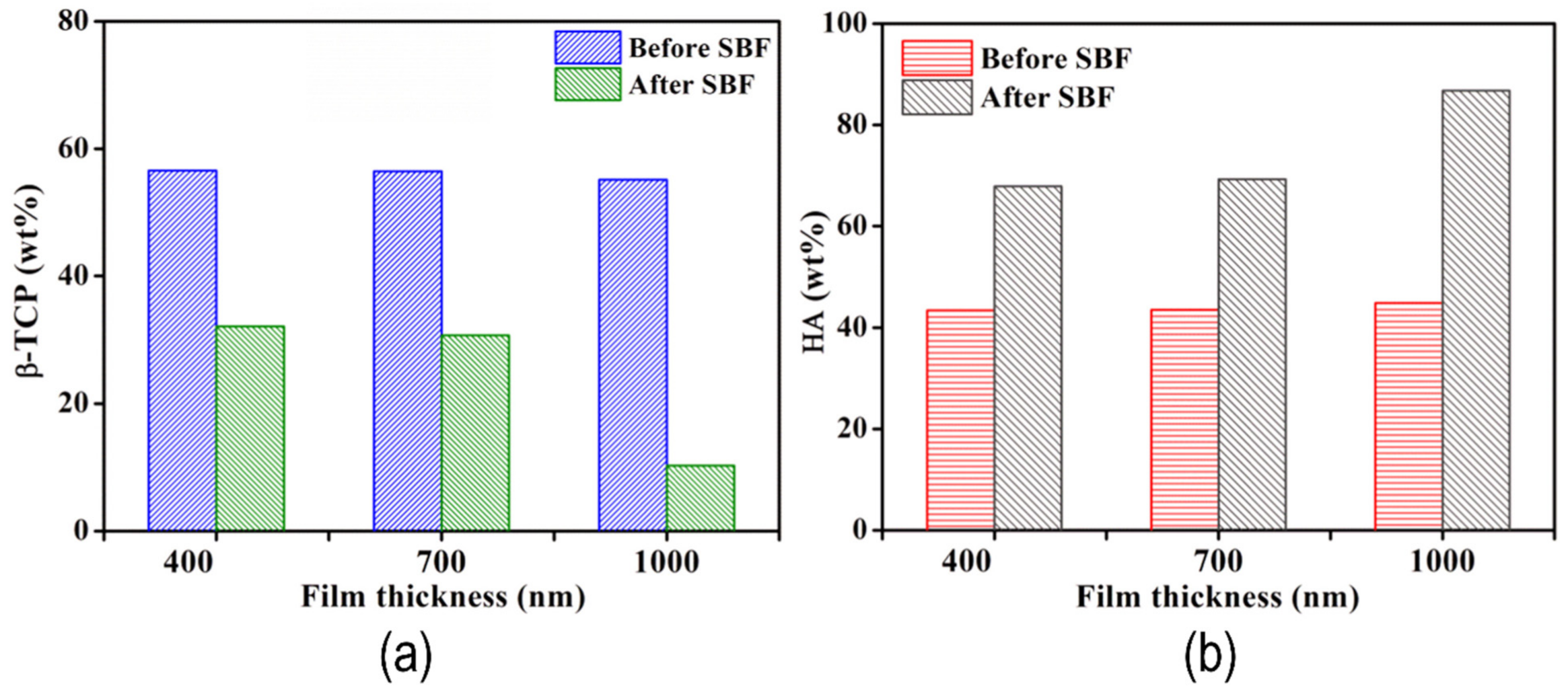
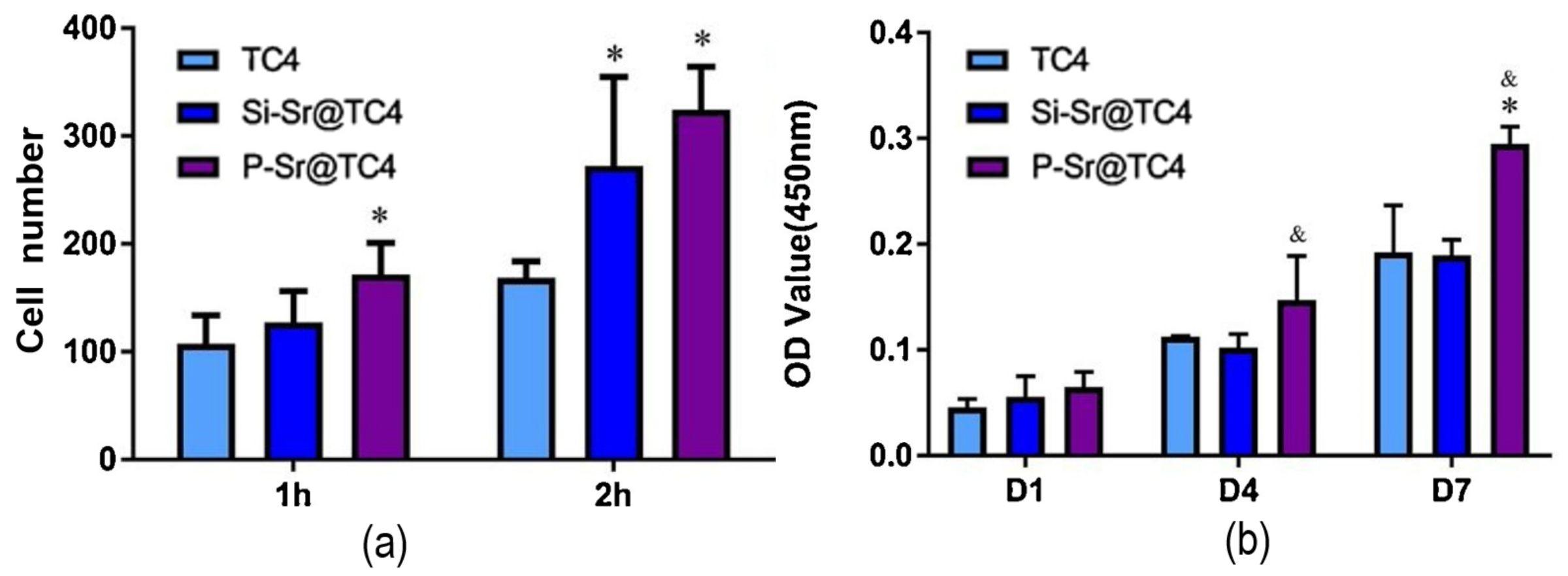

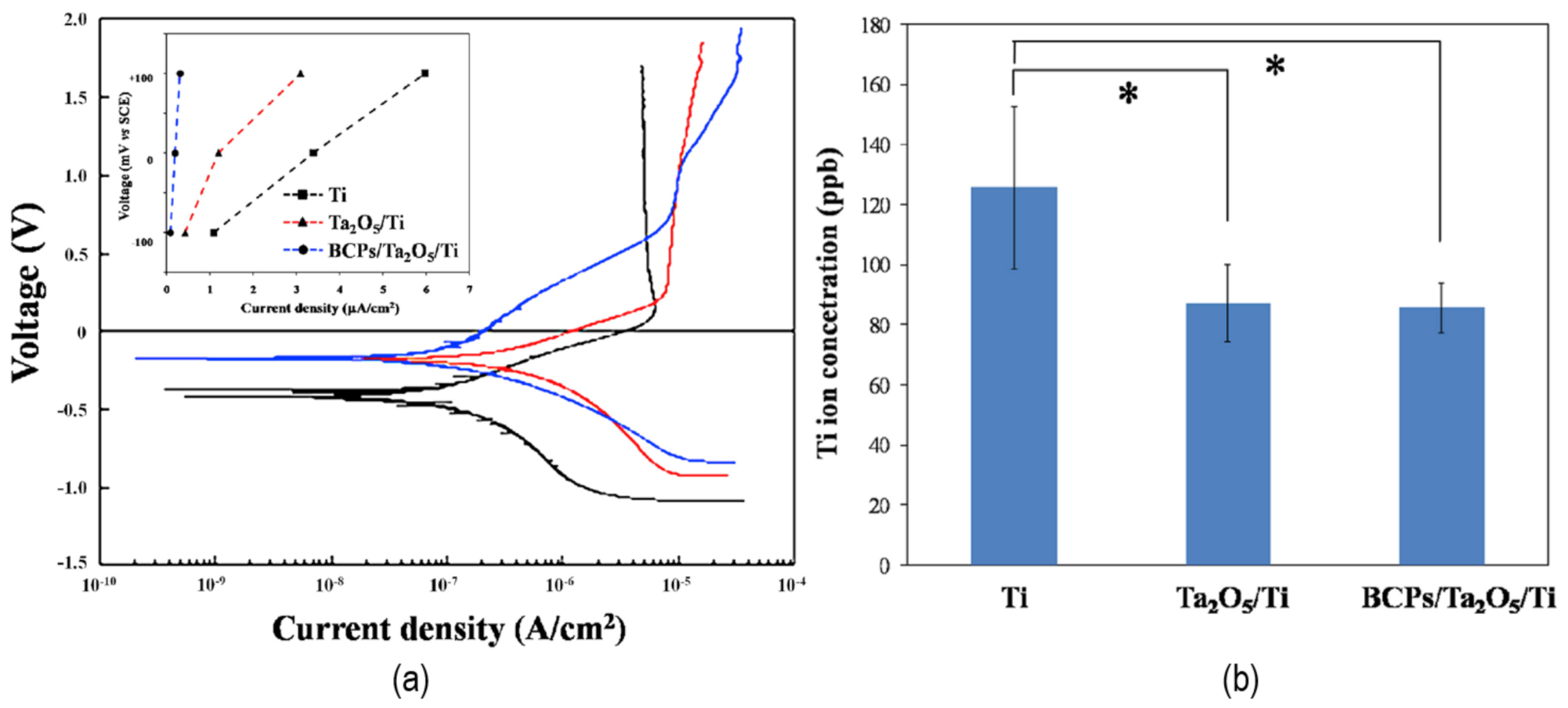
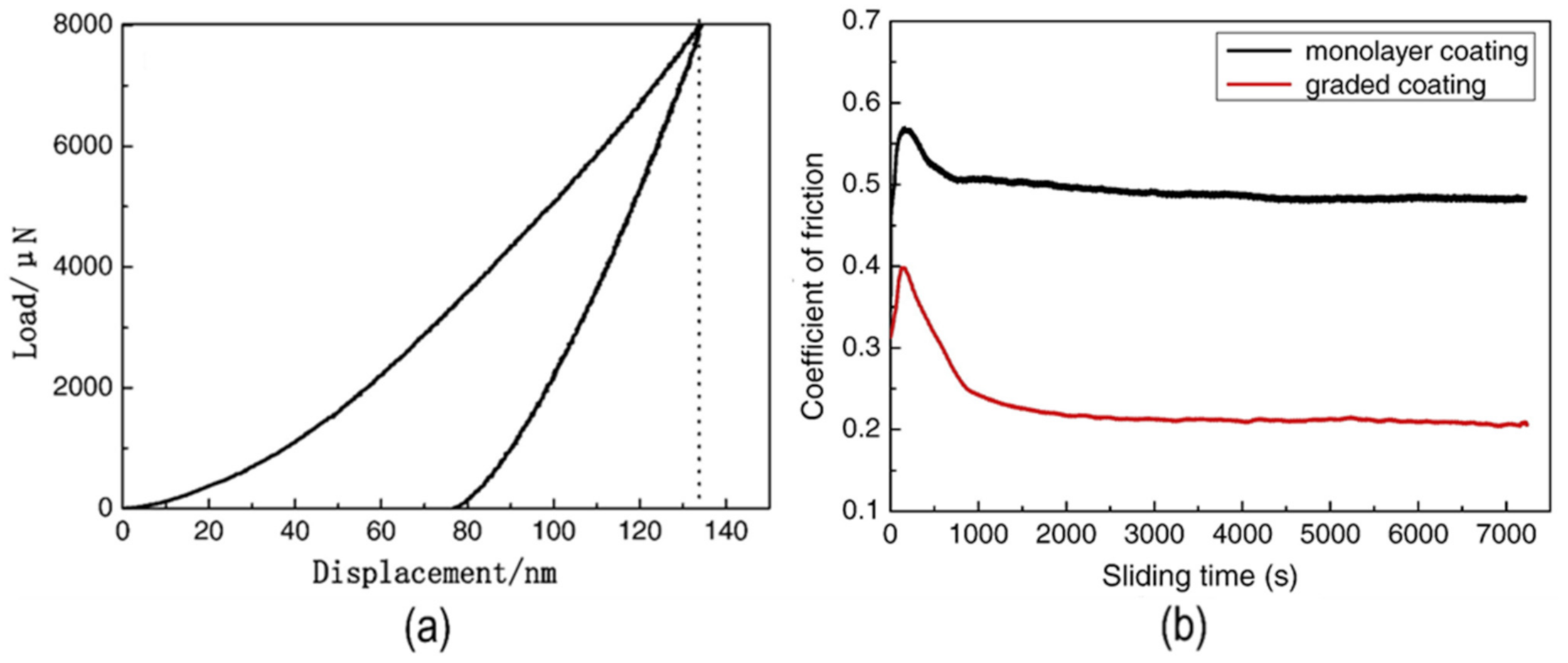
| Material | Uses | Advantages | Disadvantages | Challenges |
|---|---|---|---|---|
| Titanium alloys [31,32] | Femoral hip stems Shoulder stems Fasteners, nails, rods, screws, wires Fracture fixation plates Pedicle screws and rods for spines | Lightweight Less biological response Biocompatible High corrosion resistance | Poor bending ductility Poor wear resistance Expensive High modulus | Biodegradable Biological inertness Antibacterial Stability in mechanical properties Wear reduction |
| Stainless steel alloys [33,34] | Plates, screws, pins, wires Sliding hip screws Flexible and intramedullary nails Cerclage cables | Widely available High ductility Accepted toughness Accepted biocompatibility | Very high modulus Allergic reactivity Stress shielding effect Poor wear resistance | Corrosion resistance Wear reduction Biological inertness |
| Cobalt-chrome Molybdenum [35] | Bearing surface in metal Plates and wires Shorter-term implants | Long term biocompatibility High corrosion resistance High wear resistance High impact durability | Stress shielding effect Poor machinability Biological toxicity due to release of Ni | Wear reduction Metallic fretting Biological inertness |
