Active Casein Coatings and Films for Perishable Foods: Structural Properties and Shelf-Life Extension
Abstract
:1. Introduction
2. Protein Based Coatings and Films
3. Casein-Based Packaging
3.1. Structure
3.2. Film Forming Solution/Dispersion Properties
3.3. Composition and Preparation
4. Methodology
5. Results and Discussion
5.1. Influence of Different Additives on the Structure and Properties of Coatings and Films
5.1.1. Essential Oils
5.1.2. Probiotics
5.1.3. Phenolic Compounds
5.1.4. Plant Extracts
5.1.5. Nanoparticles
5.2. Shelf-Life Aspects of FV
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mc Carthy, U.; Uysal, I.; Badia-Melis, R.; Mercier, S.; O’Donnell, C.; Ktenioudaki, A. Global food security—Issues, challenges and technological solutions. Trends Food Sci. Technol. 2018, 77, 11–20. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- UNEP Food Waste Index Report 2021|UNEP—UN Environment Programme. Available online: https://www.unep.org/resources/report/unep-food-waste-index-report-2021 (accessed on 20 June 2021).
- Qamar, S.A.; Asgher, M.; Bilal, M.; Iqbal, H.M. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Chitravathi, K.; Unni, L.E. Bio-Based Packaging for Fresh Fruits and Vegetables; Apple Academic Press: Palm Bay, FL, USA, 2018. [Google Scholar]
- Krochta, J.M. Proteins as raw materials for films and coatings: Definitions, current status, and opportunities. Protein Based Film. Coat. 2002, 1, 1–40. [Google Scholar]
- Dangaran, K.; Tomasula, P.M.; Qi, P. Structure and Function of Protein-Based Edible Films and Coatings. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 25–56. [Google Scholar]
- Coltelli, M.B.; Wild, F.; Bugnicourt, E.; Cinelli, P.; Lindner, M.; Schmid, M.; Weckel, V.; Müller, K.; Rodriguez, P.; Staebler, A.; et al. State of the Art in the Development and Properties of Protein-Based Films and Coatings and Their Applicability to Cellulose Based Products: An Extensive Review. Coatings 2015, 6, 1. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2014, 31, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Molecular Sciences Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef] [PubMed]
- Pavlath, A.E.; Orts, W. Edible Films and Coatings: Why, What, and How? In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar]
- Ali, S.; Chatha, S.; Hussain, A.I. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review Synthesis of unnatural amino acids View project Designing of X12Y12 nanocages with late transition metals for sensing/detection of Toxic gases View project. Artic. Int. J. Biol. Macromol. 2017, 109, 1095–1107. [Google Scholar] [CrossRef]
- Lin, D.; Zhao, Y. Innovations in the Development and Application of Edible Coatings for Fresh and Minimally Processed Fruits and Vegetables. Compr. Rev. Food Sci. Food Saf. 2007, 6, 60–75. [Google Scholar] [CrossRef]
- Nayak, S.L.; Sethi, S.; Sharma, R.R.; Prajapati, U. Active Edible Coatings for Fresh Fruits and Vegetables. In Polymers for Agri-Food Applications; Springer International Publishing: Berlin, Germany, 2019; pp. 417–432. [Google Scholar]
- Ciolacu, L.; Nicolau, A.I.; Hoorfar, J. Edible coatings for fresh and minimally processed fruits and vegetables. In Global Safety of Fresh Produce: A Handbook of Best Practice, Innovative Commercial Solutions and Case Studies; Elsevier Ltd.: London, UK, 2013; pp. 233–244. ISBN 9781782420187. [Google Scholar]
- Tiwari, A.; Galanis, A.; Soucek, M.D. Biobased and Environmental Benign Coatings; Tiwari, A., Galanis, A., Soucek, M.D., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781119185055. [Google Scholar]
- Chen, H.; Wang, J.; Cheng, Y.; Wang, C.; Liu, H.; Bian, H.; Pan, Y.; Sun, J.; Han, W. Application of protein-based films and coatings for food packaging: A review. Polymers 2019, 11, 2039. [Google Scholar] [CrossRef] [Green Version]
- Fox, P.F.; Brodkorb, A. The casein micelle: Historical aspects, current concepts and significance. Int. Dairy J. 2008, 18, 677–684. [Google Scholar] [CrossRef]
- Sarode, A.R.; Sawale, P.D.; Khedkar, C.D.; Kalyankar, S.D.; Pawshe, R.D. Casein and Caseinate: Methods of Manufacture, 1st ed.; Elsevier Ltd.: London, UK, 2015; Volume 1, ISBN 9780123849533. [Google Scholar]
