Electrochemical Properties of TiWN/TiWC Multilayer Coatings Deposited by RF-Magnetron Sputtering on AISI 1060
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Coating’s Deposition
2.3. Coatings’ Characterization
2.4. Electrochemical Evaluation
2.5. Considerations for Electrochemical Evaluation
2.5.1. Open Circuit Potential
2.5.2. Corrosion Current Density
2.5.3. Corrosion Rate
2.5.4. Porosity Factor
3. Results and Discussion
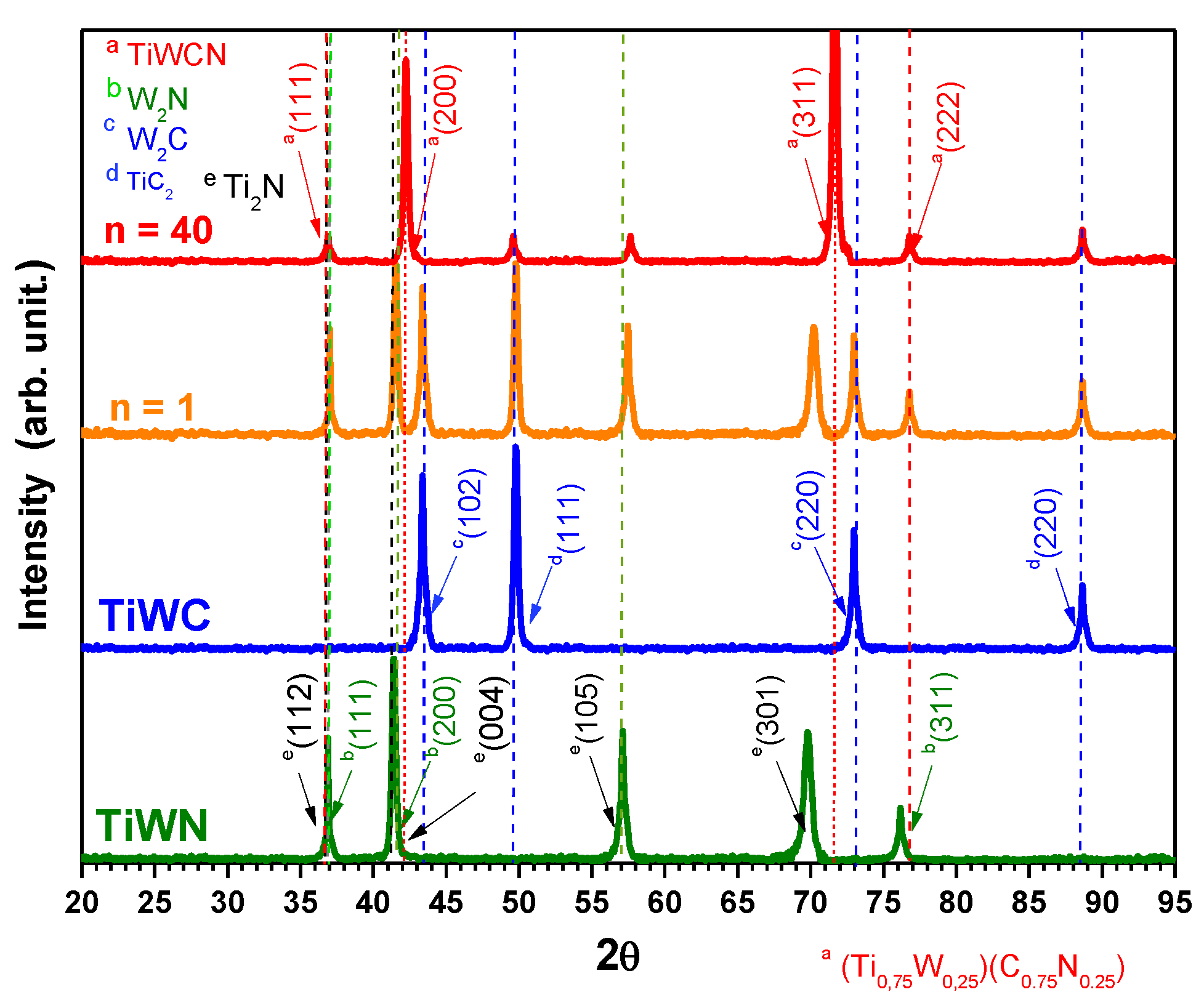
3.1. X-ray Diffraction Analysis
3.2. Microstructural Analysis
3.3. XPS Analysis
3.4. Roughness Analysis
Roughness by AFM
3.5. Electrochemical Behavior
3.5.1. Potentiodynamic Curves
3.5.2. Corrosion Rates
3.5.3. Electrochemical Impedance Spectroscopy
Nyquist Diagram
3.5.4. Determination of Porosity
3.5.5. Equivalent Electrical Circuits
4. Conclusions
- The Ti-W-N film structure consisted of a mix of two phases corresponding to Ti2N (tetragonal crystal structure) and W2N (typical simple cubic). For the n = 1 the diffraction peaks were identified as a sum of their respective phases for each layer. In the case of n = 40, the appearance of new intense peaks was observed, which were attributed to a quaternary compound Ti-W (CN) that could have been formed by the diffusion between nitride and carbide layers or the co-deposition of the used reactive gases: N2 and CH4. This quaternary compound, with the presence of mixed binary phases of WTiN and WTiC, could indicate that there is a graded zone along with the interfaces, which would be interesting to investigate by transmission electron microcopy and by small angle x-ray diffraction in future research.
