Corrosion Protection of Injection Molded Porous 440C Stainless Steel by Electroplated Zinc Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Metal Injection Molding (MIM)
2.2. Zinc Coating by Electroplating
2.3. Corrosion Testing
2.4. Characterization
3. Results and Discussion
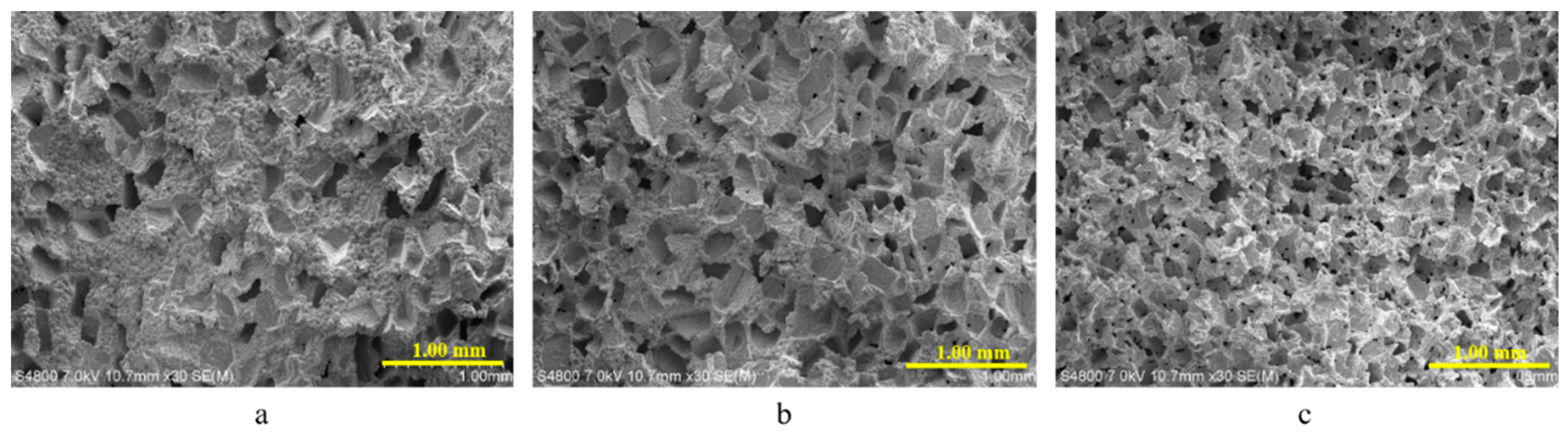
3.1. Morphology of Porous Structures
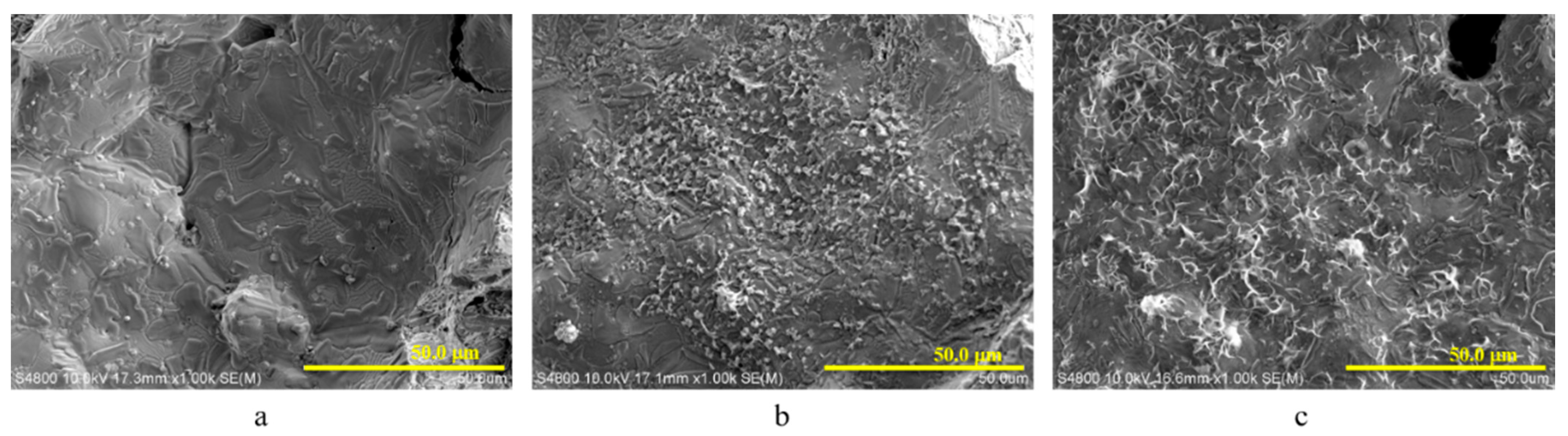
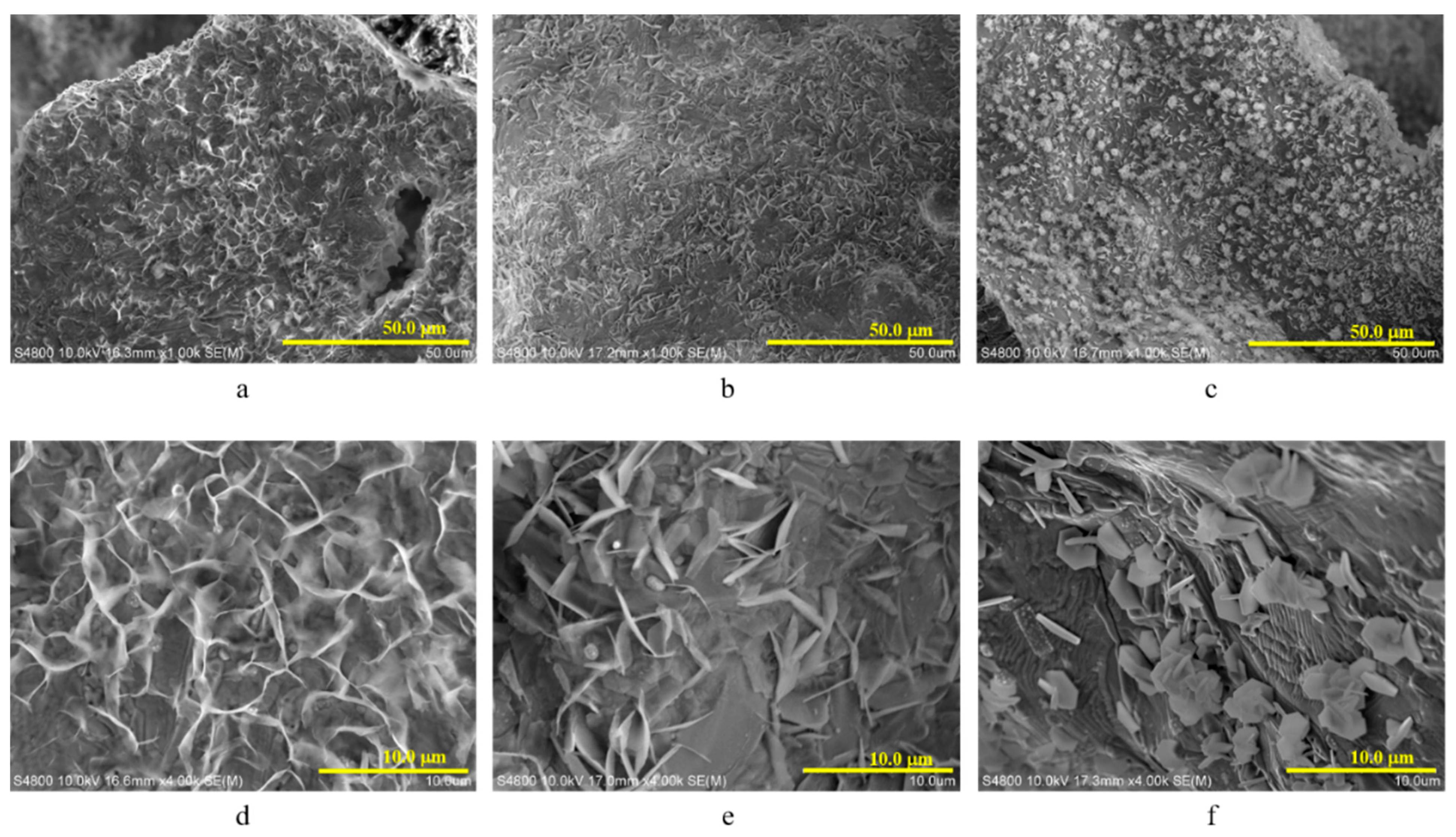
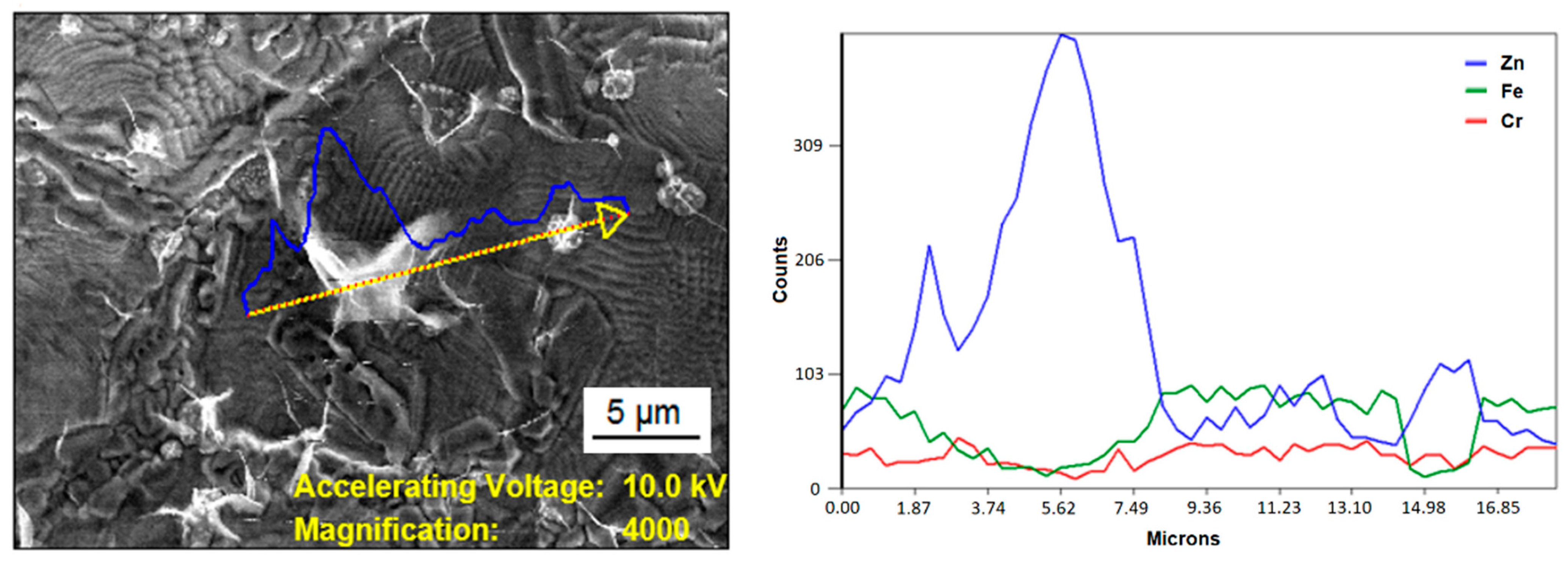
3.2. Morphology of the Electroplated Zinc Coating
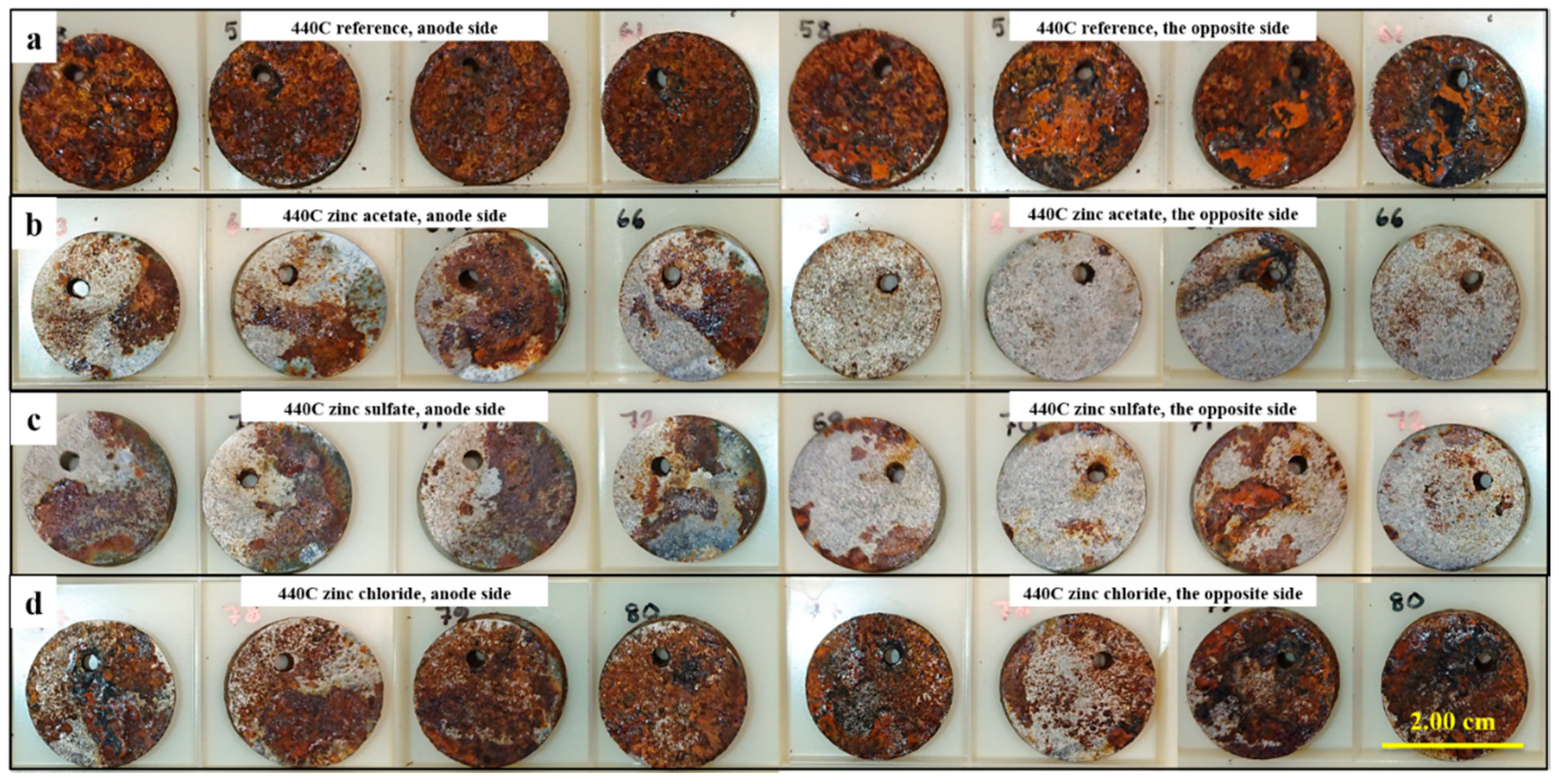
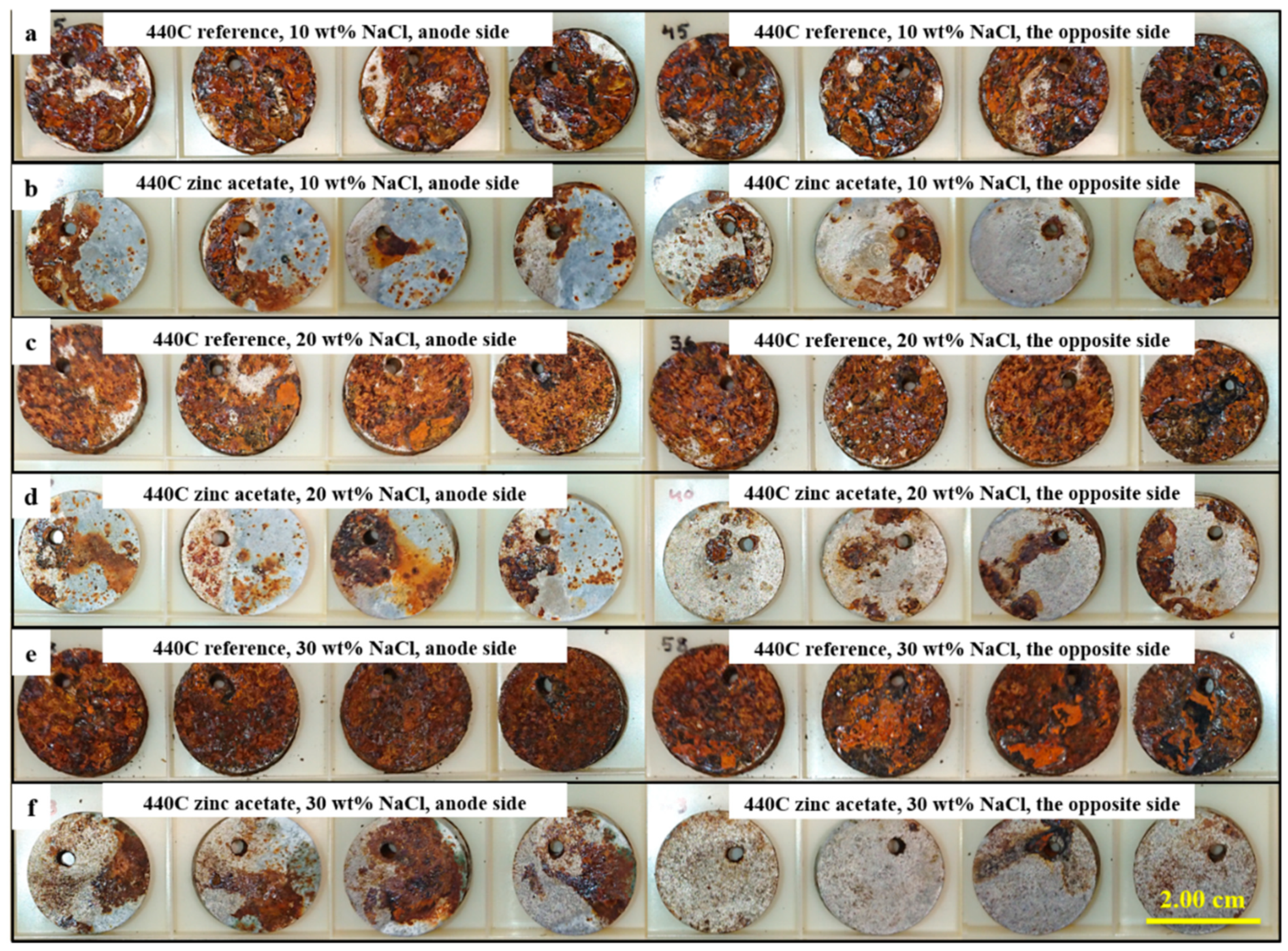
3.3. Corrosion Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mater. Sci. Eng. R. Rep. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Huang, K.T.; Chang, S.H.; Wang, C.K.; Chen, J.K. Microstructures and mechanical properties of 440C stainless steel strengthened with TaC via vacuum sintering and heat treatments. Mater. Trans. 2015, 56, 1585–1590. [Google Scholar] [CrossRef] [Green Version]
- Lo, K.H.; Cheng, F.T.; Man, H.C. Laser transformation hardening of AISI 440C martensitic stainless steel for higher cavitation erosion resistance. Surf. Coat. Technol. 2003, 173, 96–104. [Google Scholar] [CrossRef]