- Chiralt, A.; González-Martínez, C.; Vargas, M.; Atarés, L. Edible films and coatings from proteins. In Proteins in Food Processing, 2nd ed.; Elsevier Inc.: London, UK, 2018; pp. 477–500. ISBN 9780081007297. [Google Scholar]
- Mihalca, V.; Kerezsi, A.D.; Weber, A.; Gruber-Traub, C.; Schmucker, J.; Vodnar, D.C.; Dulf, F.V.; Socaci, S.A.; Fărcaș, A.; Mureșan, C.I.; et al. Protein-based films and coatings for food industry applications. Polymers 2021, 13, 769. [Google Scholar] [CrossRef]
- Głąb, T.K.; Boratyński, J. Potential of Casein as a Carrier for Biologically Active Agents. Top. Curr. Chem. 2017, 375, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Elzoghby, A.; El-Fotoh, W.S.A.; Elgindy, N.A. Casein-based formulations as promising controlled release drug delivery systems. J. Control. Release 2011, 153, 206–216. [Google Scholar] [CrossRef]
- Qi, P.X. Studies of casein micelle structure: The past and the present. Lait 2007, 87, 363–383. [Google Scholar] [CrossRef] [Green Version]
- Swaisgood, H.E. Chemistry of the Caseins. In Advanced Dairy Chemistry—1 Proteins; Springer: New York, NY, USA, 2003; pp. 139–201. [Google Scholar]
- Vaz, C.M.; Fossen, M.; Van Tuil, R.F.; De Graaf, L.A.; Reis, R.L.; Cunha, A.M. Casein and soybean protein-based thermoplastics and composites as alternative biodegradable polymers for biomedical applications. J. Biomed. Mater. Res. Part A 2003, 65, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.M.; Muhoberac, B.B.; Dubin, P.L.; Xia, J. Effects of Protein Charge Heterogeneity in Protein-Polyelectrolyte Complexation. Macromolecules 1992, 25, 290–295. [Google Scholar] [CrossRef]
- Volpe, S.; Cavella, S.; Masi, P.; Torrieri, E. Effect of solid concentration on structure and properties of chitosan-caseinate blend films. Food Packag. Shelf Life 2017, 13, 76–84. [Google Scholar] [CrossRef]
- Anal, A.K.; Tobiassen, A.; Flanagan, J. Preparation and characterization of nanoparticles formed by chitosan–caseinate interactions. Colloids Surf. B Biointerfaces 2008, 64, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ma, J.; Xu, Q. Polyelectrolyte complex from cationized casein and sodium alginate for fragrance controlled release. Colloids Surf. B Biointerfaces 2019, 178, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. J. Food Sci. 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Valentino, M.; Volpe, S.; Di Giuseppe, F.A.; Cavella, S.; Torrieri, E. Active Biopolymer Coating Based on Sodium Caseinate: Physical Characterization and Antioxidant Activity. Coatings 2020, 10, 706. [Google Scholar] [CrossRef]
- Khwaldia, K.; Banon, S.; Perez, C.; Desobry, S. Properties of sodium caseinate film-forming dispersions and films. J. Dairy Sci. 2004, 87, 2011–2016. [Google Scholar] [CrossRef]
- Sadeghi, F.; Kadkhodaee, R. Phase behavior, rheological characteristics and microstructure of sodium caseinate-Persian gum system. Carbohydr. Polym. 2018, 179, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Jahromi, M.; Niakousari, M.; Golmakani, M.T.; Mohammadifar, M.A. Physicochemical and structural characterization of sodium caseinate based film-forming solutions and edible films as affected by high methoxyl pectin. Int. J. Biol. Macromol. 2020, 165, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Volpe, S.; Torrieri, E. Use of chitosan and chitosan-caseinate coating to prolong shelf life of minimally processed apples. Ital. J. Food Sci. 2017, 30–35. [Google Scholar]
- Neirynck, N.; Dewettinck, K.; Van der Meeren, P. Undefined Influence of pH and biopolymer ratio on sodium caseinate—Guar gum interactions in aqueous solutions and in O/W emulsions. Food Hydrocoll. 2007, 21, 862–869. [Google Scholar] [CrossRef]
- Sadeghi, F.; Kadkhodaee, R.; Emadzadeh, B.; Nishinari, K. Effect of sucrose on phase and flow behavior of protein-polysaccharide mixtures. Food Hydrocoll. 2021, 113, 106455. [Google Scholar] [CrossRef]
- Ma, X.; Chatterton, D.E. Strategies to improve the physical stability of sodium caseinate stabilized emulsions: A literature review. Food Hydrocoll. 2021, 106853. [Google Scholar] [CrossRef]