- The microstructure was of the columnar growth type in all films, and the multilayers showed the smoothest surface because of the repeated nucleation processes that occurred in their growth. The same defects as cone-dome were observed in the monolayers.
- In the quantification of atomic concentration by XPS, a significantly larger presence of Ti in comparison to W was found in all the films.
- The results of the potentiodynamic prove were −0.26 V for Ecorr, and 9.2 × 10−6 (A∙cm2) for icorr. The corrosion rate (CR) was 0.19 mm·year−1. Also, n = 40 exhibited the best protection and excellent dielectric resistance (~52.86 KΩ·cm2). This behavior can be correlated to the interruption of the pores and defects that reach the substrate by the multilayer interfaces, making the coatings less permeable.
- In the equivalent electrical circuits, the n = 40 system presented a major dielectric constant throughout the adsorption of the electrolyte; hence, they have a greater capacitance. However, the simulation through the equivalent electrical circuits showed that in the TiWN system, the material presented three processes of impedance (pore resistance + film + inductance).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vera, E.; Vite, M.; Lewis, R.; Gallardo, E.; Laguna-Camacho, J. A study of the wear performance of TiN, CrN and WC/C coatings on different steel substrates. Wear 2011, 271, 2116–2124. [Google Scholar] [CrossRef]
- Montgomery, S.; Kennedy, D.; O’Dowd, N. PVD and CVD Coatings for the Metal Forming Industry. Conference Papers; Technological University Dublin: Dublin, Ireland, 2010; Available online: https://arrow.tudublin.ie/engschmeccon/?utm_source=arrow.tudublin.ie%2Fengschmeccon%2F37&utm_medium=PDF&utm_campaign=PDFCoverPages (accessed on 29 March 2021).
- Çalışkan, H.; Panjan, P.; Kurbanoglu, C. Comprehensive Materials Finishing. In Hard Coatings on Cutting Tools and Surface Finish; Chapter 3; Hashmi, M.S.J., Ed.; Elsevier: Oxford, UK, 2017; Volume 3, pp. 230–242. [Google Scholar] [CrossRef]
- Paiva, J.M.; Fox-Rabinovich, G.; Junior, E.L.; Stolf, P.; Ahmed, Y.S.; Martins, M.M.; Bork, C.; Veldhuis, S. Tribological and wear performance of nanocomposite PVD hard coatings deposited on aluminum die casting tool. Materials 2018, 11, 358. [Google Scholar] [CrossRef]
- Patel, N.S.; Menghani, J.; Pai, K.B.; Totlani, M.K. Corrosion behavior of Ti2N thin films in various corrosive environments. J. Mater. Environ. Sci. 2010, 1, 134–143. Available online: https://www.researchgate.net/publication/279585673_Corrosion_behavior_of_Ti2N_thin_films_in_various_corrosive_environments (accessed on 29 March 2021).
- Ghufran, M.; Uddin, G.M.; Arafat, S.M.; Jawad, M.; Rehman, A. Development and tribo-mechanical properties of functional ternary nitride coatings: Applications-based comprehensive review. Proc. Inst. Mech. Eng. Part J J. Eng. Tribol. 2021, 235, 196–232. [Google Scholar] [CrossRef]
- Akito, I.; Nobuhide, N.; Tomokazu, N.; Katsumi, O. Super-Abrasive Grain and Super-Abrasive Grinding Wheel. U.S. Patent Application Number 20200156213 16/635329, 12 May 2020. Available online: https://uspto.report/patent/app/20200156213 (accessed on 29 March 2021).