- Lippold, J.C. Welding Metallurgy and Weldability; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Huang, K.T.; Chang, S.H.; Hsieh, P.C. Microstructure, mechanical properties and corrosion behavior of NbC modified AISI 440C stainless steel by vacuum sintering and heat treatments. J. Alloys Compd. 2017, 712, 760–767. [Google Scholar] [CrossRef]
- Salleh, S.H.; Omar, M.Z.; Syarif, J.; Ghazali, M.J.; Abdullah, S.; Sajuri, Z. Investigation of microstructures and properties of 440C martensitic stainless steel. Int. J. Mech. Mater. Eng. 2009, 4, 123–126. [Google Scholar]
- Puli, R.; Ram, G.D.J. Microstructures and properties of friction surfaced coatings in AISI 440C martensitic stainless steel. Surf. Coat. Technol. 2012, 207, 310–318. [Google Scholar] [CrossRef]
- Kwok, C.T.; Lo, K.H.; Cheng, F.T.; Man, H.C. Effect of processing conditions on the corrosion performance of laser surface-melted AISI 440C martensic stainless steel. Surf. Coat. Technol. 2003, 166, 221–230. [Google Scholar] [CrossRef]
- Antunes, P.D.; Correa, E.O.; Barbosa, R.P.; Silva, E.M.; Padilha, A.F.; Guimaraes, P.M. Effect of weld metal chemistry on stress corrosion cracking behavior of AISI 444 ferritic stainless steel weldments in boiling chloride solution. Mater. Corros. 2013, 64, 415–421. [Google Scholar] [CrossRef]