- Belyamani, I.; Prochazka, F.; Assezat, G. Production and characterization of sodium caseinate edible films made by blown-film extrusion. J. Food Eng. 2014, 121, 39–47. [Google Scholar] [CrossRef]
- Belyamani, I.; Prochazka, F.; Assezat, G.; Debeaufort, F. Mechanical and barrier properties of extruded film made from sodium and calcium caseinates. Food Packag. Shelf Life 2014, 2, 65–72. [Google Scholar] [CrossRef]
- Colak, B.Y.; Peynichou, P.; Galland, S.; Oulahal, N.; Assezat, G.; Prochazka, F.; Degraeve, P. Active biodegradable sodium caseinate films manufactured by blown-film extrusion: Effect of thermo-mechanical processing parameters and formulation on lysozyme stability. Ind. Crops Prod. 2015, 72, 142–151. [Google Scholar] [CrossRef]
- Ranjbaryan, S.; Pourfathi, B. Reinforcing and release controlling effect of cellulose nanofiber in sodium caseinate films activated by nanoemulsified cinnamon essential oil. Food Packag. Shelf Life 2019, 21, 100341. [Google Scholar] [CrossRef]
- Gialamas, H.; Zinoviadou, K.G.; Biliaderis, C.G.; Koutsoumanis, K.P. Development of a novel bioactive packaging based on the incorporation of Lactobacillus sakei into sodium-caseinate films for controlling Listeria monocytogenes in. Food Res. Int. 2010, 43, 2402–2408. [Google Scholar] [CrossRef]
- Pereda, M.; Marcovich, N.E.; Mosiewicki, M.A. Sodium caseinate films containing linseed oil resin as oily modifier. Food Hydrocoll. 2015, 44, 407–415. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; Del, M.; Garrigós, C.; Jiménez, A. Accepted Manuscript Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol Structure and mechanical properties of sodium and calcium caseinate edible active films with carvacrol. J. Food Eng. 2012, 114, 486–494. [Google Scholar] [CrossRef] [Green Version]
- Guilbert, S.; Gontard, N.; Gorris, L.G. Prolongation of the shelf-life of perishable food products using biodegradable films and coatings. LWT Food Sci. Technol. 1996, 29, 10–17. [Google Scholar] [CrossRef]
- Suhag, R.; Kumar, N.; Trajkovska Petkoska, A.; Upadhyay, A. Film formation and deposition methods of edible coating on food products: A review Microwave Roasting of Sorghum View project Liquid Crystals View project Film formation and deposition methods of edible coating on food products: A review. Food Res. Int. 2020, 136, 109582. [Google Scholar] [CrossRef] [PubMed]
- Marín, A.; Plotto, A.; Atarés, L.; Chiralt, A. Undefined Lactic acid bacteria incorporated into edible coatings to control fungal growth and maintain postharvest quality of grapes. HortScience 2019, 54, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Olivas, G.I.I.; Barbosa-Cánovas, G. Edible Films and Coatings for Fruits and Vegetables. In Edible Films and Coatings for Food Applications; Springer: New York, NY, USA, 2009; pp. 211–244. [Google Scholar]
- Skurtys, O.; Acevedo, C.; Pedreschi, F.; Enronoe, J.; Osorio, F.; Aguilera, J.M. Food Hydrocolloid Edible Films and Coatings; Nova Science: New York, NY, USA, 2014. [Google Scholar]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-Layer Electrostatic Deposition of Edible Coating on Fresh Cut Melon Model: Anticipated and Unexpected Effects of Alginate–Chitosan Combination. Food Bioprocess Technol. 2014, 7, 1424–1432. [Google Scholar] [CrossRef]
- Murmu, S.B.; Mishra, H.N. The Effect of Edible Coating Based on Arabic Gum, Sodium Caseinate and Essential Oil of Cinnamon and Lemon Grass on Guava. Food Chem. 2017, 245, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, F.; Certel, M.; Uslu, M.K. Effects of sodium caseinate-and milk protein concentrate-based edible coatings on the postharvest quality of Bing cherries. J. Sci. Food Agric. 2004, 84, 1229–1234. [Google Scholar] [CrossRef]
- Siew, D.C.; Heilmann, C.; Easteal, A.J.; Cooney, R.P. Solution and film properties of sodium caseinate/glycerol and sodium caseinate/polyethylene glycol edible coating systems. J. Agric. Food Chem. 1999, 47, 3432–3440. [Google Scholar] [CrossRef] [PubMed]