| Polyethylene/UHMWPE [36,37,38] | Bearing surfaces | Biocompatibility Wear resistance | Wear debris Lower mechanical properties Joint infections | Fatigue life |
| Alumina/ Zirconia composites [39,40] | Bearing surfaces | High smoothness Biocompatibility | High fracture rate | Brittleness |
| Coating Method | Method Classification | Advantages | Disadvantages |
|---|---|---|---|
| Physical vapor deposition (PVD) [44,45,46,47,48,49,50,51,52,53] | Cathodic arc deposition RF magnetron sputtering DC reactive magnetron sputtering Close field magnetron sputter ion plating Cathode plasma immersion ion implantation deposition Planar magnetron sputtering Electron beam evaporation | Coat complex geometries with ease High dense High purity Increased wear resistance | Delamination Expensive Time-consuming |
| Chemical vapor deposition (CVD) [54,55,56] | Atomic layer deposition Plasma assisted chemical vapor deposition | Coat complex geometries with ease Increased wear resistance | Delamination Expensive |
| Electrochemical deposition [57,58,59,60,61,62,63] | Electrophoretic co-deposition | Low temperature Increased wear resistance | Expensive Thin layer |
| Sol-gel [64,65,66,67,68,69,70] | Dip coating | Coat complex geometries High homogeneity High purity Low temperature Low cost | Low wear resistance Low permeability Delamination Low mechanical stability Thin layer |
