Fabrication and Sterilization Characteristics of Visible Light Photocatalyst of CuO/ZrO2/CB/Coal-Tar-Pitch-SAC
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Preparation of ZrO2/CB/Coal-Tar-Pitch-SAC
2.4. Preparation of CuO/ZrO2/CB/Coal-Tar-Pitch-SAC
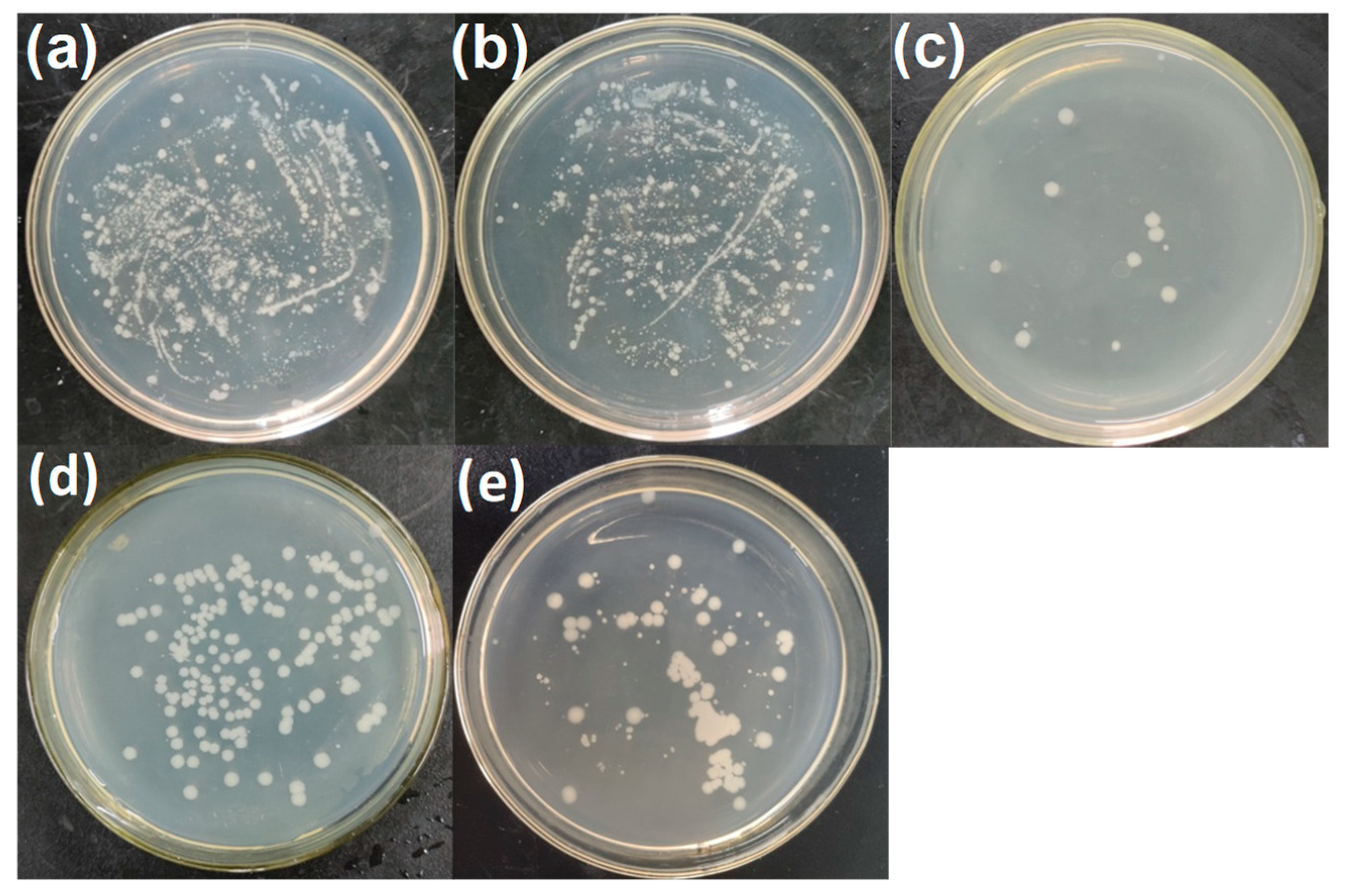
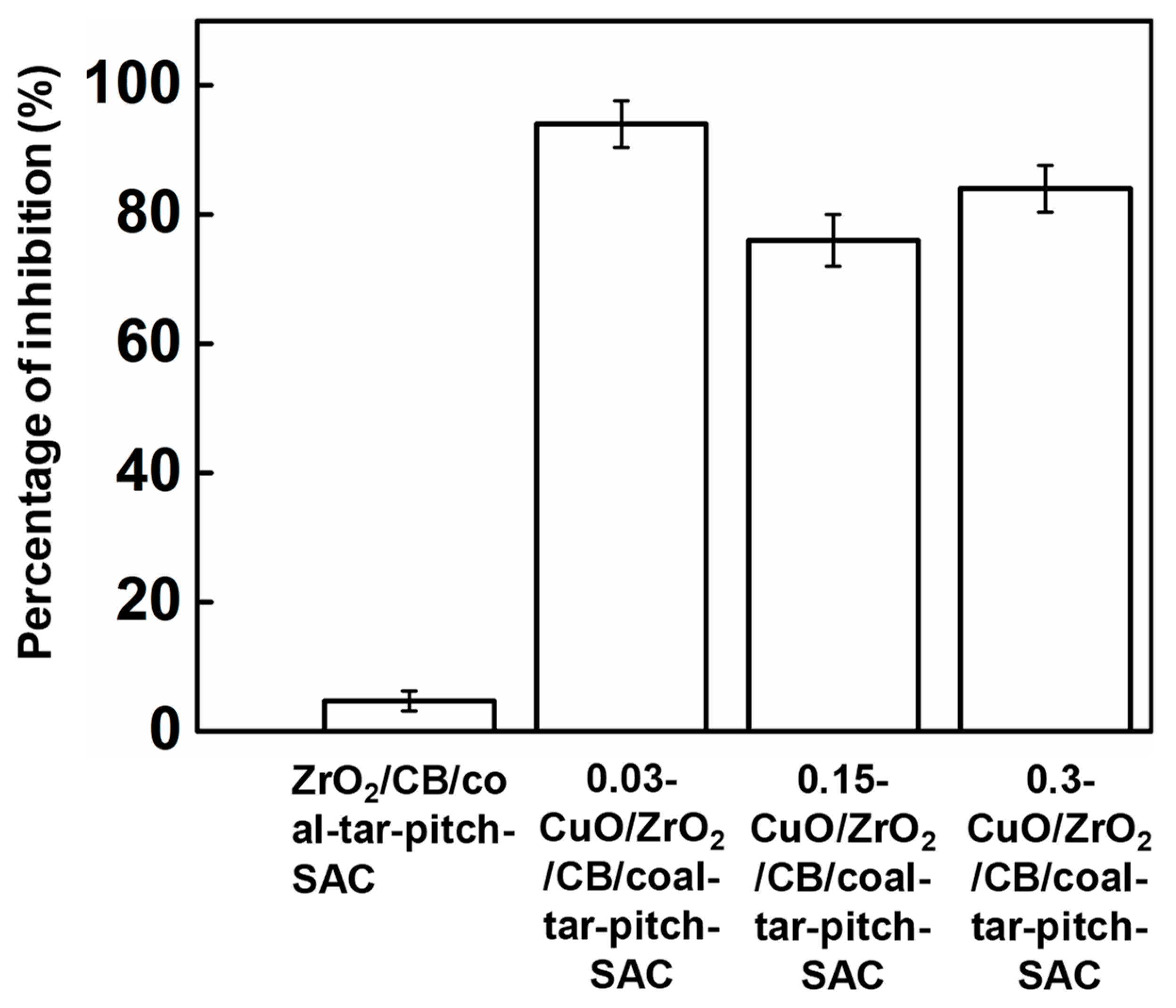
2.5. Sterilizing Tests of CuO/ZrO2/CB/Coal-Tar-Pitch-SAC
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, Q.; Song, Q.; Li, P.; Huang, W. Rejuvenated photodynamic therapy for bacterial infections. Adv. Healthc. Mater. 2019, 8, 1900608. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Tian, W.; Bai, H.; Zhang, J.; Wang, S.; Zhang, J. Sunlightdriven wearable and robust antibacterial coatings with water-soluble cellulose-based photosensitizers. Adv. Healthc. Mater. 2019, 8, 1801591. [Google Scholar] [CrossRef] [PubMed]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. Review on the improvement of the photocatalytic and antibacterial activities of ZnO. J. Alloys Compd. 2017, 727, 792–820. [Google Scholar] [CrossRef]