- Wang, Z.; Di-Franco, F.; Seyeux, A.; Zanna, S.; Maurice, V.; Marcus, P. Passivation-induced physicochemical alterations of the native surface oxide film on 316L austenitic stainless steel. J. Electrochem. Soc. 2019, 166, C3376–C3388. [Google Scholar] [CrossRef]
- Punckt, C.; Bölscher, M.; Rotermund, H.H.; Mikhailov, A.S.; Organ, L.; Budiansky, N.; Scully, J.R.; Hudson, J.L. Sudden onset of pitting corrosion on stainless steel as a critical phenomenon. Science 2004, 305, 1133–1136. [Google Scholar] [CrossRef]
- Ryan, M.P.; Williams, D.E.; Chater, R.J.; Hutton, B.M.; McPhail, D.S. Why stainless steel corrodes. Nature 2002, 415, 770–774. [Google Scholar] [CrossRef]
- Marcelin, S.; Pébère, N.; Régnier, S. Electrochemical investigations on crevice corrosion of a martensitic stainless steel in a thin-layer cell. J. Electroanal. Chem. 2015, 737, 198–205. [Google Scholar] [CrossRef] [Green Version]
- Dalmau, A.; Richard, C.; Igual-Muñoz, A. Degradation mechanisms in martensitic stainless steels: Wear, corrosion and tribocorrosion appraisal. Tribol. Int. 2018, 121, 167–179. [Google Scholar] [CrossRef]
- Parsapour, A.; Khorasani, S.N.; Fathi, M.H. Effect of Surface Treatment and Metallic Coating on Corrosion Behavior and Biocompatibility of Surgical 316L Stainless Steel Implant. J. Mater. Sci. Technol. 2012, 28, 125–131. [Google Scholar] [CrossRef]