- Fernandes, F.A.P.; Heck, S.C.; Picon, C.A.; Totten, G.E.; Casteletti, L.C. Wear and corrosion resistance of pack chromised carbon steel. Surf. Eng. 2012, 28, 313–317. [Google Scholar] [CrossRef]
- Fernandes, F.A.P.; Heck, S.C.; Totten, G.; Casteletti, L.C. Wear evaluation of pack boronized AISI 1060 steel. Mater. Perform. Charact. 2013, 2, 58–66. [Google Scholar] [CrossRef]
- Addonizio, M.L.; Castaldo, A.; Antonaia, A.; Gambale, E.; Iemmo, L. Influence of process parameters on properties of reactively sputtered tungsten nitride thin films. J. Vac. Sci. Technol. A 2012, 30, 31506. [Google Scholar] [CrossRef]
- Suetin, D.V.; Shein, I.R.; Ivanovskii, A.L. Electronic structure of cubic tungsten subnitride W2N in comparison to hexagonal and cubic tungsten mononitrides WN. J. Struct. Chem. 2010, 51, 199–203. [Google Scholar] [CrossRef]
- Bemporad, E.; Sebastiani, M.; Pecchio, C.; De Rossi, S. High thickness Ti/TiN multilayer thin coatings for wear resistant applications. Surf. Coat. Technol. 2006, 201, 2155–2165. [Google Scholar] [CrossRef]
- Shanaghi, A.; Ghasemi, S.; Chu, P.K.; Ahangarani, S.; Zhao, Y. Effect of Ti interlayer on corrosion behavior of nanostructured Ti/TiN multilayer coating deposited on TiAl6V4. Mater. Corros. 2019, 70, 2113–2127. [Google Scholar] [CrossRef]
- Jia-Hong, H.; Chi-Hsin, H.; Ge-Ping, Y. Effect of nitrogen flow rate on the structure and mechanical properties of ZrN thin films on Si (100) and stainless-steel substrates. Mater. Chem. Phys. 2007, 102, 31–38. [Google Scholar] [CrossRef]
- Panjan, P.; Drnovšek, A.; Gselman, P.; Čekada, M.; Panjan, M.; Bončina, T.; Merl, D.K. Merl influence of growth defects on the corrosion resistance of sputter-deposited TiAlN hard coatings. Coatings 2019, 9, 511. [Google Scholar] [CrossRef]
- PalDey, S.; Deevi, S. Single layer and multilayer wear resistant coatings of (Ti,Al)N: A review. Mater. Sci. Eng. A 2003, 342, 58–79. [Google Scholar] [CrossRef]
- Davis, J.R. Alloying: Understanding the basic. In Carbon and Alloys Steels, 1st ed.; The Materials Information Society Editorial; ASM International®: Materials Park, OH, USA, 2001; pp. 123–125. Available online: https://www.asminternational.org/documents/10192/1849770/ACFAAA3.pdf (accessed on 29 March 2021).
- Rorrer, R.A.L.; Mabie, H.H.; Eiss, N.S., Jr.; Furey, M.J. The wear and friction of polyvinyl chloride coatings under fretting Conditions. Tribol. Trans. 1988, 31, 98–104. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, W.; Liu, H.; Cai, C. Structural and electrical properties of Ti-W-N thin films deposited by reactive RF sputtering. Phys. Procedia 2011, 18, 66–72. [Google Scholar] [CrossRef]
- ASTM G102. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. 1994. Available online: https://www.google.com.mx/search?q=Standard+practice+for+calculation+of+corrosion+rates+and+related+information+from+electrochemical+measurements&source=hp&ei=-KJwYLihDcLSsAWkjazoDA&iflsig=AINFCbYAAAAAYHCxCJqDW4vQOw_q2oNGeUq6L0_C_zjx&oq=Standard+practice+for+calculation+of+corrosion+rates+and+related+information+from+electrochemical+measurements&gs_lcp=Cgdnd3Mtd2l6EANQxRNYxRNg4BpoAXAAeACAAQCIAQCSAQCYAQCgAQKgAQGqAQdnd3Mtd2l6sAEA&sclient=gws-wiz&ved=0ahUKEwi4_d-V6_HvAhVCKawKHaQGC80Q4dUDCAc&uact=5 (accessed on 29 March 2021).