- Correa-Betanzo, J.; Jacob, J.K.; Perez-Perez, C.; Paliyath, G. Effect of a sodium caseinate edible coating on berry cactus fruit (Myrtillocactus geometrizans) phytochemicals. Food Res. Int. 2011, 44, 1897–1904. [Google Scholar] [CrossRef]
- Jafarizadeh Malmiri, H.; Osman, A.; Tan, C.P.; Abdul Rahman, R. Effects of edible surface coatings (sodium carboxymethyl cellulose, sodium caseinate and glycerol) on storage quality of berangan banana (musa sapientum cv. Berangan) using response surface methodology. J. Food Process. Preserv. 2012, 36, 252–261. [Google Scholar] [CrossRef]
- Ramirez, M.E.; Timón, M.L.; Petrón, M.J.; Andrés, A.I. Effect of chitosan, pectin and sodium caseinate edible coatings on shelf life of fresh-cut prunus persica var. nectarine. J. Food Process. Preserv. 2015, 39, 2687–2697. [Google Scholar] [CrossRef]
- Le Tien, C.; Vachon, C.; Mateescu, M.A.; Lacroix, M. Milk protein coatings prevent oxidative browning of apples and potatoes. J. Food Sci. 2001, 66, 512–516. [Google Scholar] [CrossRef]
- Ponce, A.G.; Roura, S.I.; del Valle, C.E.; Moreira, M.R. Antimicrobial and antioxidant activities of edible coatings enriched with natural plant extracts: In vitro and in vivo studies. Postharvest Biol. Technol. 2008, 49, 294–300. [Google Scholar] [CrossRef]
- Volpe, S.; Cavella, S.; Torrieri, E. Biopolymer coatings as alternative to modified atmosphere packaging for shelf life extension of minimally processed apples. Coatings 2019, 9, 569. [Google Scholar] [CrossRef] [Green Version]
- Bush, L.; Stevenson, L.; Lane, K.E. The oxidative stability of omega-3 oil-in-water nanoemulsion systems suitable for functional food enrichment: A systematic review of the literature. Crit. Rev. Food Sci. Nutr. 2019, 59, 1154–1168. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Mohammadi, X.; Wiktor, A.; Singh, A.; Pratap Singh, A. Applications of pulsed light decontamination technology in food processing: An overview. Appl. Sci. 2020, 10, 3606. [Google Scholar] [CrossRef]
- Baldwin, E.; Hagenmaier, R.; Bai, J. Edible Coatings and Films to Improve Food Quality; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Maqbool, M.; Ali, A.; Alderson, P.G.; Mohamed, M.T.M.; Siddiqui, Y.; Zahid, N. Postharvest application of gum arabic and essential oils for controlling anthracnose and quality of banana and papaya during cold storage. Postharvest Biol. Technol. 2011, 62, 71–76. [Google Scholar] [CrossRef]
- Gupta, C.; Garg, A.P.; Uniyal, R.C.; Kumari, A. Comparative analysis of the antimicrobial activity of cinnamon oil and cinnamon extract on some food-borne microbes. Afr. J. Microbiol. Res. 2008, 2, 247–251. [Google Scholar]
- Murmu, S.B.; Mishra, H.N. Optimization of the arabic gum based edible coating formulations with sodium caseinate and tulsi extract for guava. Lebensm. Wiss. Technol. 2017, 80, 271–279. [Google Scholar] [CrossRef]
- Raghav, P.K.; Agarwal, N.; Saini, M. Edible Coating of Fruits and Vegetables: A Review Development and Standardization of Ready to Use Edible Herbal Coating for Fresh Fruits & Vegetables View Project; R&D Publication: Bangalore, India, 2016. [Google Scholar]
- Atarés, L.; Bonilla, J.; Chiralt, A. Characterization of sodium caseinate-based edible films incorporated with cinnamon or ginger essential oils. J. Food Eng. 2010, 100, 678–687. [Google Scholar] [CrossRef]
- Matsakidou, A.; Biliaderis, C.G.; Kiosseoglou, V. Preparation and characterization of composite sodium caseinate edible films incorporating naturally emulsified oil bodies. Food Hydrocoll. 2013, 30, 232–240. [Google Scholar] [CrossRef]
- Alizadeh-Sani, M.; Kia, E.M.; Ghasempour, Z.; Ehsani, A. Preparation of active nanocomposite film consisting of sodium caseinate, ZnO nanoparticles and rosemary essential oil for food packaging applications. J. Polym. Environ. 2021, 29, 588–598. [Google Scholar] [CrossRef]
- Tecnología De Alimentos, D.D.E.; Para Desarrollo, A.E.L.; Lagos, M.J.B.; Chiralt Boix Lorena Atarés Huerta, A. UNIVERSITAT POLITÈCNICA DE VALÈNCIA Development of Bioactive Edible Films and Coatings with Antioxidant and Antimicrobial Properties for Food Use TESIS DOCTORAL Presentada por (2013) ›Tesis doctoral Versión 3. Available online: https://riunet.upv.es (accessed on 20 July 2021).