| Plasma spraying [71,72,73,74,75] | ------ | Low cost Rapid deposition rate Long life span | Low adhesion Cracks Microstructure change |
| Micro arc oxidation [76,77,78,79,80] | ----- | Low cost Eco friendly Multifunctional coatings | Porosities formation Cracks Delamination |
| Ref. | Coating Material | Thickness | Coating Method | Biocompatibility/Antibacterial Properties | Corrosion/Degradation Behavior | Mechanical Results | Tribological Results |
|---|---|---|---|---|---|---|---|
| [86] | Biphasic calcium phosphate (BCP) | 1000 nm | RF magnetron sputtering | wt.% of HA—86.76% Enhancement in wt.% of apatite layer show bioactivity | ------ | Microhardness—455 HV (140%) | Roughness—153 nm (168%) Higher scratch resistance |
| [92] | BCP/Ta2O5 | 1 µm/700 nm | Electrochemical deposition/Hydration condensation | Spherical apatite layer formation in immersion test show bioactivity | Corrosion current density—0.2 µA/cm2 (5.7%) | ------ | ----- |
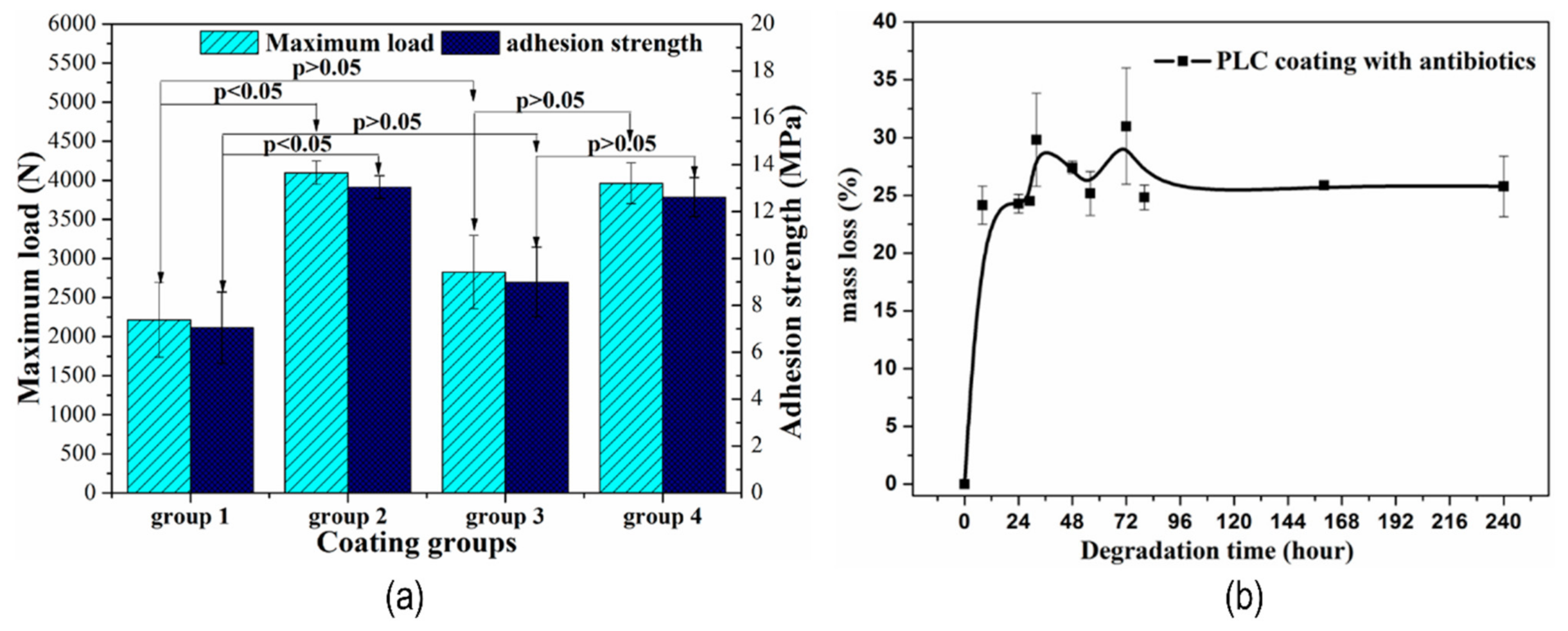
| [94] | Micro-arc oxidation & Polylactide-L-lactide-CO-ε-caprolactone (PLC) | 5.4 µm | Micro-arc oxidation/dip coating | ----- | Mass loss—24.8% | Adhesion strength—13 MPa Load to failure—4000 N | ----- |