- Calculation of Corrosion Rate. Available online: https://www.gamry.com/Framework%20Help/HTML5%20-%20Tripane%20-%20Audience%20A/Content/EFM/Introduction/Calculation%20of%20Corrosion%20Rate.htm (accessed on 1 January 2021).
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An electrochemical impedance spectroscopy study of the corrosion behaviour of PVD coated steels in 0.5N NaCl aqueous solution: Part II. EIS interpretation of corrosion behaviour. Corr. Sci. 2003, 45, 1257–1273. [Google Scholar] [CrossRef]
- Tato, W.; Landolt, D. Electrochemical determination of the porosity of single and duplex PVD coatings of titanium and titanium nitride on brass. J. Electrochem. Soc. 1998, 145, 4173–4181. [Google Scholar] [CrossRef]
- Alegre, D.; Acsente, T.; Martin-Rojo, A.B.; Oyarzabal, E.; Tabarés, F.L.; Dinescu, G.; De Temmerman, G.; Birjega, R.; Logofatu, C.; Kovac, J.; et al. Characterization of tungsten nitride layers and their erosion under plasma exposure in nano-psi. Rom. Rep. Phys. 2015, 67, 532–546. Available online: https://www.researchgate.net/publication/278385220_Characterisation_of_tungsten_nitride_layers_and_their_erosion_under_plasma_exposure_in_NANO-PSI (accessed on 7 April 2021).
- Caicedo, J.; Yate, L.; Montes, J. Improving the physicochemical surface properties on AISI D3 steel coated with Ti-W-N. Surf. Coat. Technol. 2011, 205, 2947–2953. [Google Scholar] [CrossRef]
- Sangiovanni, D.; Hultman, L.; Chirita, V.; Petrov, I.; Greene, J. Effects of phase stability, lattice ordering, and electron density on plastic deformation in cubic TiWN pseudobinary transition-metal nitride alloys. Acta Mater. 2016, 103, 823–835. [Google Scholar] [CrossRef]
- Gusev, A.I. Phase equilibria, phases and chemical compounds in the T-C system. Russ. Chem. Rev. 2002, 71, 439–463. [Google Scholar] [CrossRef]
- Jung, W.; Lee, H.; Nam, K.; Han, J. The synthesis of W–Ti–C films with a control of element composition by hybrid system. Surf. Coat. Technol. 2005, 200, 721–725. [Google Scholar] [CrossRef]
- Kwon, H.; Moon, A.; Kim, W.; Kim, J. Investigation of the conditions required for the formation of (Ti,W)(CN) during carburization of titanium under nitrogen. Mater. Lett. 2017, 209, 287–290. [Google Scholar] [CrossRef]
- Holleck, H.; Schulz, H. Preparation and behaviour of wear-resistant TiC/TiB2, TiN/TiB2 and TiC/TiN coatings with high amounts of phase boundaries. Surf. Coat. Technol. 1988, 36, 707–714. [Google Scholar] [CrossRef]
- Azadi, M.; Rouhaghdam, A.S.; Ahangarani, S.; Mofidi, H. Mechanical behavior of TiN/TiC multilayer coatings fabricated by plasma assisted chemical vapor deposition on AISI H13 hot work tool steel. Surf. Coat. Technol. 2014, 245, 156–166. [Google Scholar] [CrossRef]
- Iwai, Y.; Miyajima, T.; Mizuno, A.; Honda, T.; Itou, T.; Hogmark, S. Micro-slurry-jet erosion (MSE) testing of CVD TiC/TiN and TiC coatings. Wear 2009, 267, 264–269. [Google Scholar] [CrossRef]
- Hurkmans, T.; Trinh, T.; Lewis, D.; Brooks, J.; Münz, W.-D. Multilayered titanium tungsten nitride coatings with a superlattice structure grown by unbalanced magnetron sputtering. Surf. Coat. Technol. 1995, 76–77, 159–166. [Google Scholar] [CrossRef]
- Ramarotafika, H.; Lemperiere, G. Influence of a d.c. substrate bias on the resistivity, composition, crystallite size and microstrain of WTi and WTi-N films. Thin Solid Films 1995, 266, 267–273. [Google Scholar] [CrossRef]
- Jalali, R.; Parhizkar, M.; Bidadi, H.; Naghshara, H.; Eshraghi, M.J. Characterization of nano-crystalline Ti–W–N thin films for diffusion barrier application: A structural, microstructural, morphological and mechanical study. Appl. Phys. A 2018, 124, 810. [Google Scholar] [CrossRef]
- Djafer, Z.A.A.; Saoula, N.; Madaoui, N.; Zerizer, A. Deposition and characterization of titanium carbide thin films by magnetron sputtering using Ti and TiC targets. Appl. Surf. Sci. 2014, 312, 57–62. [Google Scholar] [CrossRef]