- Aliheidari, N.; Fazaeli, M.; Ahmadi, R.; Ghasemlou, M.; Emam-Djomeh, Z. undefined Comparative evaluation on fatty acid and Matricaria recutita essential oil incorporated into casein-based film. Int. J. Biol. Macromol. 2013, 56, 69–75. [Google Scholar] [CrossRef]
- Marco, J. Influence of nanoliposomes incorporation on properties of film forming dispersions and films based on corn starch and sodium caseinate. Food Hydrocoll. 2014, 35, 159–169. [Google Scholar] [CrossRef]
- Kaur, J.; Katopo, L.; Hung, A.; Ashton, J. Combined spectroscopic, molecular docking and quantum mechanics study of β-casein and p-coumaric acid interactions following thermal treatment. Food Chem. 2018, 252, 163–170. [Google Scholar] [CrossRef]
- Espitia, P.J.; Batista, R.A.; Azeredo, H.M.; Otoni, C.G. Probiotics and their potential applications in active edible films and coatings. Food Res. Int. 2016, 90, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Han, W.; Yu, Y.; Li, N.; Wang, L. Application and safety assessment for nano-composite materials in food packaging. Chin. Sci. Bull. 2011, 56, 1216–1225. [Google Scholar] [CrossRef] [Green Version]
- Abdollahzadeh, E.; Ojagh, S.M.; Fooladi, A.A.I.; Shabanpour, B.; Gharahei, M. Effects of probiotic cells on the mechanical and antibacterial properties of sodium-caseinate films. Appl. Food Biotechnol. 2018, 5, 155–162. [Google Scholar] [CrossRef]
- Pereira, J.O.; Soares, J.; Sousa, S.; Madureira, A.R.; Gomes, A.; Pintado, M. Edible films as carrier for lactic acid bacteria. LWT 2016, 73, 543–550. [Google Scholar] [CrossRef]
- Mozaffarzogh, M.; Misaghi, A.; Shahbazi, Y.; Kamkar, A. Evaluation of probiotic carboxymethyl cellulose-sodium caseinate films and their application in extending shelf life quality of fresh trout fillets. LWT Food Sci. Technol. 2020, 126, 109305. [Google Scholar] [CrossRef]
- Condict, L.; Kaur, J.; Hung, A.; Ashton, J.; Kasapis, S. Combined spectroscopic, molecular docking and quantum mechanics study of β-casein and ferulic acid interactions following UHT-like treatment. Food Hydrocoll. 2019, 89, 351–359. [Google Scholar] [CrossRef]
- Cao, X.; He, Y.; Kong, Y.; Mei, X.; Huo, Y.; He, Y.; Liu, J. Elucidating the interaction mechanism of eriocitrin with β-casein by multi-spectroscopic and molecular simulation methods. Food Hydrocoll. 2019, 94, 63–70. [Google Scholar] [CrossRef]
- Kadam, S.U.; Pankaj, S.K.; Tiwari, B.K.; Cullen, P.J.; O’Donnell, C.P. Development of biopolymer-based gelatin and casein films incorporating brown seaweed Ascophyllum nodosum extract. Food Packag. Shelf Life 2015, 6, 68–74. [Google Scholar] [CrossRef]
- Khezerlou, A.; Ehsani, A.; Tabibiazar, M.; Moghaddas Kia, E. Development and characterization of a Persian gum–sodium caseinate biocomposite film accompanied by Zingiber officinale extract. J. Appl. Polym. Sci. 2019, 136, 47215. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J. Antioxidant and physicochemical properties of blended films based on gelatin-sodium caseinate activated with natural extracts. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, F.; Ma, J.; Chen, T.; Zhou, J.; Simion, D.; Carmen, G. Facile synthesis of casein-based silica hybrid nano-composite for coatings: Effects of silane coupling agent. Prog. Org. Coat. 2015, 88, 1–7. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Rácz, I.; Marcovich, N.E. Structure and properties of nanocomposite films based on sodium caseinate and nanocellulose fibers. J. Food Eng. 2011, 103, 76–83. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, J.; Xu, Q.; Zhang, J. Fabrication of antibacterial casein-based ZnO nanocomposite for flexible coatings. Mater. Des. 2017, 113, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh-Sani, M.; Rhim, J.W.; Azizi-Lalabadi, M.; Hemmati-Dinarvand, M.; Ehsani, A. Preparation and characterization of functional sodium caseinate/guar gum/TiO2/cumin essential oil composite film. Int. J. Biol. Macromol. 2020, 145, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Bonnaillie, L.M.; Zhang, H.; Akkurt, S.; Yam, K.L.; Tomasula, P.M. Casein films: The effects of formulation, environmental conditions and the addition of citric pectin on the structure and mechanical properties. Polymers 2014, 6, 2018–2036. [Google Scholar] [CrossRef]
- Arfat, Y.A.; Ahmed, J.; Hiremath, N.; Auras, R.; Joseph, A. Thermo-mechanical, rheological, structural and antimicrobial properties of bionanocomposite films based on fish skin gelatin and silver-copper nanoparticles. Food Hydrocoll. 2017, 62, 191–202. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids). J. Nanopart. Res. Technol. Beijing 2007, 66, 471. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Bergin, S.; Katam, R.; Mahna, N. Improvement of postharvest quality of plum (Prunus domestica L.) using polysaccharide-based edible coatings. Plants 2020, 9, 1148. [Google Scholar] [CrossRef]
- Ncama, K.; Magwaza, L.S.; Mditshwa, A.; Tesfay, S.Z. Plant-based edible coatings for managing postharvest quality of fresh horticultural produce: A review. Food Packag. Shelf Life 2018, 16, 157–167. [Google Scholar] [CrossRef]
- McHugh, T.H.; Krochta, J.M. Milk-protein-based edible films and coatings. Food Technol. (USA) 1994, 48, 97–103. [Google Scholar]
- Torres, J.A. Edible films and coatings from proteins. Protein Funct. Food Syst. 1994, 78, 467–507. [Google Scholar]
- Moreira, M.D.R.; Pereda, M.; Marcovich, N.E.; Roura, S.I. Antimicrobial effectiveness of bioactive packaging materials from edible chitosan and casein polymers: Assessment on carrot, cheese, and salami. J. Food Sci. 2011, 76, M54–M63. [Google Scholar] [CrossRef] [PubMed]
- Lerdthanangkul, S.; Krochta, J.M. Edible coating effects on postharvest quality of green bell peppers. J. Food Sci. 1996, 61, 176–179. [Google Scholar] [CrossRef]
- EP2893813A1—Method for Applying a Coating to Fruit and/or Vegetables; Composition Suitable to Be Used in Said Method and the Use of Such Composition—Google Patents. Available online: https://patents.google.com/patent/EP2893813A1/en?oq=caseinate+edible+coating+of+fruits+and+vegetableshttps:%2F%2Fpatents.google.com%2Fpatent%2FEP2893813A1%2Fen%3Foq (accessed on 19 April 2021).
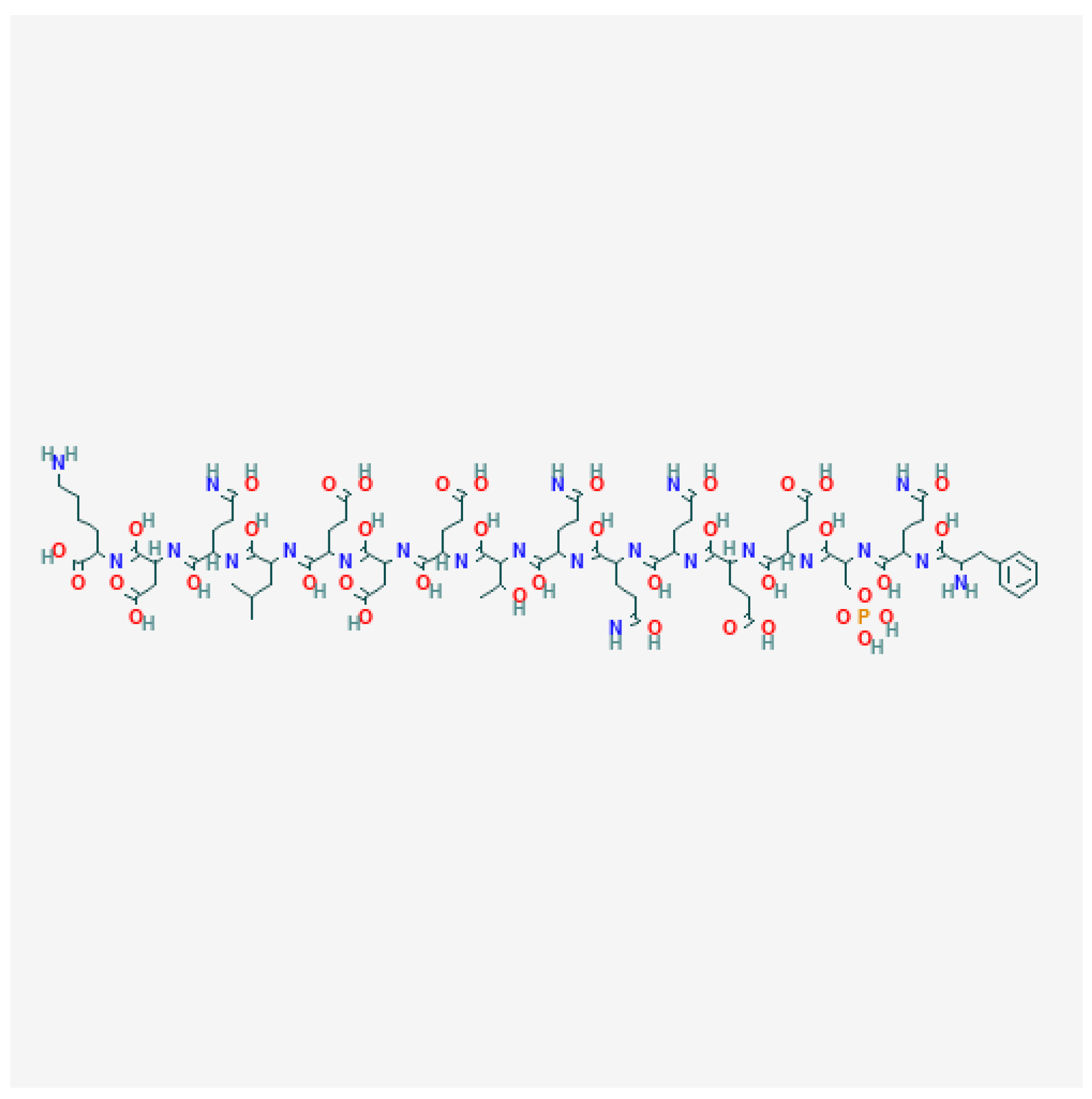



| Composition | Plasticizer Used | Formulation Conditions | Application Method | Reference | |
|---|---|---|---|---|---|
| Type | Active Additives | ||||
| Sodium caseinate (4%–14%) | Gallic acid (0.005%) and rosemary essential oil (1.5%) | Glycerol (0.4%–1.4%) | Sodium caseinate powder dissolved in deionized water with stirring for 4 h at room temperature and glycerol was added to form coating. For active coating preparation sodium caseinate was dissolved in HCl tris buffer at room temperature for 4 h by stirring. After adding glycerol, active additives were added, and solution was homogenized at 15,500 rpm for 4 min. | Dipping | [32] |