| [118] | Ti-TiN-TiAlN | 2.52 µm | Close field magnetron sputter ion plating | ----- | Corrosion current density—1.74 × 10−3 µA/cm2 (7.5%) | Hardness—37.2 GPa (775%) Elastic modulus—409 GPa (339%) Adhesion strength—48 N | COF—0.23 (50%) Wear rate—3.73 × 10−5 mm3/Nm (7.53%) |
| [93] | TiN | 5.8 µm | DC reactive magnetron sputtering | Relative growth rate of cells—+90% OD values and Hemolysis ratio show good biocompatibility. | ------ | Elastic modulus—281 GPa Hardness—0.8 GPa Bonding Force/Adhesion strength—80 N | COF—0.2 Wear rate—0.62 × 10−6 mm3/Nm Wear track depth—0.34 µm |
| [91] | TiN | 5 µm | Cathodic arc-physical deposition | Antibacterial inhabitation efficiency rates—138.2% | Corrosion current density—3.21 × 10−2 µA/cm2 (0.35%) | Hardness—38.3 GPa (726%) Elastic modulus—358 GPa Indentation depth—600 nm (47.69%) | Roughness—13 nm COF—0.448 Contact stress at failure—2.23 GPa |
| [89] | Sr incorporate calcium phosphate | 5–8 µm | Micro-arc oxidation | Better cell adhesion and cell proliferation | ----- | ----- | ----- |
| [119] | Bioactive glass (BGF18) | Similar cell adhesion Thicker biofilm growth for coated (128%) | ----- | ----- | Surface roughness—3.96 nm (1320%) | ||
| [120] | TiTaHfNbZr | ----- | RF magnetron sputtering | ----- | ----- | Hardness—3.46 GPa Elastic modulus—115 GPa | Roughness—2.78 nm (327%) COF—0.15 |
