Carbonated Hydroxyapatite-Based Honeycomb Scaffold Coatings on a Titanium Alloy for Bone Implant Application—Physicochemical and Mechanical Properties Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CHA
2.3. Synthesis of Porous CHA-Based HCB Scaffolds
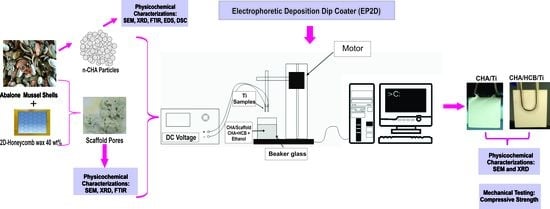
2.4. Coating Procedure for CHA/Ti and CHA/HCB/Ti
2.4.1. Preparation of Substrate for Coating Procedure
2.4.2. Preparation of CHA and Scaffold CHA/HCB Solutions
2.4.3. Coatings and Calcination Processes for CHA/Ti and CHA/HCB/Ti
2.5. Characterization of CHA Particles, CHA/Ti Coating, and CHA/HCB/Ti Coating
2.5.1. Morphology, Particle Size Distribution, Thickness, and Composition Analysis
2.5.2. Crystallographic Analysis
2.5.3. FTIR Analysis
2.5.4. Thermal Properties
2.5.5. Compressive Strength Test and Its Statistical Analysis
3. Results
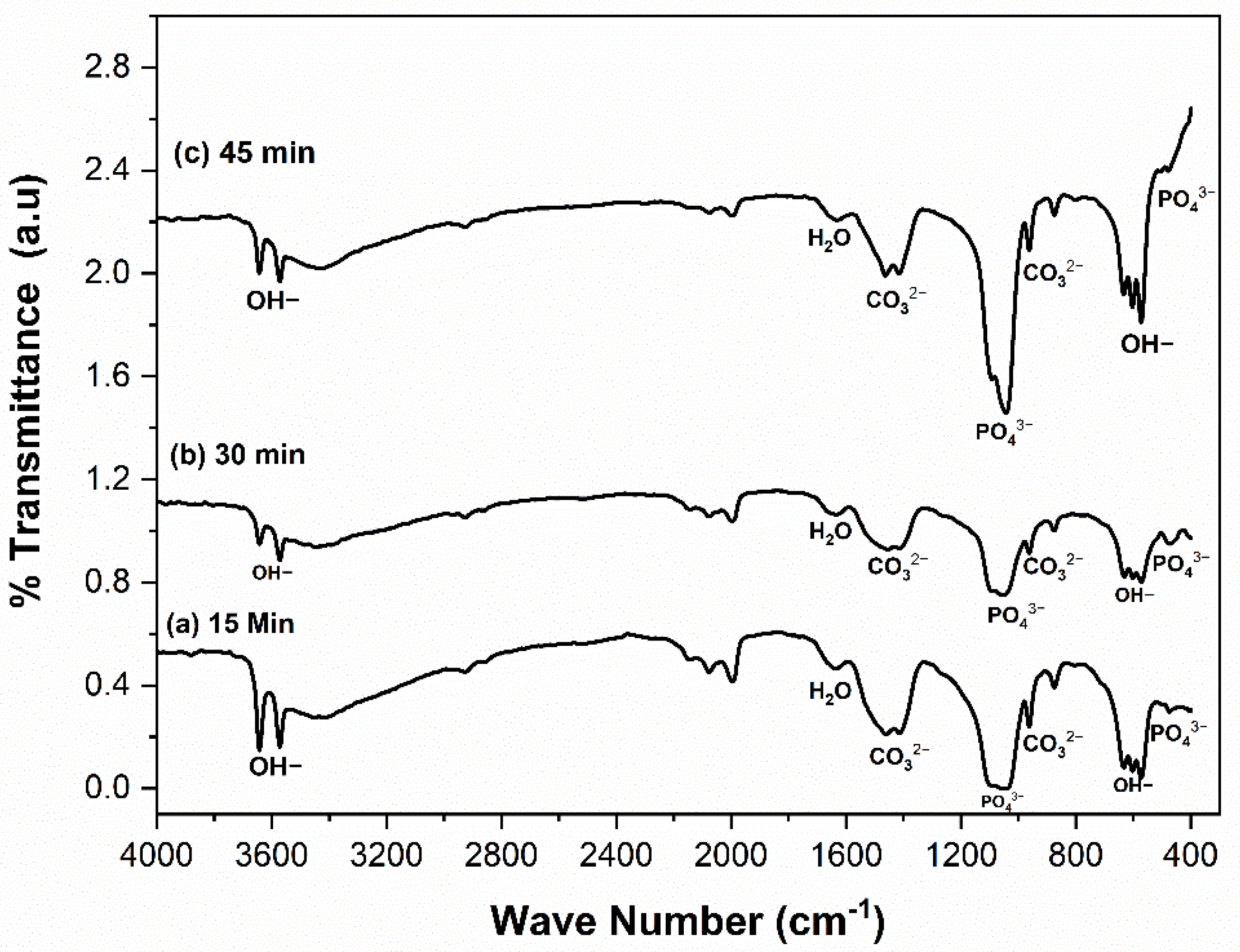
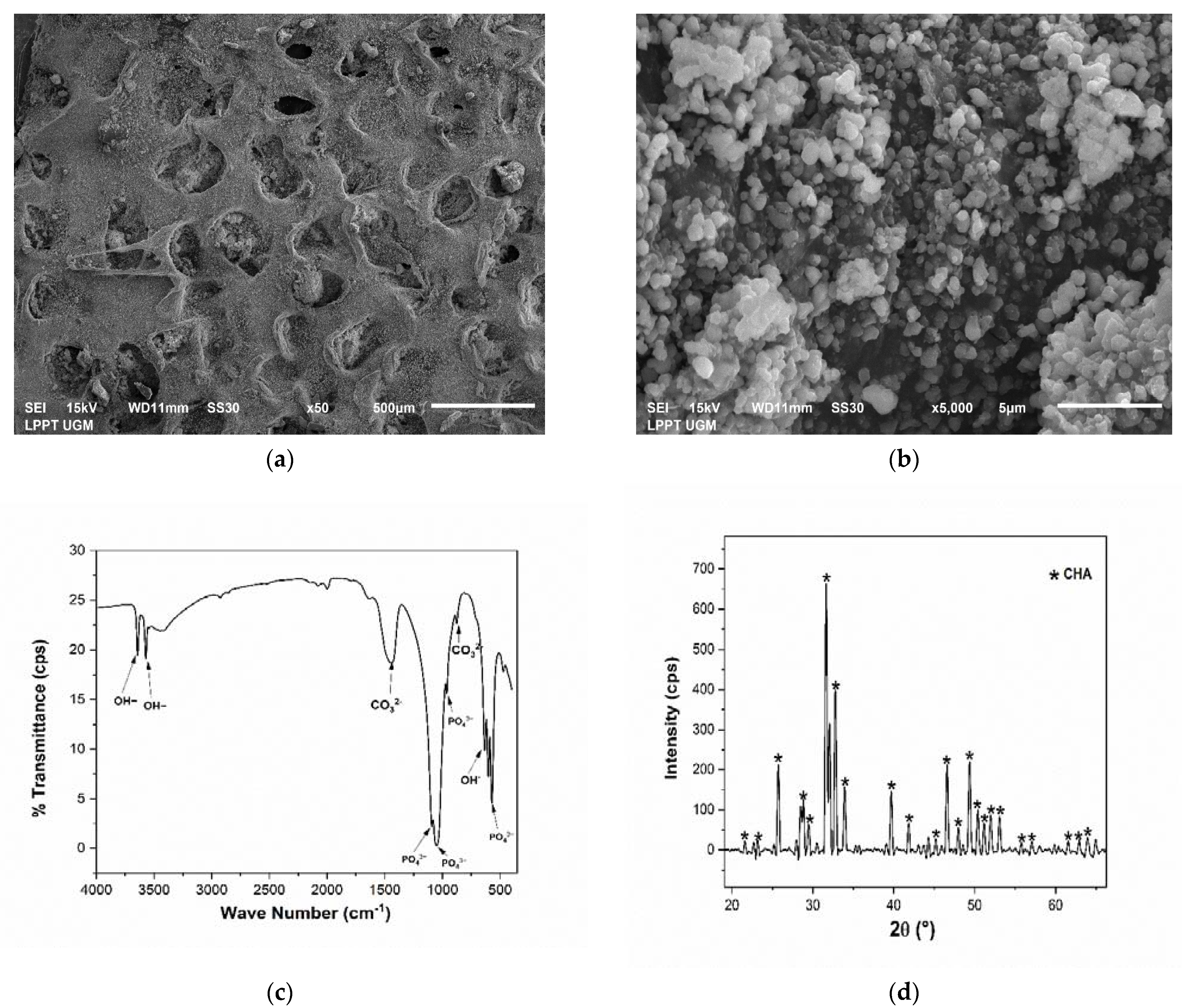
3.1. Physicochemical Analysis of CHA
3.2. Physicochemical Analysis of Scaffold CHA/HCB/40 wt.%
3.3. Electrophoretic Deposition Dip Coating (EP2D) of CHA/Ti and CHA/HCB/Ti Coatings
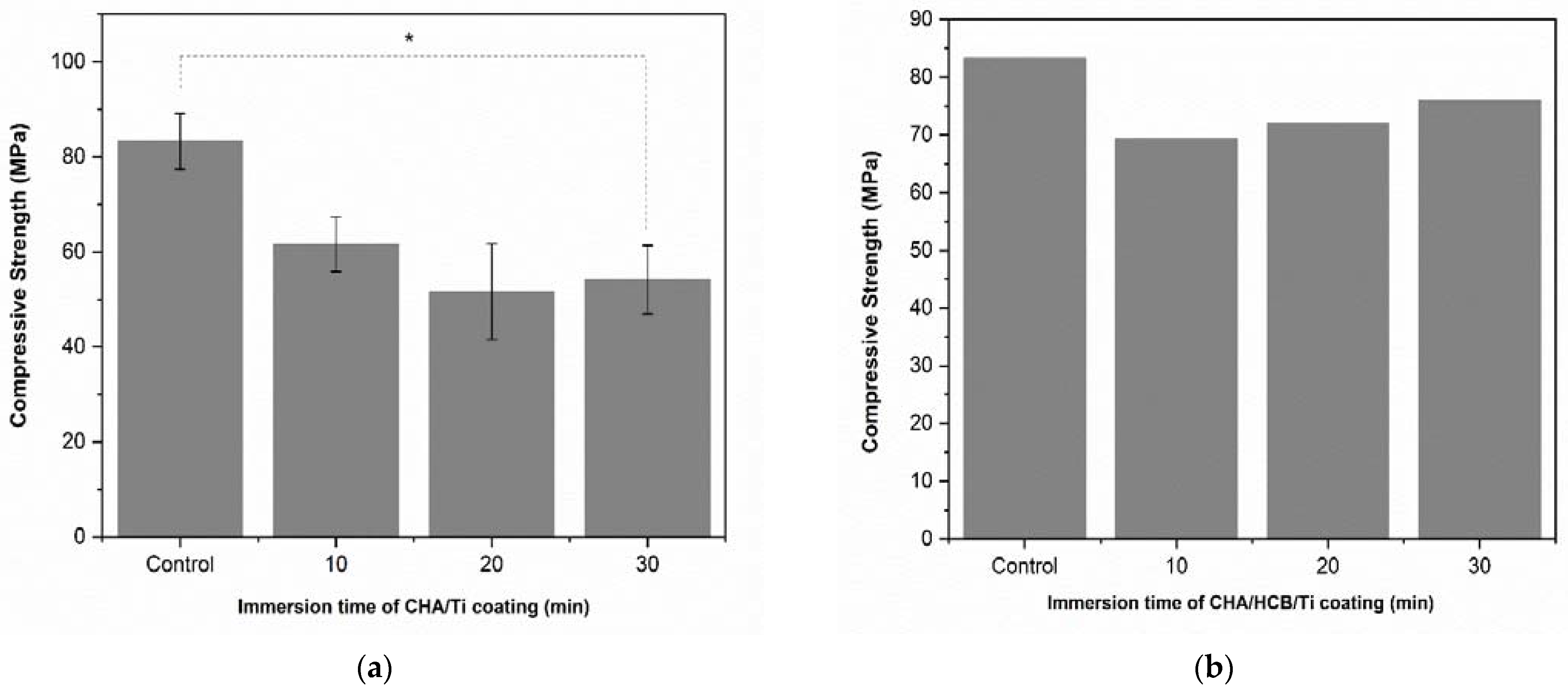
3.3.1. Compressive Strength
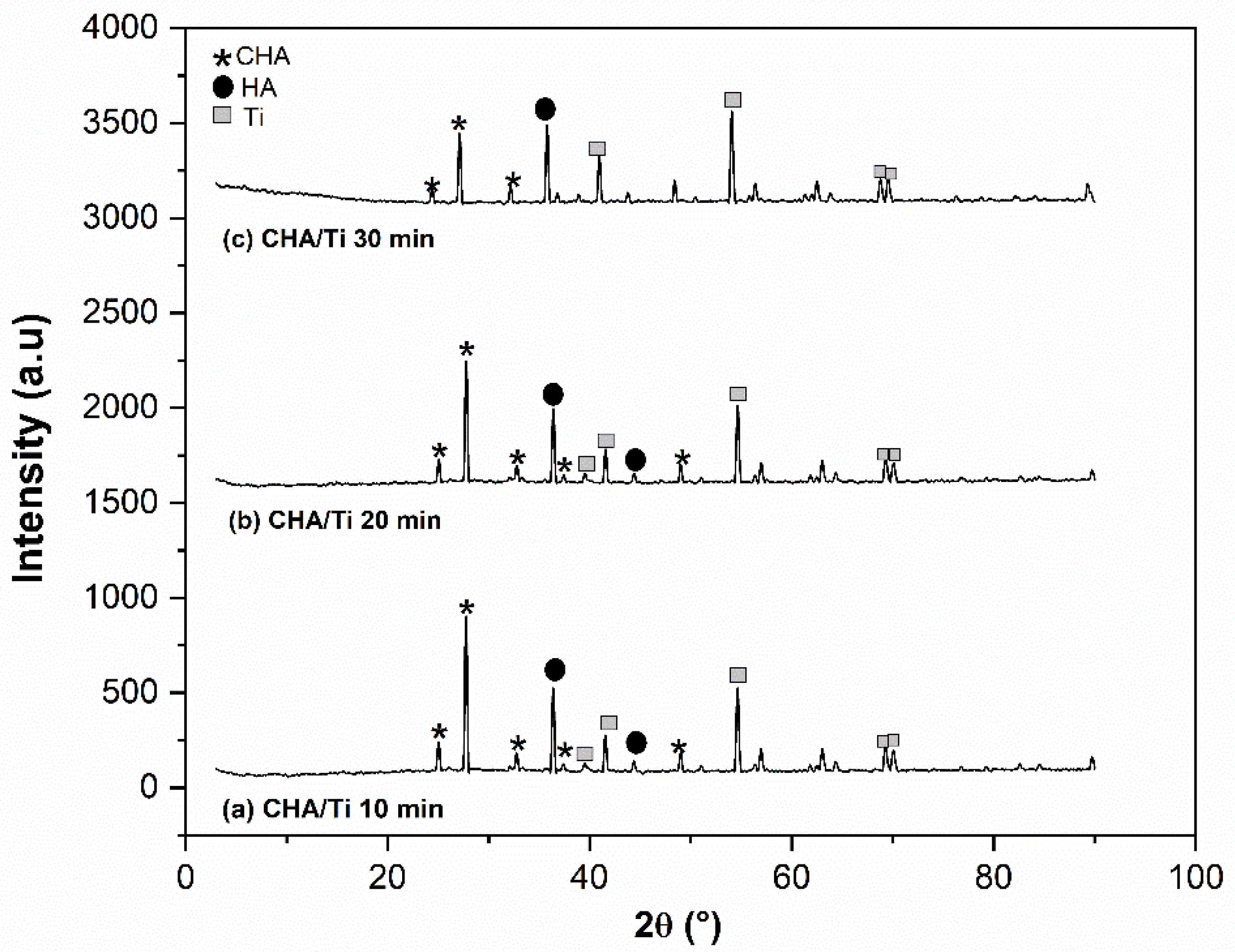
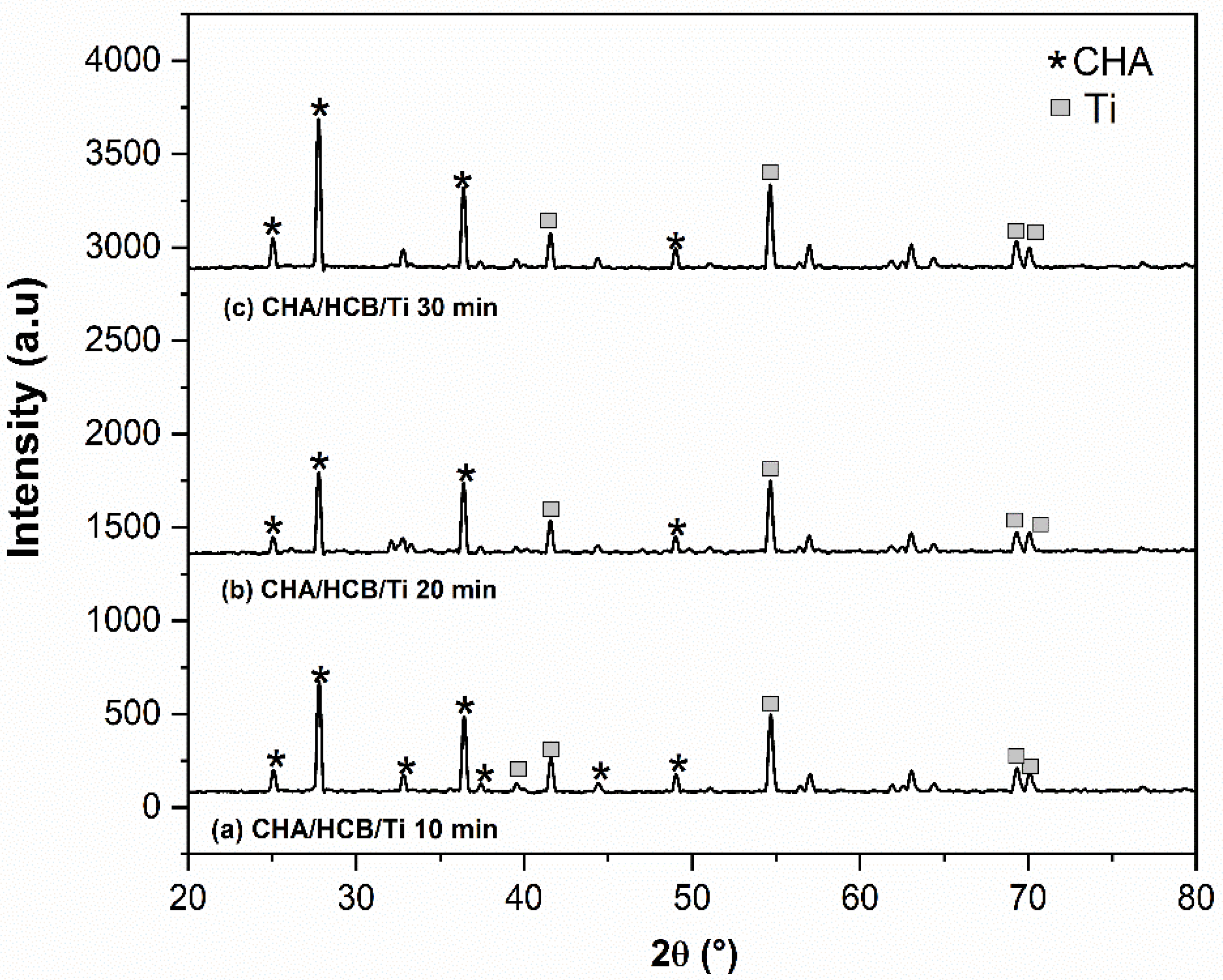
3.3.2. XRD Analysis
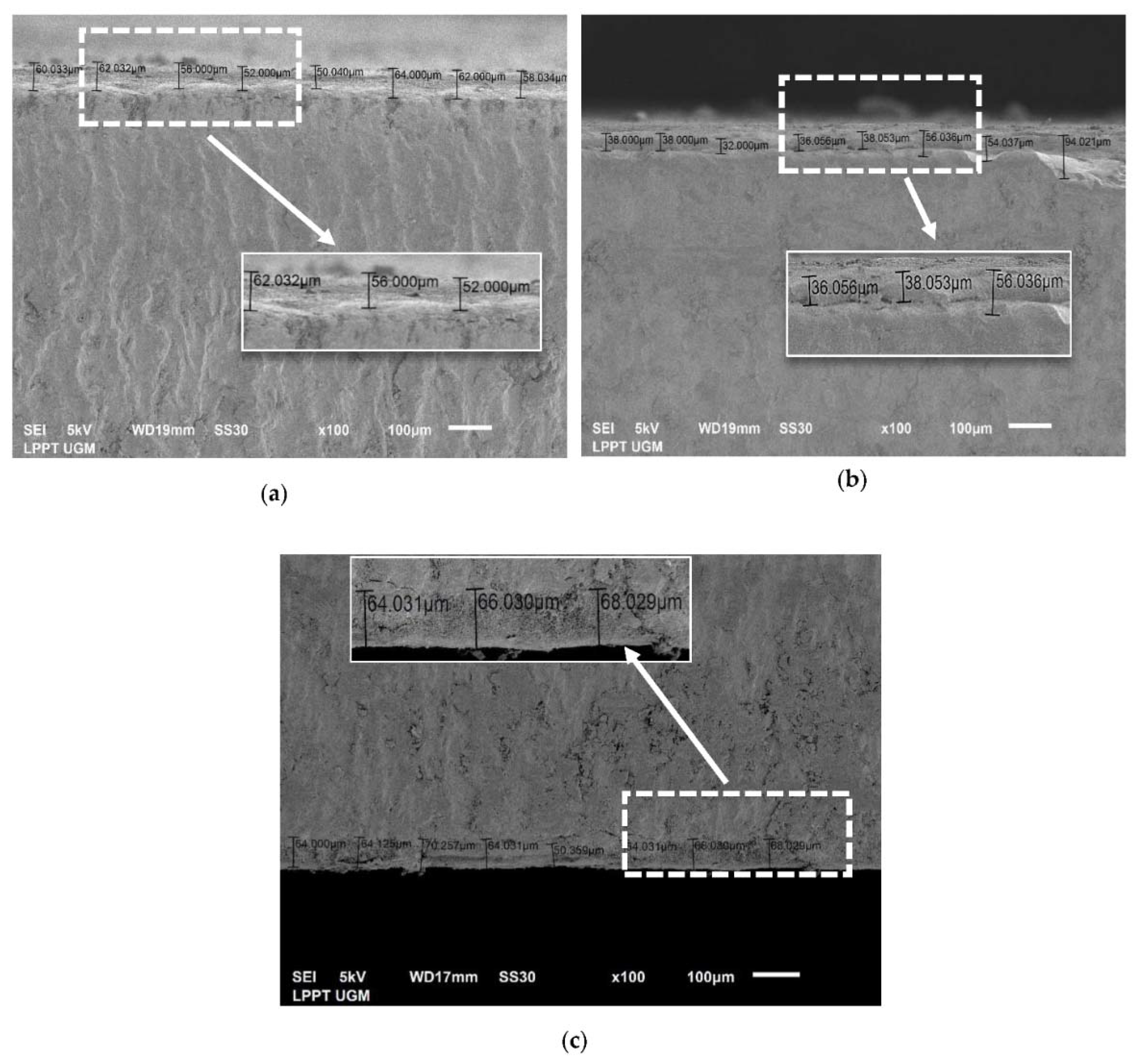
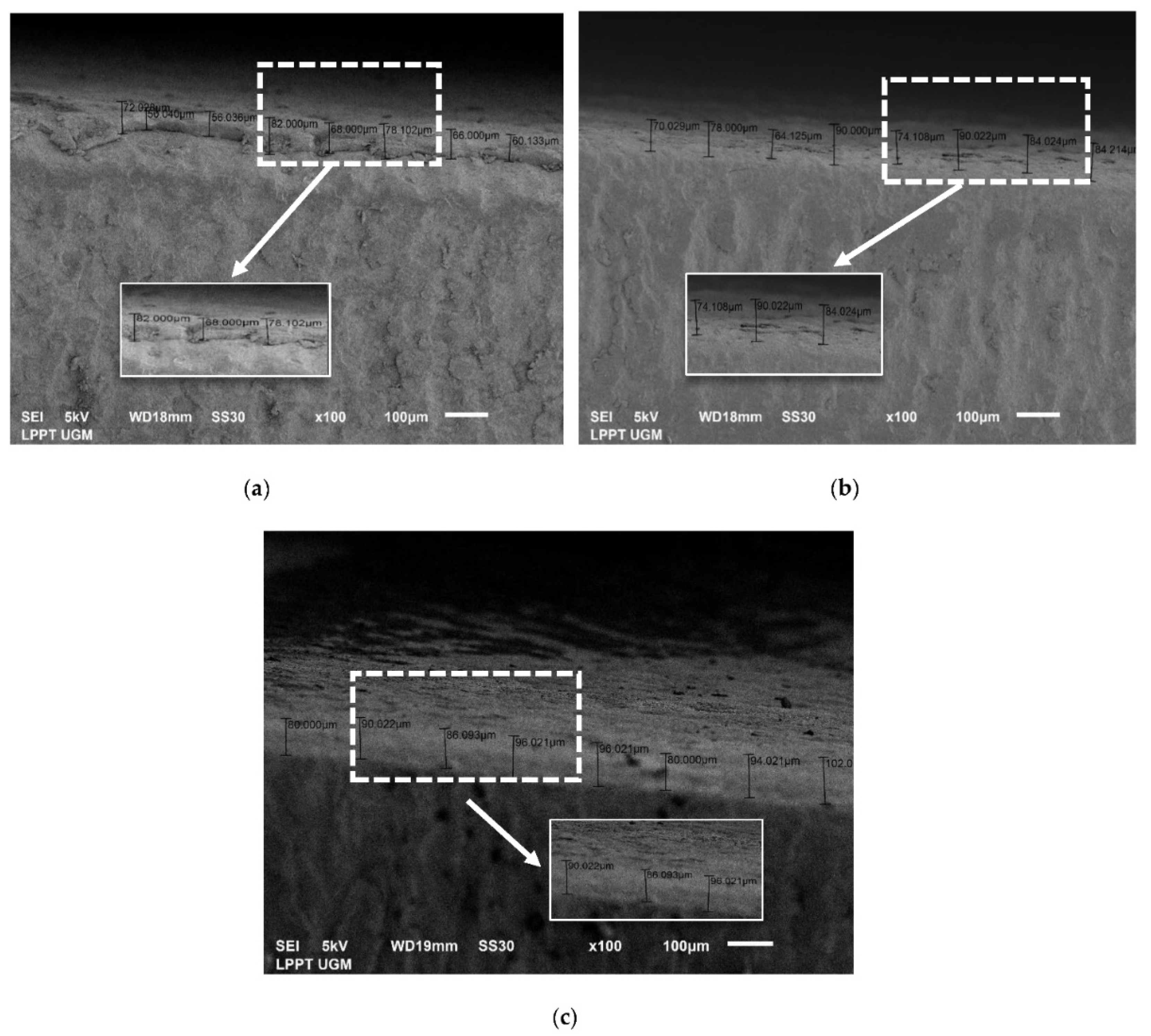
3.3.3. Thickness Analysis by SEM
4. Discussion
4.1. CHA Synthesized from Abalone Mussel Shells and Scaffold CHA/HCB 40 wt.%
4.2. Electrophoretic Deposition Dip Coating (EP2D) of CHA/Ti and CHA/HCB/Ti Coatings