- González, M.B.; Saidman, S.B. Electrodeposition of polypyrrole on 316L stainless steel for corrosion prevention. Corros. Sci. 2011, 53, 276–282. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, Y.; Zhang, H.; Sun, C.; Liang, K.; Zhang, S. Pulse electroplating of ultra-fine grained zinc coating on 316L stainless steel and its corrosion behaviour. Int. J. Electrochem. Sci. 2016, 14, 5913–5922. [Google Scholar] [CrossRef]
- Zhao, X.; Han, L.; Xiao, J.; Wang, L.; Liang, T.; Liao, X. Corrosion protection of steel elements in façade systems. Sci. Total Environ. 2020, 32, 135938. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, D.; An, M. Elevating the photo-generated cathodic protection of corrosion product layers on electrogalvanized steel through nano-electrodeposition. Chem. Phys. Lett. 2019, 722, 1–5. [Google Scholar] [CrossRef]
- Schlesinger, M.; Paunovic, M. Modern Electroplating, 5th ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Xu, M.; Lam, C.N.C.; Wong, D.; Asselin, E. Evaluation of the cathodic disbondment resistance of pipeline coatings—A review. Prog. Org. Coat. 2020, 146, 105728. [Google Scholar] [CrossRef]
- Gomes, A.; Da Silva Pereira, M.I. Pulsed electrodeposition of Zn in the presence of surfactants. Electrochim. Acta 2006, 51, 1342–1350. [Google Scholar] [CrossRef]