| [121] | Flourine doped diamond-like-carbon/Si | 1.6 µm | Cathode plasma immersion ion implantation deposition | ------ | ----- | Hardness—18.3 GPa Elastic modulus—163 GPa | Roughness—7.8 nm COF—0.11 |
| Ref. | Coating Material | Thickness | Coating Method | Biocompatibility/Antibacterial Properties | Corrosion/Degradation Behavior | Mechanical Results | Tribological Results |
|---|---|---|---|---|---|---|---|
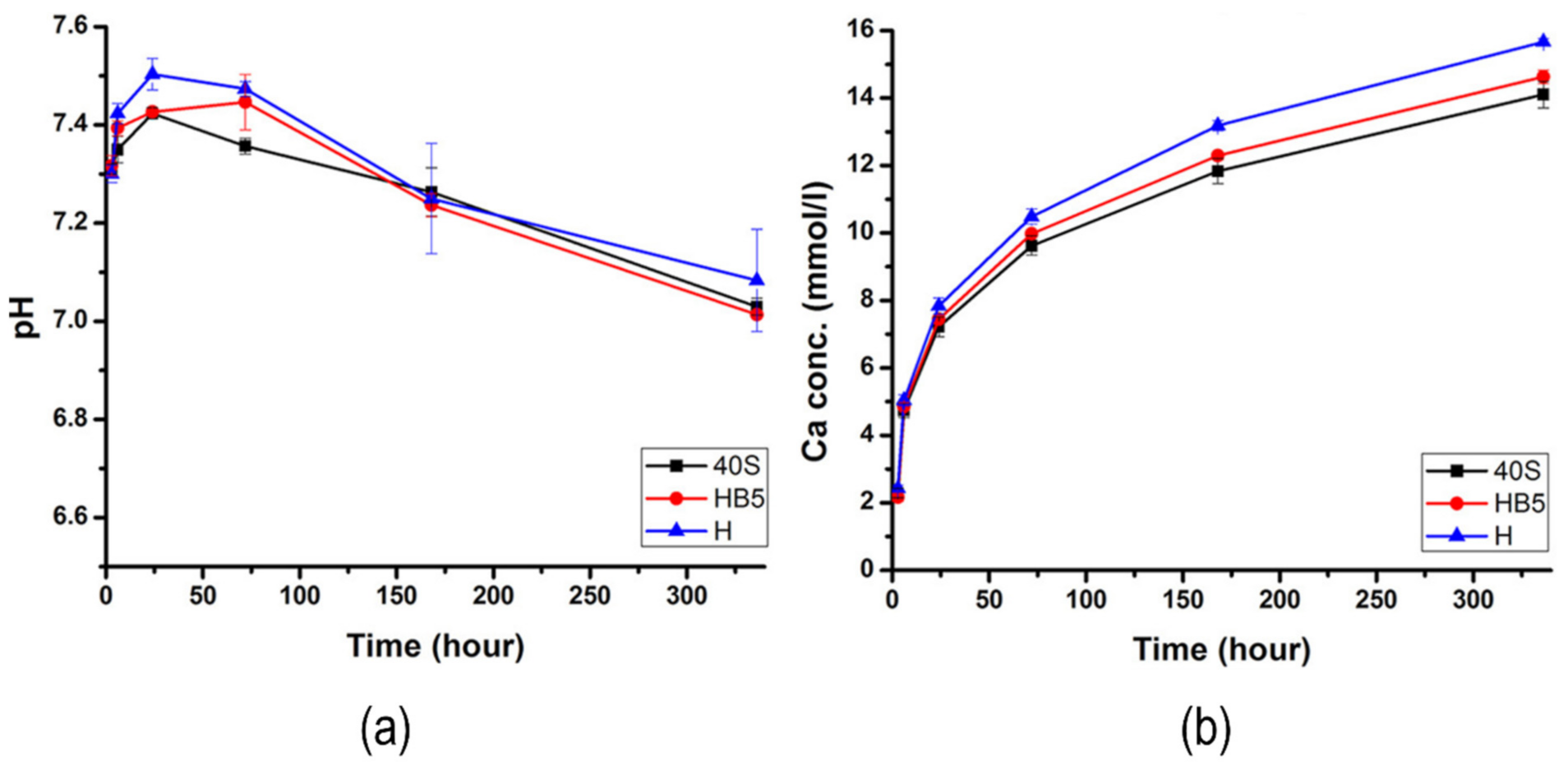
| [131] | Bioactive glass-chitosan | 143 µm | Electrophoretic co-deposition | ----- | pH—7.4–7.9 Ca ion concentration—20 × 10−2 mmm/h Corrosion current density—20.93 µA/cm2 Corrosion rate—5.02 | ----- | Roughness—170 µm (2.9%) |
| [133] | Bioactive glass/silane | 2/0.6 µm | Dip coating | Optical density—0.2 | Corrosion current density—154 µA/cm2 | Show no detachment | |
| [134] | HA-3SiC | 45 µm | Electrophoretic deposition | ----- | Corrosion potential—0.041 | Elastic modulus—728 MPa Bonding strength—1.61 MPa | ----- |
| [135] | Bioactive glass/silane | 1.2 µm | Dip coating | ------ | Improvement in corrosion resistance but show degradation in immersion test | ------- | ----- |
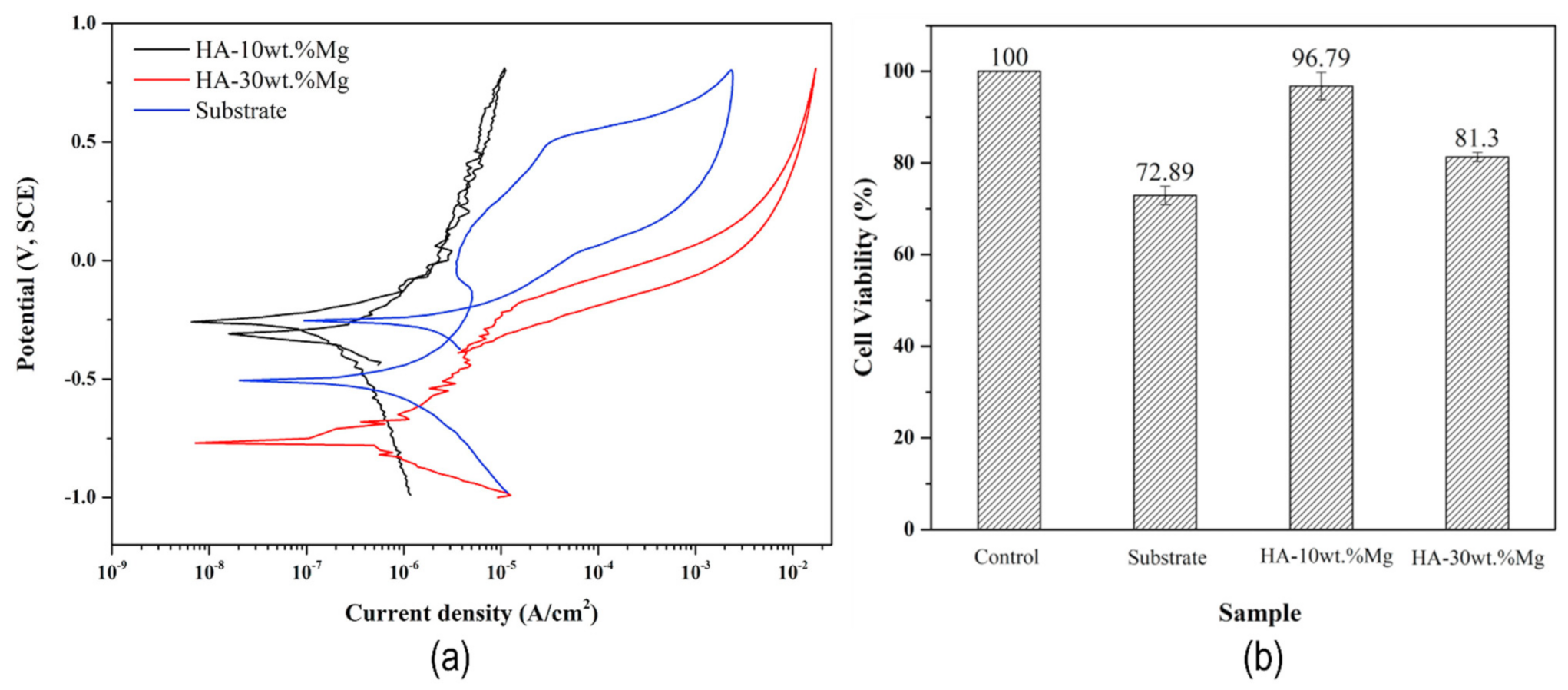
| [132] | HA-10wt.%Mg | 100 µm | Plasma spraying | Cell viability—96.7% (132%) Distribution of calcium phosphate show bioactivity | Mg ion concentration—71 ppm Corrosion potential—−0.250 Corrosion current density—0.12 µA/cm2 | ------- | ----- |