- Vernon, S.P.; Stearns, D.G.; Rosen, R.S. Ion-assisted sputter deposition of molybdenum-silicon multilayers. Appl. Opt. 1993, 32, 6969–6974. [Google Scholar] [CrossRef]
- Xu, J.; He, T.; Chai, L.; Qiao, L.; Wang, P.; Liu, W. Growth and characteristics of self-assembled MoS2/Mo-S-C nanoperiod multilayers for enhanced tribological performance. Sci. Rep. 2016, 6, 25378. [Google Scholar] [CrossRef]
- Järrendahl, K.; Ivanov, I.; Sundgren, J.-E.; Radnóczi, G.; Czigany, Z.; Greene, J.E. Microstructure evolution in amorphous Ge/Si multilayers grown by magnetron sputter deposition. J. Mater. Res. 1997, 12, 1806–1815. [Google Scholar] [CrossRef]
- Cancellieri, C.; Klyatskina, E.; Chiodi, M.; Janczak-Rusch, J.; Jeurgens, L.P.H. The Effect of interfacial Ge and RF-bias on the microstructure and stress evolution upon annealing of Ag/AlN multilayers. Appl. Sci. 2018, 8, 2403. [Google Scholar] [CrossRef]
- Soto, G.; De La Cruz, W.; Farıas, M. XPS, AES, and EELS characterization of nitrogen-containing thin films. J. Electron Spectrosc. Relat. Phenom. 2004, 135, 27–39. [Google Scholar] [CrossRef]
- Restrepo, P.E.; Arango, A.P.J.; Benavides, P.V.J. XPS structure analysis of tin/tic bilayers produced by pulsed vacuum arc discharge. Dyna 2010, 77, 64–74. Available online: http://www.scielo.org.co/scielo.php?script=sci_arttext&pid=s0012-73532010000300007&lng=en&nrm=iso> (accessed on 1 April 2021).
- Jiang, N.; Zhang, H.; Bao, S.; Shen, Y.; Zhou, Z. XPS study for reactively sputtered titanium nitride thin films deposited under different substrate bias. Phys. B Condens. Matter 2004, 352, 118–126. [Google Scholar] [CrossRef]
- Grant, J.T. Analysis of surfaces and thin films by using auger electron spectroscopy and X-ray photoelectron spectroscopy. J. Korean Phys. Soc. 2007, 51, 295–932. [Google Scholar] [CrossRef]
- Castillo, H.; Restrepo-Parra, E.; Arango-Arango, P. Chemical and morphological difference between TiN/DLC and a-C:H/DLC grown by pulsed vacuum arc techniques. Appl. Surf. Sci. 2011, 257, 2665–2668. [Google Scholar] [CrossRef]
- Del Pino, A.P.; György, E.; Marcus, I.C.; Roqueta, J.; Alonso, M.I. Effects of pulsed laser radiation on epitaxial self-assembled Ge quantum dots grown on Si substrates. Nanotechnology 2011, 22, 295304. [Google Scholar] [CrossRef]
- Petrović, S.; Bundaleski, N.; Perusko, D.; Radović, M.; Kovač, J.; Mitrić, M.; Gaković, B.; Rakočević, Z. Surface analysis of the nanostructured W–Ti thin film deposited on silicon. Appl. Surf. Sci. 2007, 253, 5196–5202. [Google Scholar] [CrossRef]
- Suseendran, J.; Halder, N.; Chakrabarti, S.; Mishima, T.; Stanley, C. Stacking of multilayer InAs quantum dots with combination capping of InAlGaAs and high temperature grown GaAs. Superlattices Microstruct. 2009, 46, 900–906. [Google Scholar] [CrossRef]
- Voronov, D.L.; Gawlitza, P.; Cambie, R.; Dhuey, S.; Gullikson, E.M.; Warwick, T.; Braun, S.; Yashchuk, V.V.; Padmore, H.A. Conformal growth of Mo/Si multilayers on grating substrates using collimated ion beam sputtering. J. Appl. Phys. 2012, 111, 093521. [Google Scholar] [CrossRef]
- Moreno, H.; Caicedo, J.; Amaya, C.; Saldaña, J.M.; Yate, L.; Esteve, J.; Prieto, P. Enhancement of surface mechanical properties by using TiN[BCN/BN]n/c-BN multilayer system. Appl. Surf. Sci. 2010, 257, 1098–1104. [Google Scholar] [CrossRef]
- Perez, N. Chapter in 6: Corrosivity and Passivity. In Electrochemistry and Corrosion Science; Springer: Cham, Switzerland, 2016; pp. 199–264. [Google Scholar] [CrossRef]
- Ricker Richard, E. Difference between Corrosion Potential and Corrosion Current. Which is More Detrimental for Corrosion? 2017. Available online: https://www.researchgate.net/post/Difference_between_corrosion_potential_and_corrosion_current_Which_is_more_detrimental_for_corrosion/59b7e6e5217e20269876a2bc/citation/download (accessed on 1 March 2021).