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Singh, G.; Bala, N. Electrophoretic deposition of hydroxyapatite-iron oxide-chitosan composite coatings on Ti–13Nb–13Zr alloy for biomedical applications. Thin. Solid. Film. 2020, 697, 137801. [Google Scholar] [CrossRef]
- Syafaat, F.Y.; Yusuf, Y. Effect of Ca:P concentration and calcination temperature on hydroxyapatite (HAp) powders from quail eggshell (Coturnix Coturnix). Int. J. Nanoelectron. Mater. 2018, 11, 51–58. [Google Scholar]
- Kriswanto, M.; Khairurrijal, M.; Wajong, D.L.J.; Kadarismanto, T.M.; Yusuf, Y. Stainless steel 316 L metal coating with capiz shell hydroxyapatite using electrophoretic deposition method as bone implant candidate. Key Eng. Mater. 2020, 840, 336–344. [Google Scholar] [CrossRef]
- Mulya, N.; Fadli, A.; Amri, A. Pengaruh penambahan hidroksiapatit dan waktu pencelupan terhadap pelapisan logam stainless steel 316L dengan metode dip coating. JOM Fteknik 2016, 3, 1–7. [Google Scholar]
- Gopi, D.; Ramya, S.; Rajeswari, D.; Kavitha, L. Corrosion protection performance of porous strontium hydroxyapatite coating on polypyrrole coated 316L stainless steel. Coll. Surf. B Biointerfaces 2013, 107, 130–136. [Google Scholar] [CrossRef]
- Ahmadi, R.; Afshar, A. In vitro study: Bond strength, electrochemical and biocompatibility evaluations of TiO2/Al2O3 reinforced hydroxyapatite sol–gel coatings on 316L SS. Surf. Coat. Technol. 2021, 405, 126594. [Google Scholar] [CrossRef]
- Ana, I.D.; Satria, G.A.P.; Dewi, A.H. Bioceramics for Clinical Application in Regenerative Dentistry; Springer: Singapore, 2018; pp. 309–316. [Google Scholar]
- Sari, M.; Yusuf, Y. Synthesis and characterization of hydroxyapatite based on green mussel shells (Perna viridis) with calcination temperature variation using the precipitation method. Int. J. Nanoelectron. Mater. 2018, 11, 357–370. [Google Scholar]