- Lee, M.M.S.; Xu, W.; Zheng, L.; Yu, B.; Leung, A.C.S.; Kwok, R.T.K.; Lam, J.W.Y.; Xu, F.J.; Wang, D.; Tang, B.Z. Ultrafast discrimination of Gram-positive bacteria and highly efficient photodynamic antibacterial therapy using near-infrared photosensitizer with aggregation-induced emission characteristics. Biomaterials 2020, 230, 119582. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Ojha, A.K.; Singh, A.; Hirsch, F.; Fischer, I.; Patrice, D.; Materny, A. Well-controlled in-situ growth of 2D WO3 rectangular sheets on reduced graphene oxide with strong photocatalytic and antibacterial properties. J. Hazard. Mater. 2018, 347, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.C.; Jeong, E.; Kim, E.; Hong, S.W. Bio-organic-inorganic hybrid photocatalyst, TiO2 and glucose oxidase composite for enhancing antibacterial performance in aqueous environments. Appl. Catal. 2019, 242, 194–201. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, D. On the controllable soft-templating approach to mesoporous silicates. Chem. Rev. 2007, 107, 2821–2831. [Google Scholar]
- Yamamoto, O.; Sawai, J.; Sasamoto, T. Activated carbon sphere with antibacterial characteristics. Mater. Trans. 2005, 43, 1069–1073. [Google Scholar] [CrossRef] [Green Version]
- Shen, L.Z.; Xu, G.Y.; Han, B.B.; Kato, S.K.; Dai, Y.Y.; Ge, H.; Wang, K.; Sun, F.; Zhou, W.M. Study on fabrication and sterilization characteristics of novel composite spherical activated carbon (TiO2/CB/SAC). J. Chem. Eng. Jpn. 2020, 53, 526–532. [Google Scholar] [CrossRef]
- Hirvonen, A.; Nowak, R.; Yamamoto, Y.; Sekino, T.; Niihara, K. Fabrication, structure, mechanical and thermal properties of zirconia-based ceramic nanocomposites. J. Eur. Ceram. Soc. 2006, 26, 1497–1505. [Google Scholar] [CrossRef]
- Chandra, N.; Singh, D.K.; Sharma, M.; Upadhyay, R.K.; Amritphale, S.S.; Sanghi, S.K. Synthesis and characterization of nano-sized zirconia powder synthesized by single emulsion-assisted direct precipitation. J. Colloid. Interf. Sci. 2010, 342, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Kaviyarasu, K.; Kotsedi, L.; Simo, A.; Fuku, X.; Mola, G.T.; Kennedy, J.; Maaza, M. Photocatalytic activity of ZrO2 doped lead dioxide nanocomposites: Investigation of structural and optical microscopy of RhB organic dye. Appl. Surf. Sci. 2016, 421, 234–239. [Google Scholar] [CrossRef]