- Zha, L.; Li, H.; Wang, N. In situ electrochemical study of the growth kinetics of passive film on TC11 alloy in sulfate solution at 300 °C/10 MPa. Materials 2020, 13, 1135. [Google Scholar] [CrossRef]
- Anthony, E.H.; Johannes, M.C.M.; Zheludkevich, L.; Rudolph, G.B. Book charter in: G.S. Frankel, Part I Fundamentals. Fundamentals of corrosion kinetics. In Active Protective Coatings: New-Generation Coatings for Metals; Springer: Berlin/Heidelberg, Germany, 2016; pp. 17–57. Available online: http://www.daryatamin.com/uploads/Books%20File/Active%20Protective%20Coatings.pdf (accessed on 1 March 2021).
- Senna, L.F.; Achete, C.A.; Hirsch, T.; Freire, F.L., Jr. Structural, chemical and corrosion resistance characterization of TiCN coatings prepared by magnetron sputtering. Surf. Coat. Technol. 1997, 94–95, 390–397. [Google Scholar] [CrossRef]
- Alves, V.A.; Brett, C.M.; Cavaleiro, A. Electrochemical corrosion of magnetron sputtered WTiN-coated mild steels in a chloride medium. Surf. Coat. Technol. 2002, 161, 257–266. [Google Scholar] [CrossRef][Green Version]
- Madaoui, N.; Saoula, N.; Zaid, B.; Saidi, D.; Ahmed, A.S. Structural, mechanical and electrochemical comparison of TiN and TiCN coatings on XC48 steel substrates in NaCl 3.5% water solution. Appl. Surf. Sci. 2014, 312, 134–138. [Google Scholar] [CrossRef]
- Rahmati, B.; Sarhan, A.A.D.; Zalnezhad, E.; Kamiab, Z.; Dabbagh, A.; Choudhury, D.; Abas, W. Development of tantalum oxide (Ta-O) thin film coating on biomedical Ti-6Al-4V alloy to enhance mechanical properties and biocompatibility. Ceram. Int. 2016, 42, 466–480. [Google Scholar] [CrossRef]
- Massiani, Y.; Medjahed, A.; Gravier, P.; Argème, L.; Fedrizzi, L. Electrochemical study of titanium nitride films obtained by reactive sputtering. Thin Solid Films 1990, 191, 305–316. [Google Scholar] [CrossRef]
- Jehn, H. Improvement of the corrosion resistance of PVD hard coating–substrate systems. Surf. Coat. Technol. 2000, 125, 212–217. [Google Scholar] [CrossRef]
- Grips, V.W.; Selvi, V.E.; Barshilia, H.; Rajam, K. Effect of electroless nickel interlayer on the electrochemical behavior of single layer CrN, TiN, TiAlN coatings and nanolayered TiAlN/CrN multilayer coatings prepared by reactive dc magnetron sputtering. Electrochim. Acta 2006, 51, 3461–3468. [Google Scholar] [CrossRef]
- Barsoukov, E.; Macdonald, J.R. Book chapter in: 1.3 Elementary analysis of impedance spectra. In Impedance Spectroscopy Theory, Experiment, and Applications, 2nd ed.; Wiley-Interscience, Jonn Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; pp. 13–20. ISBN 0-471-64749-7. [Google Scholar]
- Waters, N.; Connolly, R.; Brown, D.; Laskowski, B. Electrochemical impedance spectroscopy for coating evaluation using a micro sensor. In Proceedings of the Annual Conference of the Prognostics and Health Management Society, Fort Worth, TX, USA, 29 September–2 October 2014; Volume 5, pp. 1–6. Available online: https://www.semanticscholar.org/paper/Electrochemical-Impedance-Spectroscopy-for-Coating-Waters-Connolly/7c58d6b539466073b319333db9b10c8a2374f7ad (accessed on 1 March 2021).