- Sari, M.; Hening, P.; Chotimah; Ana, I.D.; Yusuf, Y. Bioceramic hydroxyapatite-based scaffold with a porous strructure using honeycomb as a natural polymeric porogen for bone tissue engineering. Biomater. Res. 2021, 25, 1–13. [Google Scholar] [CrossRef]
- Singh, A.; Singh, G.; Chawla, V.J. Characterization and mechanical behaviour of reinforced hydroxyapatite coatings deposited by vacuum plasma spray on SS-316L alloy. J. Mech. Behav. Biomed. Mater. 2018, 79, 273–282. [Google Scholar] [CrossRef]
- Permatasari, H.A.; Supii, A.I.; Suparta, G.B.; Yusuf, Y. Characteristics of abalone mussel shells (Halioitis asinina) with calcination temperature variations as a basic material for synthesis of carbonated hydroxyapatite. Key Eng. Mater. 2019, 818, 31–36. [Google Scholar] [CrossRef]
- Youness, R.A.; Taha, M.A.; Ibrahim, M.A. Effect of sintering temperatures on the in vitro bioactivity, molecular structure and mechanical properties of titanium/carbonated hydroxyapatite nanobiocomposites. J. Mol. Struct. 2017, 1150, 188–195. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Ramesh, S.; Tan, C.Y.; Chandran, H.; Noor, A.F.M.; Krishnasamy, S.; Alengaram, U.J.; Ramesh, S. Effect of multi-ions doping on the properties of carbonated hydroxyapatite bioceramic. Ceram. Int. 2019, 45, 3473–3477. [Google Scholar] [CrossRef]
- Permatasari, H.A.; Yusuf, Y. Characteristics of carbonated hydroxyapatite based on abalone mussel shells (Halioitis asinina) synthesized by precipitation method with aging time variations. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042031. [Google Scholar] [CrossRef]