- Qin, Y.M.; Ding, Z.Y.; Guo, W.W.; Guo, X.L.; Hou, C.; Jiang, B.P.; Liu, C.G.; Shen, X.C. A full solar light spectrum responsive B@ZrO2–OV photocatalyst: A synergistic strategy for visible-to-NIR photon harvesting. ACS Sustain. Chem. Eng. 2020, 8, 13039–13047. [Google Scholar] [CrossRef]
- Fechete, I.; Ye, W.; Védrine, J.C. The past, present and future of heterogeneous catalysis. Catal. Today 2012, 189, 2–27. [Google Scholar] [CrossRef]
- Yu, S.; Liu, J.; Yan, Z.; Richard, D.W.; Yan, X.L. Effect of synthesis method on the nanostructure and solar-driven photocatalytic properties of TiO2-CuS composites. ACS Sustain. Chem. Eng. 2017, 5, 1347–1357. [Google Scholar] [CrossRef]
- Li, J.X.; Guan, R.Q.; Zhang, J.K.; Zhao, Z.; Zhai, H.J.; Sun, D.W.; Qi, Y.F. Preparation and photocatalytic performance of dumbbell Ag2CO3−ZnO heterojunctions. ACS Omega 2020, 5, 570–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.Q.; Cui, M.M.; Guo, Z.; Liu, Z.X.; Zhu, Z.F. Synthesis of dumbbell-like CuO–BiVO4 heterogeneous nanostructures with enhanced visible-light photocatalytic activity. Mater. Lett. 2014, 130, 36–39. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Xiao, Z.Y.; Liu, D.; Wang, S.; Zhang, J.J.; Hao, Y.T.; Zhang, W.Z. Hollow sphere TiO2–ZrO2 prepared by self-assembly with polystyrene colloidal template for both photocatalytic degradation and H2 evolution from water splitting. ACS Sustain. Chem. Eng. 2016, 4, 2037–2046. [Google Scholar] [CrossRef]
- Ayodeji, P.A.; Reyes-Lopéz, S.Y. ZrO2−ZnO nanoparticles as antibacterial agents. ACS Omega 2019, 4, 19216–19224. [Google Scholar]
- Kargar, A.; Jing, Y.; Kim, S.J.; Riley, C.T.; Pan, X.Q.; Wang, D. ZnO/CuOheterojunction branched nanowires for photoelectrochemical hydrogen generation. ACS Nano 2013, 7, 11112–11120. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Liu, Z.; Xia, P.; Xie, Y.; Xiong, D. Synthesis of 0D/3D CuO/ZnOheterojunction with enhanced photocatalytic activity. J. Phys. Chem. B. 2018, 122, 9531–9539. [Google Scholar]
- Raees, A.; Jamal, M.A.; Ahmed, I.; Silanpaa, M.; Algarni, T.S. Synthesis and characterization of CeO2/CuOnanocomposites for photocatalytic degradation of methylene blue in visible light. Coatings 2021, 11, 305. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Yang, Z.; Yang, H. Theoretical and practical discussion of measurement accuracy for physisorption with micro- and mesoporous materials. Chin. J. Catal. 2013, 34, 1797–1810. [Google Scholar] [CrossRef]
- Hu, S.X.; Hsieh, Y.L. Lignin derived activated carbon particulates as an electric supercapacitor: Carbonization and activatioon porous structures and microstructures. RSC Adv. 2017, 7, 30459–30468. [Google Scholar] [CrossRef] [Green Version]
- Kupgan, G.; Liyana-Arachchi, T.P.; Colina, C.M. NLDFT Pore Size Distribution in Amorphous Microporous Materials. Langmuir 2017, 33, 11138–11145. [Google Scholar] [CrossRef] [Green Version]
- Gong, J.; Zhao, Z.J.; Wang, Z.; Luo, R.; Wang, Y. Strong electronic oxidesupport interaction over In2O3/ZrO2 for highly selective CO2 hydrogenation to methanol. J. Am. Chem. Soc. 2020, 142, 19523–19531. [Google Scholar]
- Nova, C.V.; Reis, K.A.; Pinheiro, A.L.; Dalmaschio, C.J.; Chiquito, A.J.; Teodoro, M.D.; Rodrigues, A.D. Synthesis, characterization, photocatalytic, and antimicrobial activity of ZrO2 nanoparticles and Ag@ZrO2nanocomposite prepared by the advanced oxidative process/hydrothermal route. J. Sol. Gel. Sci. Technol. 2021, 98, 113–126. [Google Scholar] [CrossRef]
- Lou, X.; Shang, J.; Liang, W.; Feng, H.; Hao, W.; Wang, T.; Du, Y. Enhanced photocatalytic activity of Bi24O31Br10: Constructing heterojunction with BiOI. J. Mater. Sci. Technol. 2017, 33, 281–284. [Google Scholar] [CrossRef]

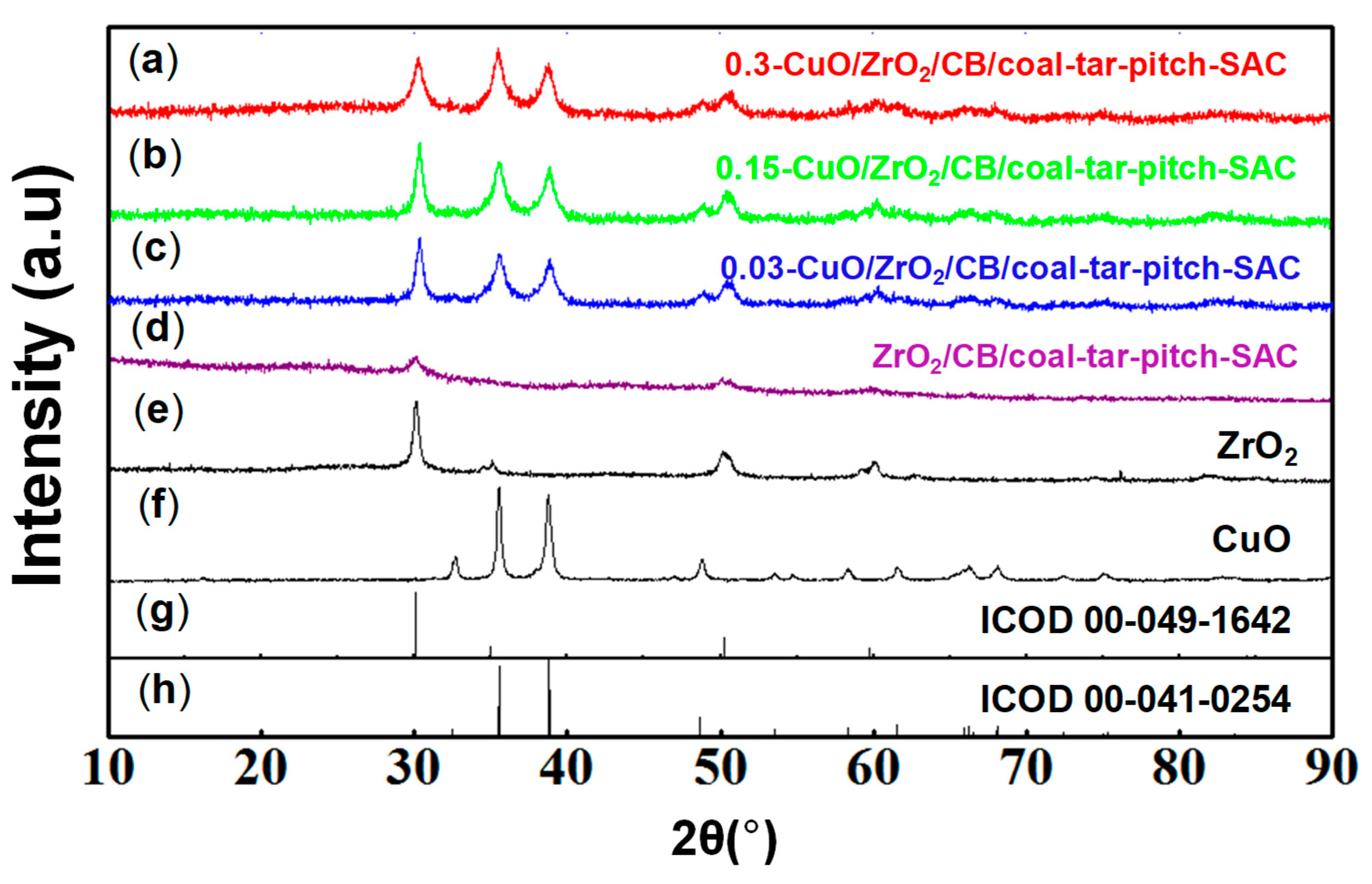

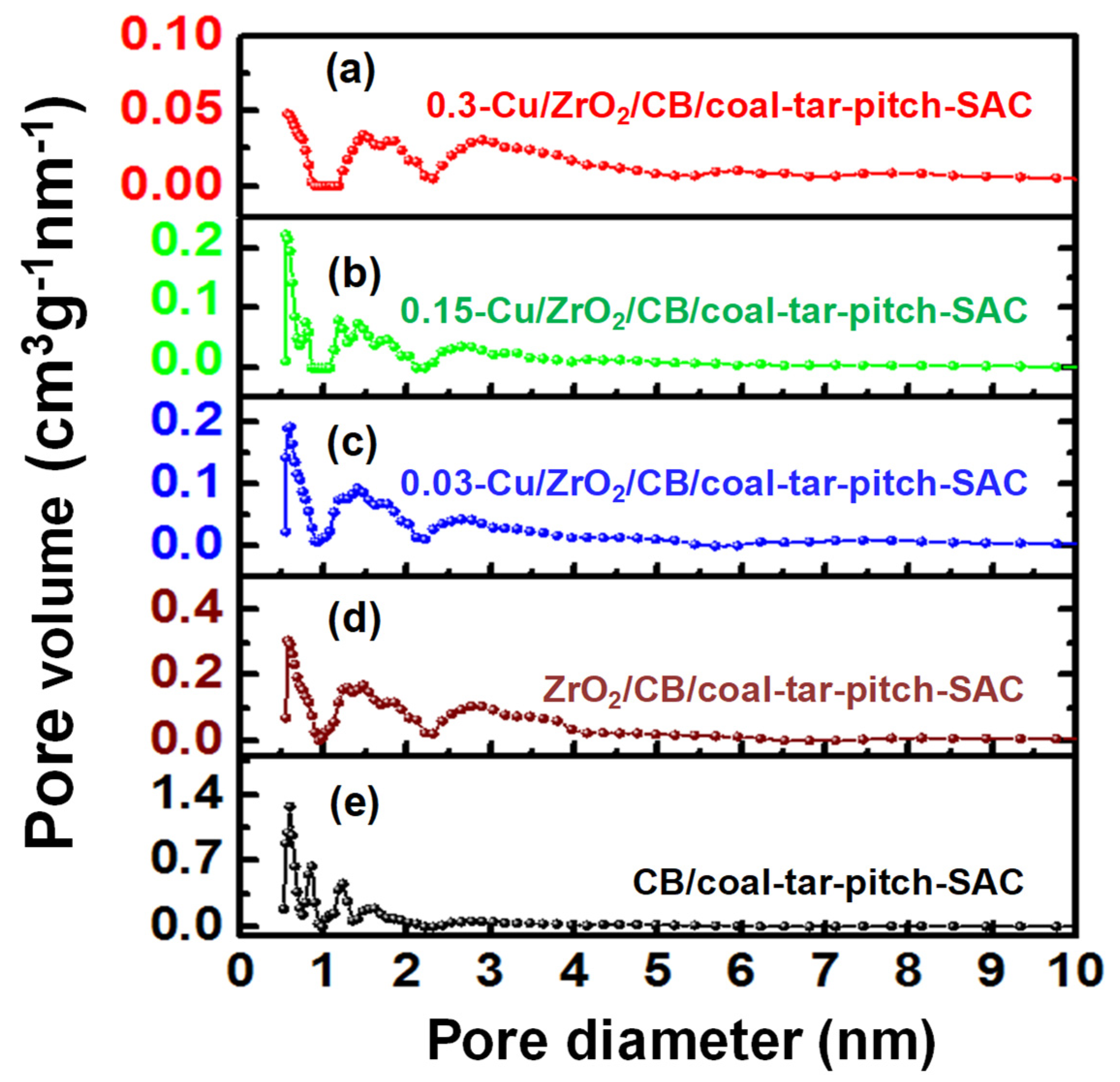
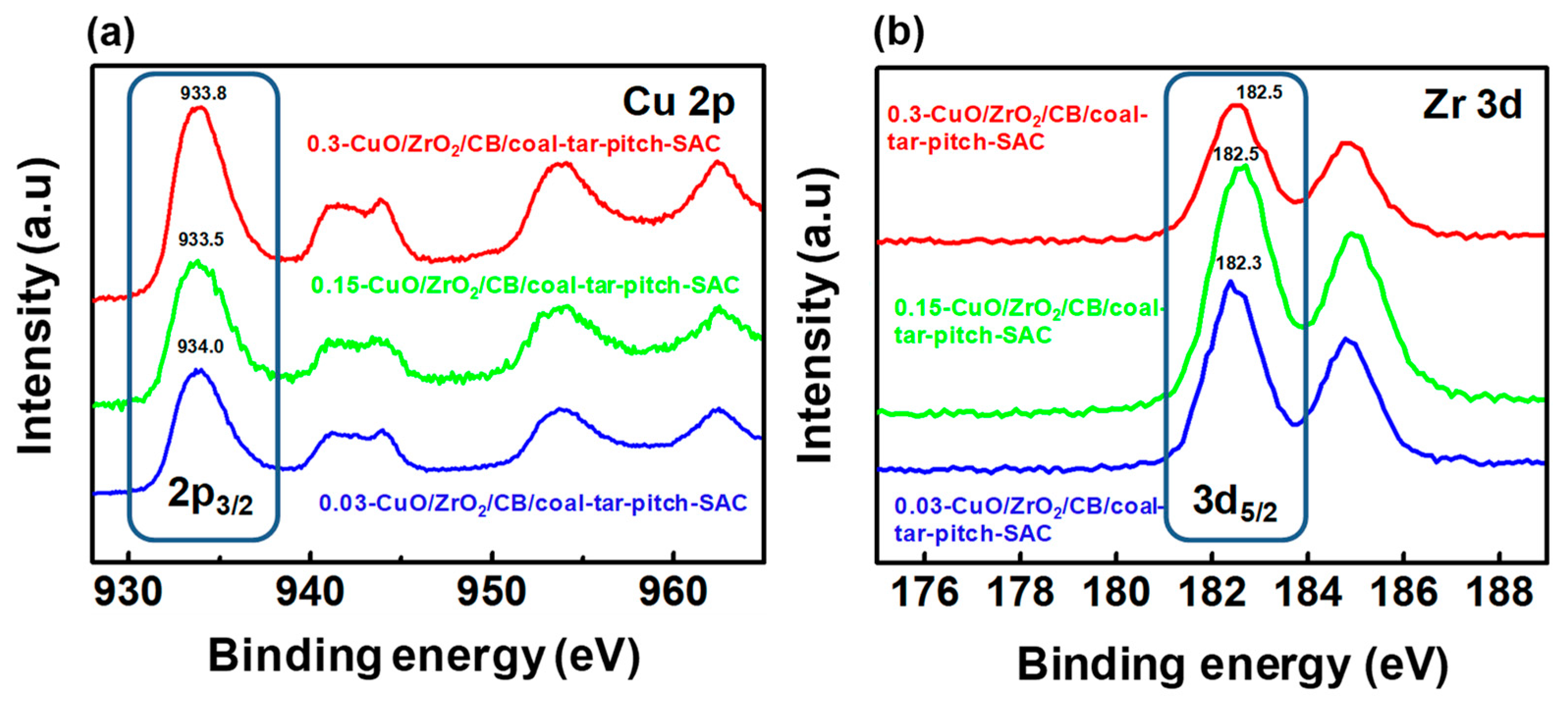

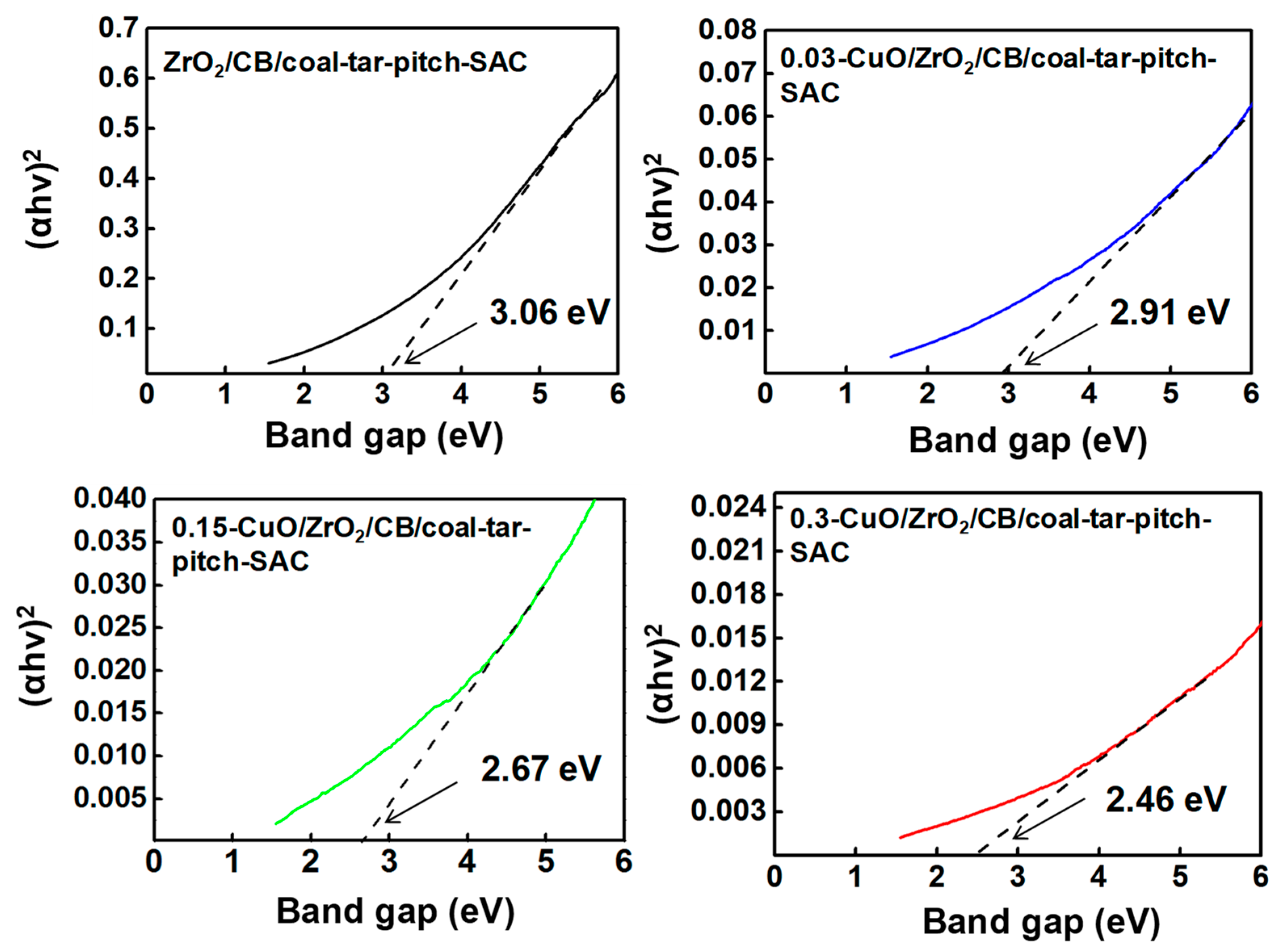
| Samples | SBET (m2·g−1) | Vtotal (cm3·g−1) |
|---|---|---|
| CB/coal-tar-pitch-SAC | 1321.8 | 0.52 |
| ZrO2/CB/coal-tar-pitch-SAC | 631.6 | 0.49 |
| 0.03-CuO/ZrO2/CB/coal-tar-pitch-SAC | 330.8 | 0.24 |
| 0.15-CuO/ZrO2/CB/coal-tar-pitch-SAC | 210.6 | 0.19 |