| Parameter | Ti | Ti-W-N | Ti-W-C | n = 1 | n = 40 |
|---|---|---|---|---|---|
| Power (W) | |||||
| Ti | 450 | 350 | 350 | 350 | 350 |
| W | – | 420 | 420 | 420 | 420 |
| Flux (sccm) | |||||
| Ar | 50 | 50 | 50 | 50/50 | 50/50 |
| N2 | 0 | 12 | 0 | 12/0 | 12/0 |
| CH4 | 0 | 0 | 16 | 0/16 | 0/16 |
| Deposition time (min.) | 20 | 180 | 180 | 90/90 | (2.25/2.25) |
| Total Thickness (μm) | 0.21 | 2.81 | 3.54 | 1.57 + 1.20 = 2.77 | 3.57 |
| Material Type | Element | |||
|---|---|---|---|---|
| C | N | W | Ti | |
| Ti-W-N | – | 24.16 | 35.72 | 40.12 |
| Ti-W-C | 20.30 | – | 36.97 | 42.72 |
| Material Type | Ecorr (V vs. Ag/AgCl) | icorr (A·cm2) | CR (mm·year−1) |
|---|---|---|---|
| AISI 1060 | −1.13 | 4.2 × 10−5 | 3.12 |
| Ti-W-N | −0.86 | 5.7 × 10−6 | 0.58 |
| Ti-W-C | −0.69 | 2.2 × 10−6 | 0.42 |
| n = 1 | −0.55 | 2.0 × 10−6 | 0.35 |
| n = 40 | −0.26 | 1.08 × 10−6 | 0.19 |
| Material Type | Rp (kΩ·cm2) | CPE1 S × sn | n1 | CPE2 S × sn | n2 |
|---|---|---|---|---|---|
| AISI 1060 | 0.32 | 1.87 × 10−9 | 0.72 | 26.09 × 10−9 | 0.92 |
| Ti-W-N | 12.15 | 8.70 × 10−7 | 0.84 | 119.7 × 10−6 | 0.85 |
| Ti-W-C | 16.48 | 0.74 × 10−6 | 0.94 | 133.6 × 10−6 | 0.86 |
| n = 1 | 32.02 | 6.66 × 10−6 | 0.82 | 177.9 × 10−6 | 0.86 |
| n = 40 | 56.82 | 8.70 × 10−6 | 0.92 | 20.97 × 10−6 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Hernández, A.; Morales-Cepeda, A.B.; Flores, M.; Caicedo, J.C.; Aperador, W.; Amaya, C. Electrochemical Properties of TiWN/TiWC Multilayer Coatings Deposited by RF-Magnetron Sputtering on AISI 1060. Coatings 2021, 11, 797. https://doi.org/10.3390/coatings11070797
González-Hernández A, Morales-Cepeda AB, Flores M, Caicedo JC, Aperador W, Amaya C. Electrochemical Properties of TiWN/TiWC Multilayer Coatings Deposited by RF-Magnetron Sputtering on AISI 1060. Coatings. 2021; 11(7):797. https://doi.org/10.3390/coatings11070797
Chicago/Turabian StyleGonzález-Hernández, Andrés, Ana Beatriz Morales-Cepeda, Martín Flores, Julio C. Caicedo, William Aperador, and César Amaya. 2021. "Electrochemical Properties of TiWN/TiWC Multilayer Coatings Deposited by RF-Magnetron Sputtering on AISI 1060" Coatings 11, no. 7: 797. https://doi.org/10.3390/coatings11070797
APA StyleGonzález-Hernández, A., Morales-Cepeda, A. B., Flores, M., Caicedo, J. C., Aperador, W., & Amaya, C. (2021). Electrochemical Properties of TiWN/TiWC Multilayer Coatings Deposited by RF-Magnetron Sputtering on AISI 1060. Coatings, 11(7), 797. https://doi.org/10.3390/coatings11070797