- Almukarrama; Yusuf, Y. Development carbonated hydroxyapatite powders from oyster shells (Crassostrea gigas) by sintering time controlling. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042001. [Google Scholar] [CrossRef]
- Laonapakul, T. Synthesis of hydroxyapatite from biogenic wastes. KKU Eng. J. 2015, 42, 269–4275. [Google Scholar]
- Wati, R.; Yusuf, Y. Effect of sintering temperature on carbonated hydroxyapatite derived from common cockle shells (Cerastodermaedule): Composition and crystal characteristics. Key Eng. Mater. 2019, 818, 37–43. [Google Scholar] [CrossRef]
- Anggraini, R.M.; Supii, A.I.; Suparta, G.B.; Yusuf, Y. The effect of pH on the characteristics of carbonate hydroxyapatite based on pearl shell (Pinctada maxima). Key Eng. Mater. 2019, 818, 44–49. [Google Scholar] [CrossRef]
- Zhou, W.Y.; Wang, M.; Cheung, W.L.; Guo, B.C.; Jia, D.M. Synthesis of carbonated hydroxyapatite nanospheres through nanoemulsion. J. Mater. Sci. Mater. Med. 2008, 19, 103–110. [Google Scholar] [CrossRef]
- Ezekiel, I.; Kasim, S.R.; Ismail, Y.M.B.; Noor, A.F.M. Nanoemulsion synthesis of carbonated hydroxyapatite nanopowders: Effect of variant CO32−/PO43− molar ratios on phase, morphology, and bioactivity. Ceram. Int. 2018, 44, 13082–13089. [Google Scholar] [CrossRef]
- Rajabi-Zamani, A.H.; Behnamghader, A.; Kazemzadeh, A. Synthesis of nanocrystalline carbonated hydroxyapatite powder via nonalkoxide sol–gel method. Mater. Sci. Eng. C 2008, 28, 1326–1329. [Google Scholar] [CrossRef]
- Lala, S.; Brahmachari, S.; Das, P.K.; Das, D.; Kar, T.; Pradhan, S.K. Biocompatible nanocrystalline natural bonelike carbonated hydroxyapatite synthesized by mechanical alloying in a record minimum time. Mater. Sci. Eng. C 2014, 42, 647–656. [Google Scholar] [CrossRef]