| Sodium caseinate + potato starch (2%) | L. plantarum (5 × 107 CFU/ml) | Glycerol (-) | The coatings were prepared by dispersing 2% biopolymers in deionized water at room temperature for 2 h, oleic acid was added, at a ratio of biopolymer: oleic acid (1:0.1), homogenized at 13,600 rpm and sterilized. After cooling, L. plantarum was added into dispersions. | Spraying | [49] |
| Sodium caseinate (1%) + arabic gum (5%) | Cinnamon and lemon grass oils (1%–2%) | Sorbitan monooleate (1%) | After arabic gum was dissolved in deionized water for 90 min at low heat by stirring, plasticizer was added. The pH was adjusted to 5.6 by using 1 N NaOH. Similarly, sodium caseinate was dissolved in deionized water. This formulation was added into the formulation of arabic gum and stirred. Finally, essential oils were added and the mixtures were homogenized to obtain coatings. | Dipping | [53] |
| Sodium caseinate (11.1%) + bees wax (5%–15% of protein content) + stearic and palmitic acid blend (5%–15% of protein content) | - | Glycerol (3.3%) | Dipping solutions were prepared by dissolving sodium caseinate in distilled water. After adding the plasticizer, beeswax and stearic + palmitic blend was added. Finally, the solutions were homogenized at 22,000 rpm for 5 min to get coating. | Dipping | [54] |
| Sodium caseinate (10%) | - | Glycerol/PEG | The system was prepared by adding sodium caseinate gradually into distilled water. Finally, glycerol/PEG was added. | - | [55] |
| Sodium caseinate | - | Sorbitol and glycerol | Coatings were prepared separately from sodium caseinate + glycerol and sodium caseinate + sorbitol. | Spraying | [56] |
| Sodium caseinate (0%–1%) and sodium carboxy methyl cellulose (0.1%–1.5%) | - | Glycerol (0%–2%) | Sodium caseinate and sodium carboxy methyl cellulose were dissolved in distilled water, after that glycerol was added. The solutions were homogenized for 3 min at 21,500 rpm. | Dipping | [57] |
| Sodium caseinate (8%–13%) + sodium azide (0.02%) | - | Glycerol (10%–30% w/w of total solids) | Sodium caseinate and sodium azide were dispersed in distilled water for 30 min at 60 °C while stirring. Glycerol was added into the dispersion and the mixture was again stirred for 30 min at room temperature. | - | [33] |
| Sodium caseinate (2%) | - | Glycerol (28% w/w of total solids) | Sodium caseinate was dispersed in deionized water with magnetic stirring at ambient temperature. After glycerol was added, the coatings solution was obtained after filtration. | Dipping | [58] |
| Calcium caseinate (5%) + carboxy methyl cellulose (0.25%) + CaCl2 (0.125%) | - | Glycerol (2.5%) | The components were mixed in distilled water to get homogenized mixture, heated for 30 min at 80 °C and cooled. | Dipping | [59] |
| Sodium caseinate (5%) | Oleoresins | Glycerol (25% of total solids) | Sodium caseinate was added gradually into distilled water and stirred continually for 3 h to get coating. | Dipping | [60] |
| Sodium caseinate (2%) + chitosan (1%) | 1% citric acid, ascorbic acid and calcium chloride as anti-browning agents | Glycerol (10% of total solids) | 1% and 2% chitosan and caseinate solutions were prepared respectively. Blend solution was prepared with 1:1 ratio of caseinate and chitosan. | Dipping | [36] |
| Sodium caseinate (4%) + chitosan (2%) | 1% citric acid, ascorbic acid and calcium chloride as anti-browning agents | Glycerol (10% of total solids) | 2% and 4% chitosan and caseinate solutions were prepared respectively. Blend solution was prepared with 1:1 ratio of caseinate and chitosan. | Dipping | [61] |
| Packaging Composition | Food Product | Impact on Shelf-Life | Reference |
|---|---|---|---|