- Chen, L.J.; Li, T.; Li, Y.M.; He, H.; Hu, Y.H. Porous titanium implants fabricated by metal injection molding. Trans. Nonferr. Met. Soc. China (Engl. Ed.) 2009, 19, 1174–1179. [Google Scholar] [CrossRef]
- Dehghan-Manshadi, A.; Yu, P.; Dargusch, M.; StJohn, D.; Qian, M. Metal injection moulding of surgical tools, biomaterials and medical devices: A review. Powder Technol. 2020, 364, 189–204. [Google Scholar] [CrossRef]
- Nishiyabu, K.; Matsuzaki, S.; Tanaka, S. Fabrication of micro porous metal components by metal injection molding based powder space holder method. High Temp. Mater. Process. 2007, 26, 257–267. [Google Scholar] [CrossRef]
- Gülsoy, H.Ö.; German, R.M. Production of micro-porous austenitic stainless steel by powder injection molding. Scr. Mater. 2008, 58, 295–298. [Google Scholar] [CrossRef]
- Manonukul, A.; Muenya, N.; Léaux, F.; Amaranan, S. Effects of replacing metal powder with powder space holder on metal foam produced by metal injection moulding. J. Mater. Process. Technol. 2010, 210, 529–535. [Google Scholar] [CrossRef]
- Li, D.; Hou, H.; Liang, L.; Lee, K. Powder injection molding 440C stainless steel. Int. J. Adv. Manuf. Technol. 2010, 49, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Harun, W.; Toda, K.; Osada, T.; Kang, H.; Tsumori, F.; Miura, H. Effect of MIM Processing Parameters on the Properties of 440C Stainless Steel. J. Jpn. Soc. Powder Powder Metall. 2012, 59, 264–271. [Google Scholar] [CrossRef] [Green Version]