| [136] | Carbon nano tubes (CNTs) | 80 µm | FEM | ---- | ---- | Static stress—87.574 MPa (181%) | ------ |
| [137] | Chitosan-20 Polyvinyl alcohol (PVA)-BG | --- | Electrophoretic deposition | HA forming ability show best bioactivity | Corrosion potential—−0.7265 | Optimum adhesive strength for 20 wt. % PVA coating | ------ |
| [138] | ZNO | 220 nm | Atomic layer deposition | Corrosion potential—−0.131 Corrosion current density—0.04 µA/cm2 | Indenter load to failure—0.85 N | Surface roughness—0.40 µm | |
| [139] | Amorphous carbon: Niobium | 2 µm | Planar magnetron sputtering | Enhancement in corrosion protection | Hardness—16.5 GPa Young modulus—44 GPa | COF—0.10 | |
| [140] | 5 Ag-Sr-Chitosan-Gelatin | --- | Electrophoretic deposition | Suitable proliferation of osteoblast cells Antibacterial | ----- | Suitable adhesion strength | ----- |
| [141] | Ag-CaSZ nanocomposites | --- | E-beam evaporation | Hemocompatible Calcite precipitation | Improved corrosion resistance | ------ | ----- |
| Ref. | Coating Material | Thickness | Coating Method | Biocompatibility/Antibacterial Properties | Corrosion/Degradation Behavior | Mechanical Results | Tribological Results |
|---|---|---|---|---|---|---|---|
