Effect of Vacuum Annealing on Microstructure and Hot-Salt Corrosion Behavior of CoNiCrAlY/YSZ/LaMgAl11O19 Double-Ceramic Coating
Abstract
:1. Introduction
2. Materials and Methods
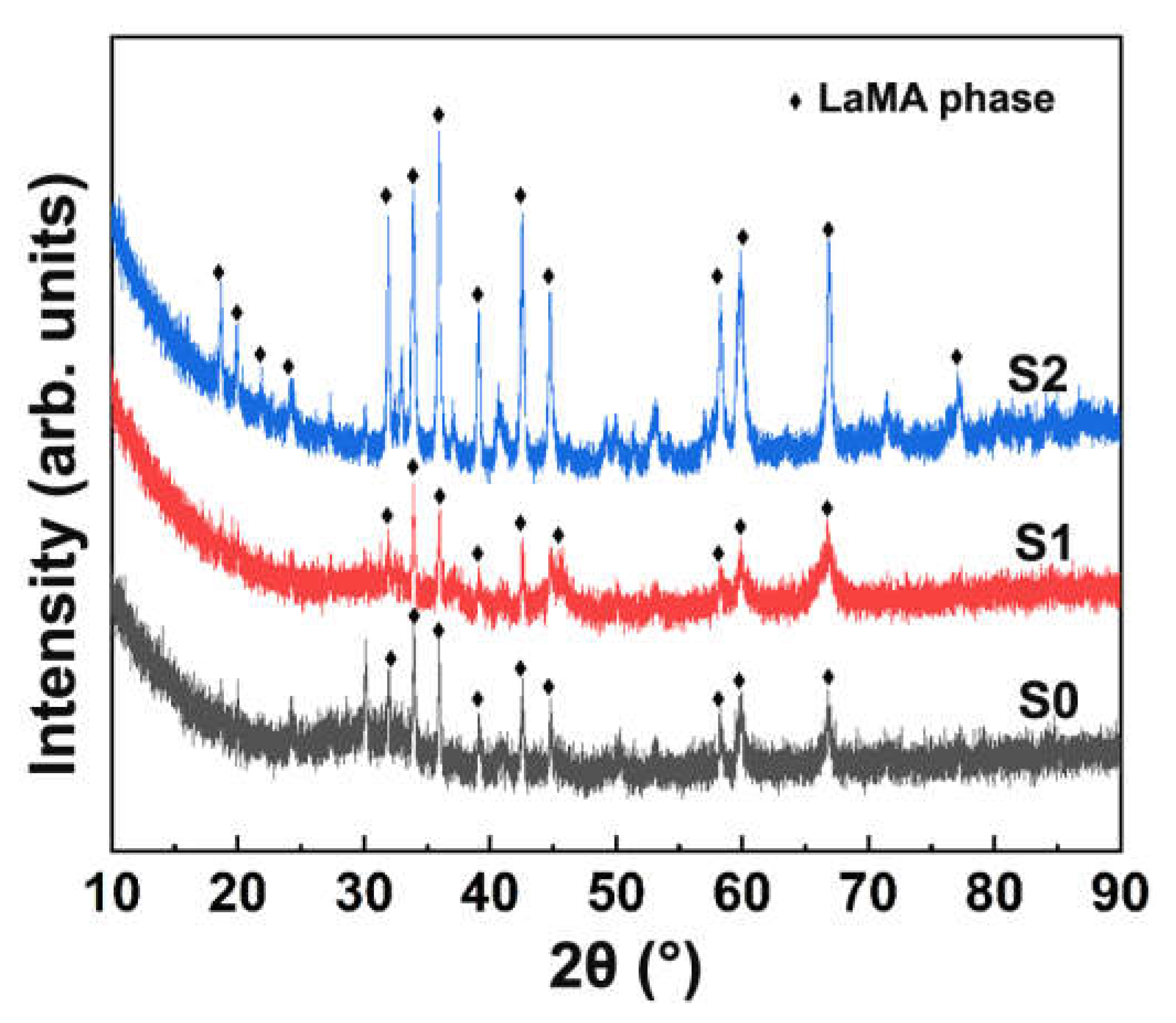
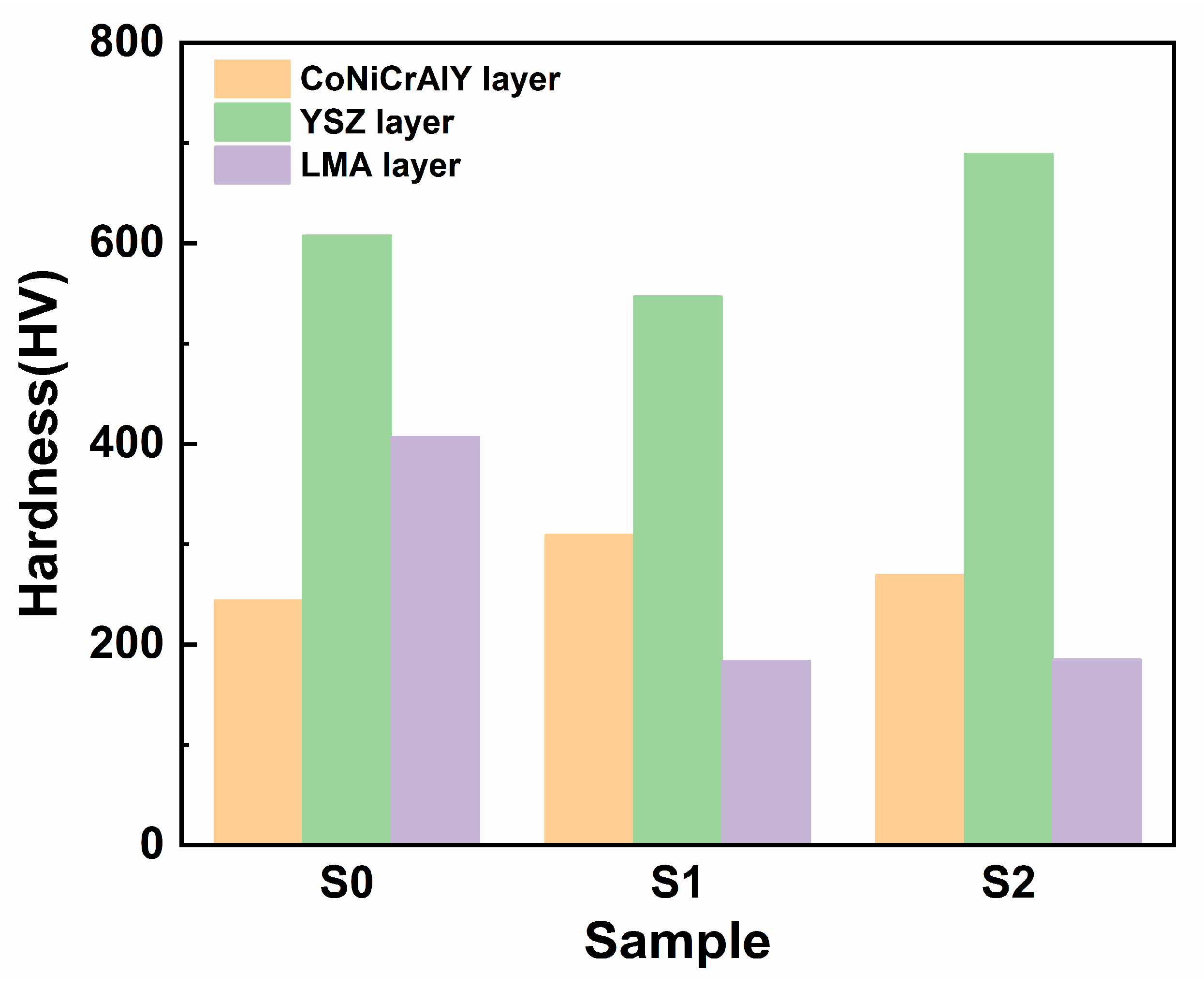