- García, C.; Martín, F.; de Tiedra, P.; Cambronero, L.G. Pitting corrosion behaviour of PM austenitic stainless steels sintered in nitrogen-hydrogen atmosphere. Corros. Sci. 2007, 49, 1718–1736. [Google Scholar] [CrossRef]
- Leisner, P.; Leu, R.C.; Møller, P. Electroplating of porous PM compacts. Powder Metall. 1997, 40, 207–210. [Google Scholar] [CrossRef]
- Seah, K.H.W.; Thampuran, R.; Teoh, S.H. The influence of pore morphology on corrosion. Corros. Sci. 1998, 40, 547–556. [Google Scholar] [CrossRef]
- Garcia-Cabezon, C.; Garcia-Hernandez, C.; Rodriguez-Mendez, M.L.; Martin-Pedrosa, F. A new strategy for corrosion protection of porous stainless steel using polypyrrole films. J. Mater. Sci. Technol. 2020, 37, 85–95. [Google Scholar] [CrossRef]
- Heaney, D.F. Handbook of Metal Injection Molding, 1st ed.; Woodhead Publishing Limited: Cambridge, UK, 2012. [Google Scholar]
- Gibson, L.J.; Ashby, M. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Winand, R. Electrodeposition of Zinc and Zinc Alloys. In Modern Electroplating, 5th ed.; Schlesinger, M., Paunovic, M., Eds.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2011; pp. 285–307. [Google Scholar]