| [144] | HA/oxide | 12.73/51.03 µm | Sol-gel dip coating | ---- | ---- | Adhesion strength—8.63 N Increases by increasing sintering temperature. | ---- |
| [142] | TiN | 5.5 µm | Physical vapor deposition | Osteoblast viability—145.6% | ----- | Tensile strength—22 MPa Shear strength—20 MPa | Roughness—50 µm |
| [145] | Fluorohydroxyapatite | 6.22 µm | Sol-gel procedure | Corrosion potential—0.264 V Corrosion density—3.7 × 10−3 µA/cm2 | ---- | Show high adhesion | Roughness—0.477 µm |
| [146] | Tantalum | 1.5 µm | Magnetron sputtering | ---- | ------ | Multilayered Ta film shows best adhesion | ---- |
| [147] | TiSIN | 1.89 µm | Cathodic arc evaporation | Reduction in fretting volume—1000 times Reduction in Co Ion release—90% | Elastic modulus—396 GPa Hardness—41.6 GPa Residual stress—−8.00 GPa Critical load—0.329 N | Roughness—0.0406 µm | |
| [147] | ZrN | 2.37 µm | Cathodic arc evaporation | Reduction in fretting volume—10 times Reduction in Co Ion release—90% | Elastic modulus—409 GPa Hardness—29.3 GPa Residual stress—−8.00 GPa Critical load—0.175 N | Roughness—0.0134 µm | |
| [148] | Graphene | 5.76 µm | Chemical vapor deposition | Improved cell proliferation | ----- | Adhesion strength—1152 µN | ---- |