- Kumar, T.S.; Manjubala, I.; Gunasekaran, J. Synthesis of carbonated calcium phosphate ceramics using microwave irradiation. Biomaterials 2000, 21, 1623–1629. [Google Scholar] [CrossRef]
- Kong, W.; Zhao, K.; Gao, C.; Zhu, P. Synthesis and characterization of carbonated hydroxyapatite with layered structure. Mater. Lett. 2019, 255, 126552. [Google Scholar] [CrossRef]
- Avcu, E.; Bastan, F.E.; Abdullah, H.Z.; Rehman, M.A.U.; Avcu, Y.Y.; Boccaccini, A.R. Electrophoretic deposition of chitosan-based composite coatings for biomedical applications: A review. Prog. Mater. Sci. 2019, 103, 69–108. [Google Scholar] [CrossRef]
- Ishikawa, K.; Munar, M.L.; Tsuru, K.; Miyamoto, Y. Fabrication of carbonate apatite honeycomb and its tissue response. J. Biomed. Mater. Res. Part A 2019, 107, 1014–1020. [Google Scholar] [CrossRef]
- Thomas, T.; Tiwari, G. Crushing behaviour of honeycomb structure: A review. Int. J. Crashworthiness 2019, 24, 1–25. [Google Scholar] [CrossRef]
- Ananth, K.P.; Suganya, S.; Mangalara, D.; Ferreira, J.M.F.; Balamurugan, A. Electrophoretic bilayer deposition of zirconia and reinforced bioglass system on Ti6Al4V for implant applications: An in vitro investigation. Mater. Sci. Eng. C 2013, 33, 4160–4166. [Google Scholar] [CrossRef] [PubMed]
- Aminatun; Apsari, R.; Yusuf, Y.; Suhariningsih. Synthesis and characterization of hydroxyapatite layer on cobalt alloys through dip coating method as a prosthetic bone implant candidate. J. Optoelectron. Biomed. Mater. 2015, 7, 11–18. [Google Scholar]