| 0.3-CuO/ZrO2/CB/coal-tar-pitch-SAC | 131.9 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Xu, G.; Han, B.; Wang, K.; Ge, H.; An, B.; Ju, D.; Chai, M.; Li, L.; Zhou, W. Fabrication and Sterilization Characteristics of Visible Light Photocatalyst of CuO/ZrO2/CB/Coal-Tar-Pitch-SAC. Coatings 2021, 11, 816. https://doi.org/10.3390/coatings11070816
Xu Z, Xu G, Han B, Wang K, Ge H, An B, Ju D, Chai M, Li L, Zhou W. Fabrication and Sterilization Characteristics of Visible Light Photocatalyst of CuO/ZrO2/CB/Coal-Tar-Pitch-SAC. Coatings. 2021; 11(7):816. https://doi.org/10.3390/coatings11070816
Chicago/Turabian StyleXu, Ziang, Guiying Xu, Beibei Han, Kun Wang, Hui Ge, Baigang An, Dongying Ju, Maorong Chai, Lixiang Li, and Weimin Zhou. 2021. "Fabrication and Sterilization Characteristics of Visible Light Photocatalyst of CuO/ZrO2/CB/Coal-Tar-Pitch-SAC" Coatings 11, no. 7: 816. https://doi.org/10.3390/coatings11070816
APA StyleXu, Z., Xu, G., Han, B., Wang, K., Ge, H., An, B., Ju, D., Chai, M., Li, L., & Zhou, W. (2021). Fabrication and Sterilization Characteristics of Visible Light Photocatalyst of CuO/ZrO2/CB/Coal-Tar-Pitch-SAC. Coatings, 11(7), 816. https://doi.org/10.3390/coatings11070816