| [143] | TiN | 1.7 µm | Cathodic arc evaporation | Hardness—30 GPa Penetration modulus—514 GPa Critical load—10 N | Roughness—0.0045 µm COF—0.094 Wear rate—4 × 10−15 m3/mN | ||
| [143] | Diamond-like carbon | 0.7 µm | Cathodic arc evaporation | ----- | ----- | Hardness—74 GPa Penetration modulus—680 GPa Critical load—3 N | Roughness—0.0285 µm COF—0.023 |
| [149] | TiNbN | 3–6 µm | Physical vapor deposition | Viability—above 100% | Reduction in Co/Cr/Mo ion released—80.1/62.5/48% | ------ | ----- |
| [150] | TiN | 2 µm | Plasma assisted chemical vapor deposition | ----- | Corrosion rate—0.793 µm/y | ----- | ------- |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.; Askari Rizvi, S.H.; Abbas, N.; Sajjad, U.; Shad, M.R.; Badshah, M.A.; Malik, A.I. Recent Developments in Coatings for Orthopedic Metallic Implants. Coatings 2021, 11, 791. https://doi.org/10.3390/coatings11070791
Hussain M, Askari Rizvi SH, Abbas N, Sajjad U, Shad MR, Badshah MA, Malik AI. Recent Developments in Coatings for Orthopedic Metallic Implants. Coatings. 2021; 11(7):791. https://doi.org/10.3390/coatings11070791
Chicago/Turabian StyleHussain, Muzamil, Syed Hasan Askari Rizvi, Naseem Abbas, Uzair Sajjad, Muhammad Rizwan Shad, Mohsin Ali Badshah, and Asif Iqbal Malik. 2021. "Recent Developments in Coatings for Orthopedic Metallic Implants" Coatings 11, no. 7: 791. https://doi.org/10.3390/coatings11070791