- Bartmanski, M.; Cieslik, B.; Glodowska, J.; Kalka, P.; Pawlowski, L.; Pieper, M.; Zielinski, A. Electrophoretic deposition (EPD) of nanohydroxyapatite-nanosilver coatings on Ti13Zr13Nb alloy. Ceram. Int. 2017, 43, 11820–11829. [Google Scholar] [CrossRef]
- Li, M.; Xiong, P.; Mo, M.; Cheng, Y.; Zheng, Y. Electrophoretic-deposited novel ternary silk fibroin/graphene oxide/hydroxyapatite nanocomposite coatings on titanium substrate for orthopedic applications. Front. Mater. Sci. 2016, 10, 270–280. [Google Scholar] [CrossRef]
- Balaji, J.; Oh, T.W.; Sethurahman, M.G. Effects of pH on inhibitor-doped hybrid protective sol–gel coatings on the copper electrode surface. J. Taiwan Inst. Chem. Eng. 2021, 119, 2–11. [Google Scholar] [CrossRef]
- Sari, M.; Hening, P.; Chotimah; Ana, I.D.; Yusuf, Y. Porous structure of bioceramics carbonated hydroxyapatite-based honeycomb scaffold for bone tissue engineering. Mater. Today Commun. 2021, 26, 102135. [Google Scholar] [CrossRef]
- Tsuchida, T.; Kubo, J.; Yoshioka, T.; Sakuma, S.; Takeguchi, T.; Ueda, W. Reaction of ethanol over hydroxyapatite affected by Ca/P ratio of catalyst. J. Catal. 2008, 259, 183–189. [Google Scholar] [CrossRef]
- Lovon-Quintana, J.J.; Rodriguez-Guerrero, J.K.; Valenca, P.G. Carbonated Hydroxyapatite as a catalyst for ethanol conversion to hydrocarbon fuels. Appl. Catal. A Genera 2017, 542, 136–145. [Google Scholar] [CrossRef]
- Igathinathane, C.; Pordesimo, L.O.; Columbus, E.P.; Batchelor, W.D.; Methuku, S.R. Shape identification and particles size distribution from basic shape parameters using ImageJ. Comp. Electron. Agric. 2008, 63, 168–182. [Google Scholar] [CrossRef]
- Writing ImageJ Plugins-A Tutorial. Available online: https://imagej.nih.gov/ij/index.html (accessed on 20 July 2021).