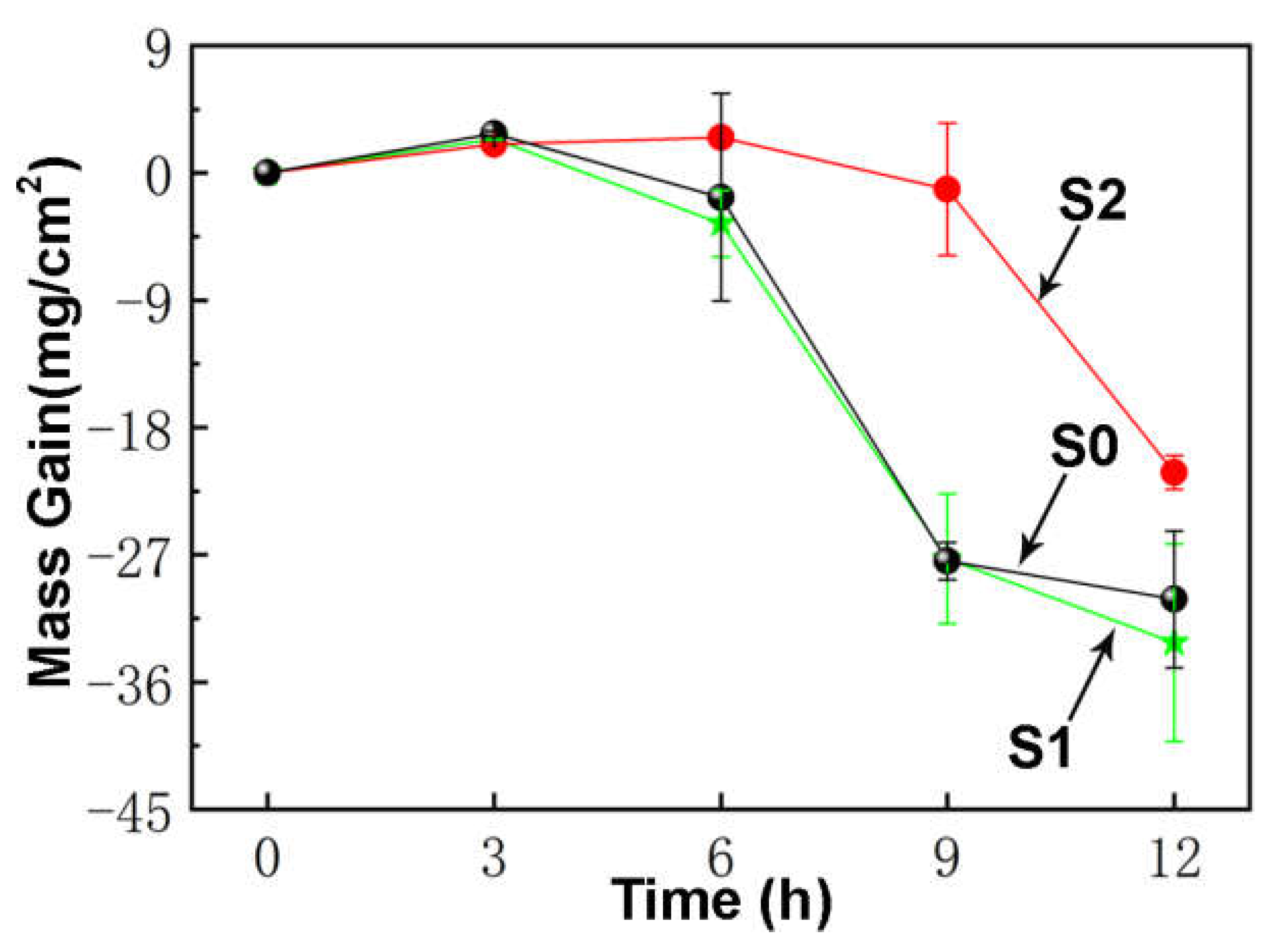
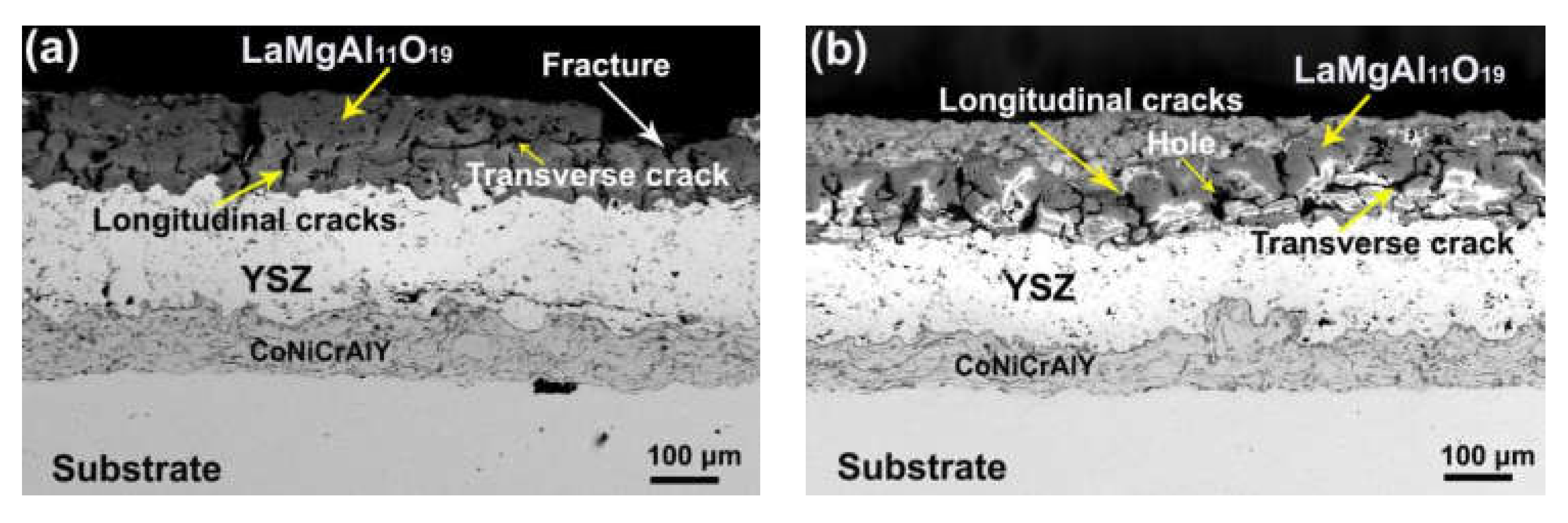
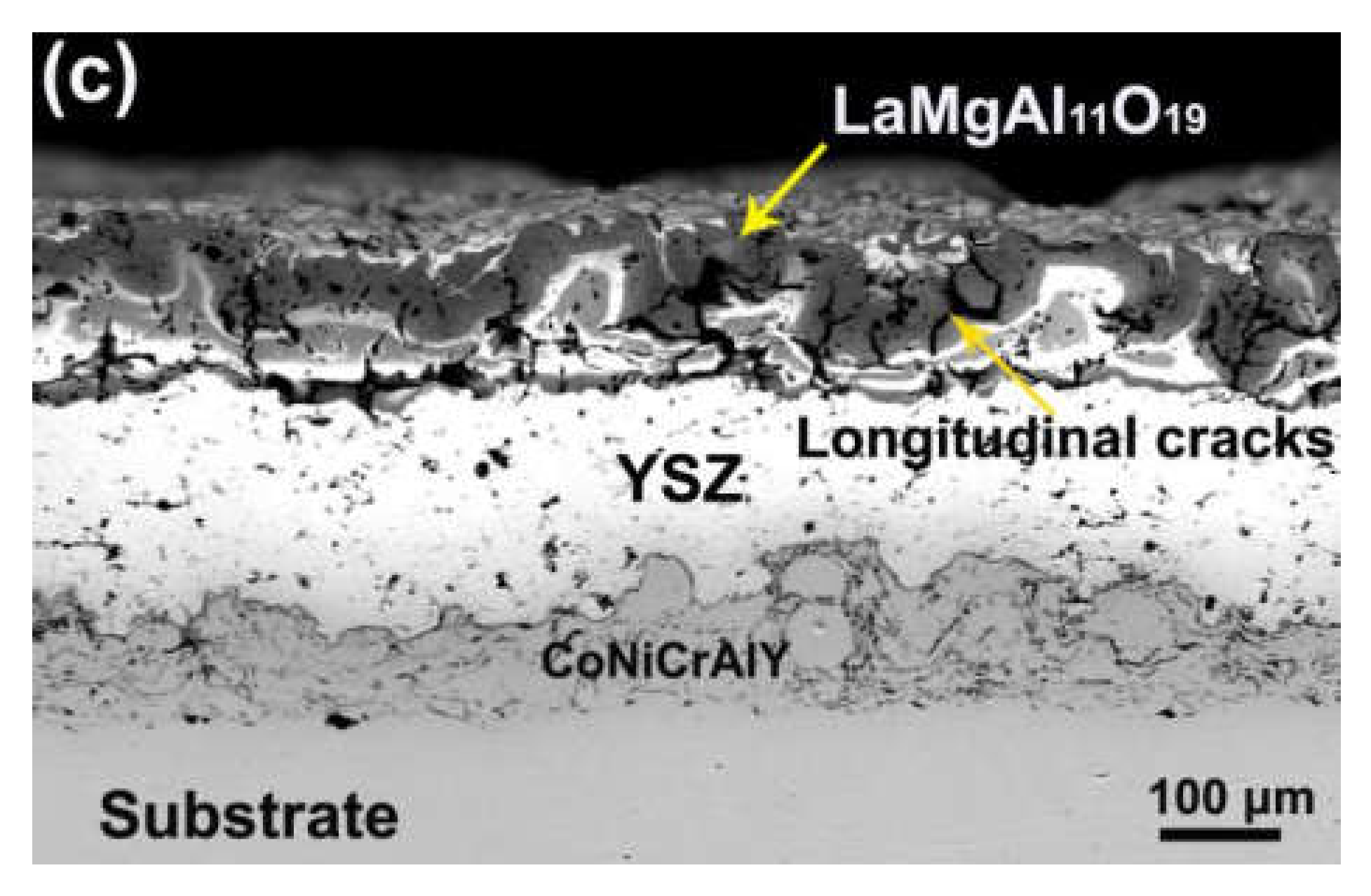
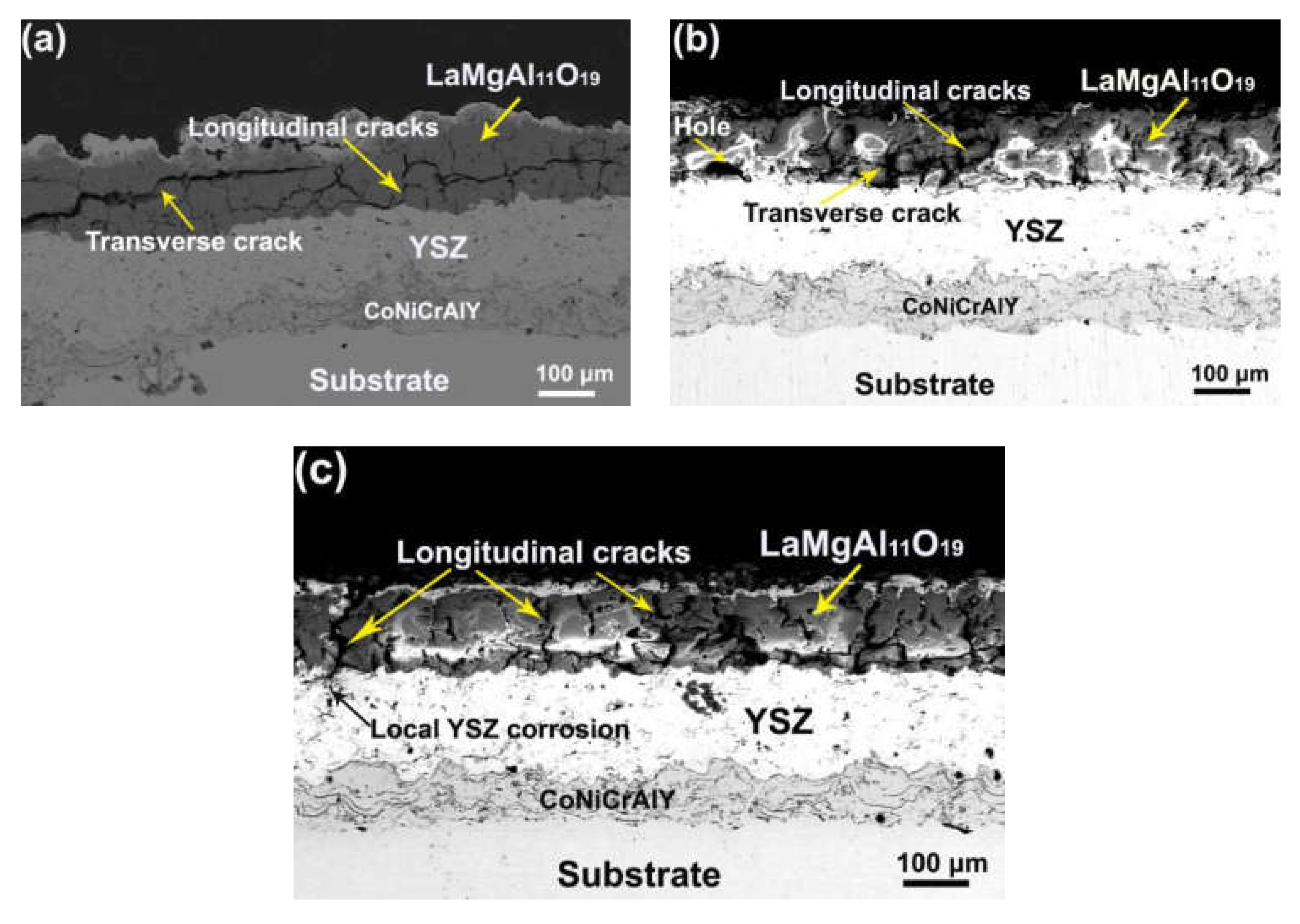
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clarke, D.; Levi, C. Materials design for the next generation thermal barrier coatings. Annu. Rev. Mater. Res. 2003, 33, 383–417. [Google Scholar] [CrossRef]
- Padture, N.P.; Gell, M.; Jordan, E.H. