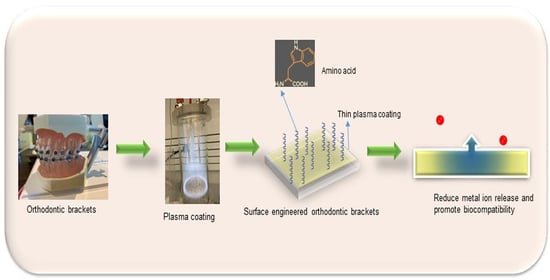
Bioactive Plasma Coatings on Orthodontic Brackets: In Vitro Metal Ion Release and Cytotoxicity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Plasma Coating of Orthodontic Brackets
2.2.2. Surface Functionalisation of Orthodontic Brackets
2.2.3. Physico-Chemical Characterisation
3. Results and Discussion
3.1. Surface Roughness
3.2. Bulk Chemistry
3.3. Surface Chemistry
3.4. ToF-SIMS
3.5. Quantification of Adsorbed Amino Acids
3.6. Metal Ion Release
3.7. Cytotoxicity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ntasi, A.; Al Jabbari, Y.S.; Silikas, N.; Eliades, T.; Zinelis, S. Multitechnique characterization of conventional and experimental Ag-based brazing alloys for orthodontic applications. Dent. Mater. 2018, 34, e25–e35. [Google Scholar] [CrossRef]
- Redlich, M.; Katz, A.; Rapoport, L.; Wagner, H.; Feldman, Y.; Tenne, R. Improved orthodontic stainless steel wires coated with inorganic fullerene-like nanoparticles of WS2 impregnated in electroless nickel–phosphorous film. Dent. Mater. 2008, 24, 1640–1646. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef] [Green Version]
- Tracana, R.B.; Sousa, J.; Carvalho, G.S. Mouse inflammatory response to stainless steel corrosion products. J. Mater. Sci. Mater. Electron. 1994, 5, 596–600. [Google Scholar] [CrossRef]
- Tracana, R.B.; Pereira, M.D.L.; Abreu, A.M.; Sousa, J.; Carvalho, G.S. Stainless steel corrosion products cause alterations on mouse spleen cellular populations. J. Mater. Sci. Mater. Electron. 1995, 6, 56–61. [Google Scholar] [CrossRef]
- Ortiz, A.J.; Fernández, E.; Vicente, A.; Calvo, J.L.; Ortiz, C. Metallic ions released from stainless steel, nickel-free, and tita-nium orthodontic alloys: Toxicity and DNA damage. Am. J. Orthod. Dentofacial. Orthop. 2011, 140, e115–e122. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implants. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Qi, P.; Ding, Y.; Maitz, M.F.; Yang, Z.; Tu, Q.; Xiong, K.; Leng, Y.; Huang, N. A biocompatible and functional adhesive amine-rich coating based on dopamine polymerization. J. Mater. Chem. B 2014, 3, 72–81. [Google Scholar] [CrossRef] [Green Version]
- Metin-Gürsoy, G.; Taner, L.; Akca, G. Nanosilver coated orthodontic brackets: In vivo antibacterial properties and ion re-lease. Eur. J. Orthod. 2016, 39, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, H.-S.; Bae, I.-H.; Lee, K.-G.; Hwang, H.-S.; Lee, K.-H.; Koh, J.-T.; Cho, J.-H. Antibacterial effect of silver-platinum coating for orthodontic appliances. Angle Orthod. 2011, 82, 151–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joseph, B.; Ninan, N.; Visalakshan, R.M.; Denoual, C.; Bright, R.; Kalarikkal, N.; Grohens, Y.; Vasilev, K.; Thomas, S. Insights into the biomechanical properties of plasma treated 3D printed PCL scaffolds decorated with gold nanoparticles. Compos. Sci. Technol. 2021, 202, 108544. [Google Scholar] [CrossRef]
- Forget, A.; Staehly, C.; Ninan, N.; Harding, F.J.; Vasilev, K.; Voelcker, N.H.; Blencowe, A. Oxygen-Releasing Coatings for Improved Tissue Preservation. ACS Biomater. Sci. Eng. 2017, 3, 2384–2390. [Google Scholar] [CrossRef]
- Gillam, T.; Goh, C.; Ninan, N.; Bilimoria, K.; Shirazi, H.; Saboohi, S.; Al-Bataineh, S.; Whittle, J.; Blencowe, A. Iodine complexed poly(vinyl pyrrolidone) plasma polymers as broad-spectrum antiseptic coatings. Appl. Surf. Sci. 2021, 537, 147866. [Google Scholar] [CrossRef]
- Macgregor-Ramiasa, M.N.; Cavallaro, A.; Vasilev, K. Properties and reactivity of polyoxazoline plasma polymer films. J. Mater. Chem. B 2015, 3, 6327–6337. [Google Scholar] [CrossRef]
- MacGregor, M.N.; Michelmore, A.; Shirazi, H.S.; Whittle, J.; Vasilev, K. Secrets of Plasma-Deposited Polyoxazoline Functionality Lie in the Plasma Phase. Chem. Mater. 2017, 29, 8047–8051. [Google Scholar] [CrossRef]
- Park, H.; Kim, K.M.; Lee, A.; Ham, S.; Nam, W.; Chin, J. Bioinspired Chemical Inversion of l-Amino Acids to d-Amino Acids. J. Am. Chem. Soc. 2007, 129, 1518–1519. [Google Scholar] [CrossRef] [PubMed]