| Composition | Fe | C | Ni | Cr | Mo | Mn | Si | Nb | S | P |
|---|---|---|---|---|---|---|---|---|---|---|
| > | - | 0.85 | - | 16.0 | - | - | - | 1.0 | - | - |
| < | Balance | 1.0 | 0.6 | 18.0 | 0.75 | 1.0 | 1.0 | 2.0 | 0.03 | 0.04 |
| NaCl (wt.%) | Before Debinding (g) | After Debinding (g) | Mass Loss (%) |
|---|---|---|---|
| 10 | 12.62 ± 0.08 | 10.90 ± 0.08 | 13.6 ± 0.6 |
| 20 | 11.33 ± 0.09 | 8.72 ± 0.09 | 23.0 ± 0.5 |
| 30 | 10.18 ± 0.10 | 6.82 ± 0.13 | 33.0 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kultamaa, M.; Mönkkönen, K.; Saarinen, J.J.; Suvanto, M. Corrosion Protection of Injection Molded Porous 440C Stainless Steel by Electroplated Zinc Coating. Coatings 2021, 11, 949. https://doi.org/10.3390/coatings11080949
Kultamaa M, Mönkkönen K, Saarinen JJ, Suvanto M. Corrosion Protection of Injection Molded Porous 440C Stainless Steel by Electroplated Zinc Coating. Coatings. 2021; 11(8):949. https://doi.org/10.3390/coatings11080949
Chicago/Turabian StyleKultamaa, Matti, Kari Mönkkönen, Jarkko J. Saarinen, and Mika Suvanto. 2021. "Corrosion Protection of Injection Molded Porous 440C Stainless Steel by Electroplated Zinc Coating" Coatings 11, no. 8: 949. https://doi.org/10.3390/coatings11080949