| Sodium caseinate (4%–14%) + gallic acid (0.005%) and rosemary essential oil (1.5%) | Fennel | - | [32] |
| Sodium caseinate + potato starch (2%) + L. plantarum (5 × 107 CFU/ml) | Grapes | Higher weight loss (~3%) and maturity index (67) for grape samples packed in casein coating (incorporated with probiotics) as compared to control. | [49] |
| Sodium caseinate (1%) + arabic gum (5%) + cinnamon and lemon grass oils (1%–2%) | Guava | Highest pulp firmness values (10.01) for samples coated with (2% concentration of both lemon grass and cinnamon essential oils). However, highest polyphenol peroxidase activity (~7 units/100 mg protein) was also observed due to toxic effects. | [53] |
| Sodium caseinate (11.1%) + bees wax (5%–15% of protein content) + stearic and palmitic acid blend (5%–15% of protein content) | Bing cherries | Improved firmness (4.2−4.5) and appearance (4.2−4.6) as compared to control. | [54] |
| Sodium caseinate | Berry cactus | Total polyphenol content of berries was not affected by casein coatings. | [56] |
| Sodium caseinate (0%–1%) + sodium carboxy methyl cellulose (0.1%–1.5%) | Berangan banana | Increased biopolymer concentration led to decrease in weight loss of banana samples. | [57] |
| Sodium caseinate (2%) | Fresh-Cut nectarine | Lower weight loss (3.09%) of nectarine samples wrapped in caseinate packaging as compared to control. | [58] |
| Calcium caseinate (5%) + carboxy methyl cellulose (0.25%) + CaCl2 (0.125%) | Potatoes and apples | Coating effectively delayed browning by acting as oxygen scavengers. | [59] |
| Sodium caseinate (5%) + oleoresins | Butternut squash | - | [60] |
| Sodium caseinate 2% + chitosan 1% + 1% citric acid, ascorbic acid and calcium chloride | Apples | Hardness remained relatively stable (20−22 N) for blended coatings throughout the storage period. | [36] |
| Sodium caseinate (4%) + chitosan (2%) + 1% citric acid, ascorbic acid and calcium chloride | Apples | Better hardness values (>95 N) as compared to control (<80 N) at the end of storage. | [61] |
| Sodium caseinate (2.5%) and chitosan (2%) + sodium caseinate (2.5%) | Cheese, carrot and salami | A slight inhibitory influence of film forming solution (10−12 mm) and films (4.2 cm2) (of casein and chitosan) was observed on cheese and salami microflora. However, inhibitory effect on carrot was observed when packed in composite packaging film (which may be due to direct contact of film). | [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.R.; Volpe, S.; Valentino, M.; Miele, N.A.; Cavella, S.; Torrieri, E. Active Casein Coatings and Films for Perishable Foods: Structural Properties and Shelf-Life Extension. Coatings 2021, 11, 899. https://doi.org/10.3390/coatings11080899
Khan MR, Volpe S, Valentino M, Miele NA, Cavella S, Torrieri E. Active Casein Coatings and Films for Perishable Foods: Structural Properties and Shelf-Life Extension. Coatings. 2021; 11(8):899. https://doi.org/10.3390/coatings11080899
Chicago/Turabian StyleKhan, Muhammad Rehan, Stefania Volpe, Marika Valentino, Nicoletta Antonella Miele, Silvana Cavella, and Elena Torrieri. 2021. "Active Casein Coatings and Films for Perishable Foods: Structural Properties and Shelf-Life Extension" Coatings 11, no. 8: 899. https://doi.org/10.3390/coatings11080899
APA StyleKhan, M. R., Volpe, S., Valentino, M., Miele, N. A., Cavella, S., & Torrieri, E. (2021). Active Casein Coatings and Films for Perishable Foods: Structural Properties and Shelf-Life Extension. Coatings, 11(8), 899. https://doi.org/10.3390/coatings11080899