- Yazici, H.; O’Neill, M.B.; Kacar, T.; Wilson, B.R.; Oren, E.E.; Sarikaya, M.; Tamerler, C. Engineered Chimeric Peptides as Antimicrobial Surface Coating Agents toward Infection-Free Implants. ACS Appl. Mater. Interfaces 2016, 8, 5070–5081. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A.K.; Choi, J.; Moon, E.; Baek, K.-H. Tryptophan-Rich and Proline-Rich Antimicrobial Peptides. Molecules 2018, 23, 815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bataineh, S.A.; Cavallaro, A.A.; Michelmore, A.; Macgregor, M.N.; Whittle, J.D.; Vasilev, K. Deposition of 2-oxazoline-based plasma polymer coatings using atmospheric pressure helium plasma jet. Plasma Process. Polym. 2019, 16, 1900104. [Google Scholar] [CrossRef]
- Ninan, N.; Albrecht, H.; Blencowe, A. Characterization of Nanomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 129–166. [Google Scholar]
- Ninan, N.; Forget, A.; Shastri, V.P.; Voelcker, N.H.; Blencowe, A. Antibacterial and Anti-Inflammatory pH-Responsive Tannic Acid-Carboxylated Agarose Composite Hydrogels for Wound Healing. ACS Appl. Mater. Interfaces 2016, 8, 28511–28521. [Google Scholar] [CrossRef] [PubMed]
- Vega-Avila, E.; Pugsley, M.K. An overview of colorimetric assay methods used to assess survival or proliferation of mammalian cells. Proc. West. Pharmacol. Soc. 2011, 54, 10–14. [Google Scholar]
- De La Rosa, V.R. Poly(2-oxazoline)s as materials for biomedical applications. J. Mater. Sci. Mater. Med. 2014, 25, 1211–1225. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Vasilev, K.; Shirazi, H.S.; McNicholas, K.; Li, J.; Gleadle, J.; MacGregor, M. Biosensor device for the pho-to-specific detection of immuno-captured bladder cancer cells using hexaminolevulinate: An ex-vivo study. Photodiagnosis Photodyn. Ther. 2019, 28, 238–247. [Google Scholar] [CrossRef]
- MacGregor, M.; Sinha, U.; Visalakshan, R.M.; Cavallaro, A.; Vasilev, K. Preserving the reactivity of coatings plasma depos-ited from oxazoline precursors − An in depth study. Plasma Process. Polym. 2019, 16, 1800130. [Google Scholar] [CrossRef]
- Barrea, R.A.; Mainardi, R.T. Standardless XRF analysis of stainless-steel samples. X-Ray Spectrom. 1998, 27, 111–116. [Google Scholar] [CrossRef]
- Henry, M.; Bertrand, P. Surface composition of insulin and albumin adsorbed on polymer substrates as revealed by multi-variate analysis of ToF-SIMS data. Surf. Interface Anal. 2009, 41, 105–113. [Google Scholar] [CrossRef]
- Tamulienė, J.; Romanova, L.G.; Vukstich, V.S.; Papp, A.V.; Snegursky, A.V. Electron-impact-induced tryptophan molecule fragmentation. Eur. Phys. J. D 2015, 69, 21. [Google Scholar] [CrossRef]
- Visalakshan, R.M.; Cavallaro, A.A.; MacGregor, M.N.; Lawrence, E.P.; Koynov, K.; Hayball, J.D.; Vasilev, K. Nanotopog-raphy-Induced Unfolding of Fibrinogen Modulates Leukocyte Binding and Activation. Adv. Funct. Mater. 2019, 29, 1807453. [Google Scholar] [CrossRef]
- Li, W.; Zhou, J.; Xu, Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015, 3, 617–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumarasinghe, L.S.; Ninan, N.; Dabare, P.R.L.; Cavallaro, A.; Doğramacı, E.J.; Rossi-Fedele, G.; Dreyer, C.; Vasilev, K.; Zilm, P. Bioactive Plasma Coatings on Orthodontic Brackets: In Vitro Metal Ion Release and Cytotoxicity. Coatings 2021, 11, 857. https://doi.org/10.3390/coatings11070857
Kumarasinghe LS, Ninan N, Dabare PRL, Cavallaro A, Doğramacı EJ, Rossi-Fedele G, Dreyer C, Vasilev K, Zilm P. Bioactive Plasma Coatings on Orthodontic Brackets: In Vitro Metal Ion Release and Cytotoxicity. Coatings. 2021; 11(7):857. https://doi.org/10.3390/coatings11070857
Chicago/Turabian StyleKumarasinghe, Lasni Samalka, Neethu Ninan, Panthihage Ruvini Lakshika Dabare, Alex Cavallaro, Esma J. Doğramacı, Giampiero Rossi-Fedele, Craig Dreyer, Krasimir Vasilev, and Peter Zilm. 2021. "Bioactive Plasma Coatings on Orthodontic Brackets: In Vitro Metal Ion Release and Cytotoxicity" Coatings 11, no. 7: 857. https://doi.org/10.3390/coatings11070857
APA StyleKumarasinghe, L. S., Ninan, N., Dabare, P. R. L., Cavallaro, A., Doğramacı, E. J., Rossi-Fedele, G., Dreyer, C., Vasilev, K., & Zilm, P. (2021). Bioactive Plasma Coatings on Orthodontic Brackets: In Vitro Metal Ion Release and Cytotoxicity. Coatings, 11(7), 857. https://doi.org/10.3390/coatings11070857