- Heimann, R.B. Materials Science of Crystalline Bioceramics: A Review of Basic Properties and Applications. CMU J. 2002, 1, 23–46. [Google Scholar]
- Abdeltawab, A.A.; Shoeib, M.A.; Mohamed, S.G. Electrophoretic deposition of hydroxyapatite coatings on titanium from dimethylformamide suspensions. Surf. Coat. Technol. 2011, 206, 43–50. [Google Scholar] [CrossRef]
- Mawuntu, V.J.; Yusuf, Y. Porous structure engineering of bioceramic hydroxyapatite-based scaffolds using PVA, PVP, and PEO as polymeric porogens. J. Asian Ceram. Soc. 2019, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sari, M.; Yusuf, Y. Synthesis and characterization of hydroxyapatite based on green mussel shells (perna viridis) with the variation of stirring time using the precipitation method. IOP Conf. Ser. Mater. Sci. Eng. 2018, 432, 012046. [Google Scholar] [CrossRef] [Green Version]
- Cheng, M.; Wahafu, T.; Jiang, G.; Liu, W.; Qiao, Y.; Peng, X.; Cheng, T.; Zhang, X.; He, G.; Liu, X. A novel open-porous magnesium scaffold with controllable microstructures and properties for bone regeneration. Nat. Publ. Gr. 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Besra, L.; Liu, M. A review on fundamentals and applications of electrophoretic deposition (EPD). Prog. Mater. Sci. 2007, 52, 1–61. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Alias, J.; Zulkifli, F.H.; Kadirgama, K.; Ghani, S.A.C.; Shariffuddin, J.H.M. A comprehensive review of hydroxyapatite-based coatings adhesion on metallic biomaterials. Ceram. Int. 2018, 44, 1250–1268. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, S.; Arcos, D.; Vallet-Regi, M. Upgrading calcium phosphate scaffolds for tissue engineering applications. Key Eng. Mater. 2008, 377, 19–42. [Google Scholar] [CrossRef]
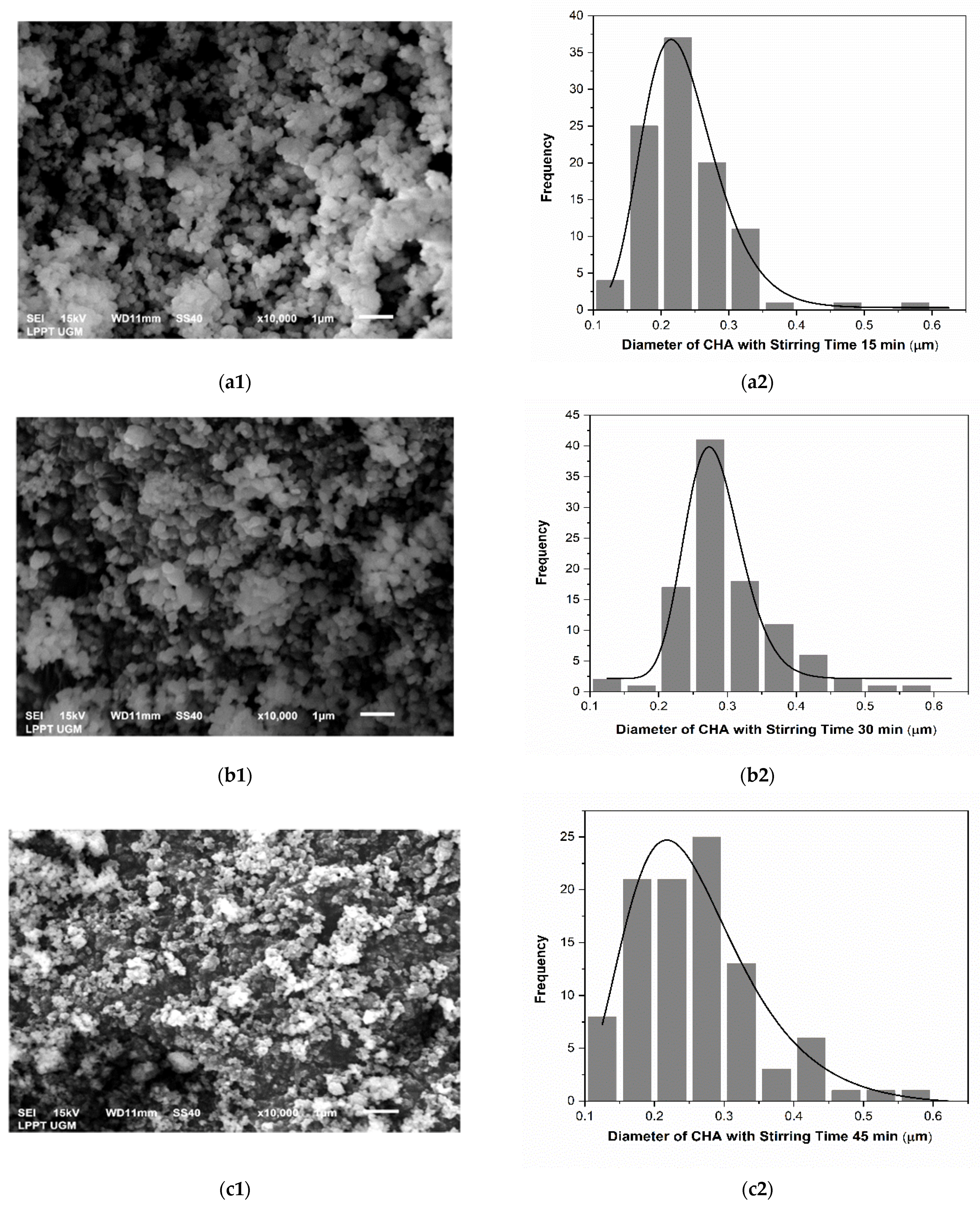

| No. | CHA with Stirring Time Variations (min) | Particle Size (μm) |
|---|---|---|
| 1 | 15 | 0.227 ± 0.002 |
| 2 | 30 | 0.280 ± 0.003 |
| 3 | 45 | 0.247 ± 0.012 |
| No. | CHA with Stirring Time Variations (min) | Ca and P (%) | Molar Ratio of Ca/P | |
|---|---|---|---|---|
| P | Ca | |||
| 1 | 15 | 14.81 | 31.91 | 1.67 |
| 2 | 30 | 11.72 | 27.52 | 1.81 |
| 3 | 45 | 13.79 | 31.87 | 1.79 |
| Composite | Macropore Size (μm) | Micropore Size (μm) | FTIR Analysis | XRD Analysis | |||||
|---|---|---|---|---|---|---|---|---|---|
| Transmittance (cm−1) | Description | S ± ∆s (nm) | Microstrain (ε) | Lattice Parameter (Å) | X-ray Density (g/cm3) | ||||
| a | c | ||||||||
| CHA/HCB 40 wt.% | 102.02 ± 9.88 | 1.08 ± 0.086 | 602–570 and 1091–962 | PO43− Absorption | 30.48 ± 2.97 | 0.0042 | 9.455 | 6.916 | 7.892 |
| 1446–876 | B-type CO3-HCB | ||||||||
| 3643–3571 and 633 | OH− Absorption | ||||||||
| No. | Immersion Times of CHA/Ti (min) | Compressive Strength (MPa) | p-Value |
|---|---|---|---|
| Mean ± SD | |||
| 1 | Control | 83.30 ± 10.15 | 0.059 |
| 2 | 10 | 61.60 ± 9.92 | 0.059 |
| 3 | 20 | 51.59 ± 17.47 | 0.059 |
| 4 | 30 | 54.17 ± 12.51 | 0.059 |
| No. | CHA/Ti and CHA/HCB/Ti Coatings with Immersion Times Variation (min) | S ± ∆s (nm) | Microstrain (ε) | Lattice Parameter (Å) | ||
|---|---|---|---|---|---|---|
| a | c | c/a | ||||
| 1 | CHA/Ti 10 | 16.65 ± 2.00 | 0.0087 | 9.45 | 7.10 | 0.75 |
| 2 | CHA/Ti 20 | 16.31 ± 2.01 | 0.0088 | 9.46 | 7.10 | 0.74 |
| 3 | CHA/Ti 30 | 18.15 ± 2.85 | 0.0042 | 9.63 | 7.29 | 0.76 |
| 4 | CHA/HCB/Ti 10 | 16.99 ± 2.11 | 0.085 | 9.44 | 7.09 | 0.75 |
| 5 | CHA/HCB/Ti 20 | 17.83 ± 2.51 | 0.004 | 9.47 | 7.10 | 0.75 |
| 6 | CHA/HCB/Ti 30 | 20.39 ± 2.46 | 0.007 | 9.50 | 7.11 | 0.74 |
| No. | CHA/Ti and CHA/HCB/Ti Coatings with Immersion Times Variation (min) | Average Thickness Value (μm) |
|---|---|---|
| 1 | CHA/Ti 10 | 56.80 ± 4.82 |
| 2 | CHA/Ti 20 | 41.73 ± 8.66 |
| 3 | CHA/Ti 30 | 63.86 ± 5.54 |
| 4 | CHA/HCB/Ti 10 | 71.04 ± 7.35 |
| 5 | CHA/HCB/Ti 20 | 77.79 ± 8.36 |
| 6 | CHA/HCB/Ti 30 | 88.88 ± 6.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sari, M.; Kristianto, N.A.; Chotimah; Ana, I.D.; Yusuf, Y. Carbonated Hydroxyapatite-Based Honeycomb Scaffold Coatings on a Titanium Alloy for Bone Implant Application—Physicochemical and Mechanical Properties Analysis. Coatings 2021, 11, 941. https://doi.org/10.3390/coatings11080941
Sari M, Kristianto NA, Chotimah, Ana ID, Yusuf Y. Carbonated Hydroxyapatite-Based Honeycomb Scaffold Coatings on a Titanium Alloy for Bone Implant Application—Physicochemical and Mechanical Properties Analysis. Coatings. 2021; 11(8):941. https://doi.org/10.3390/coatings11080941
Chicago/Turabian StyleSari, Mona, Nicholas Adi Kristianto, Chotimah, Ika Dewi Ana, and Yusril Yusuf. 2021. "Carbonated Hydroxyapatite-Based Honeycomb Scaffold Coatings on a Titanium Alloy for Bone Implant Application—Physicochemical and Mechanical Properties Analysis" Coatings 11, no. 8: 941. https://doi.org/10.3390/coatings11080941
APA StyleSari, M., Kristianto, N. A., Chotimah, Ana, I. D., & Yusuf, Y. (2021). Carbonated Hydroxyapatite-Based Honeycomb Scaffold Coatings on a Titanium Alloy for Bone Implant Application—Physicochemical and Mechanical Properties Analysis. Coatings, 11(8), 941. https://doi.org/10.3390/coatings11080941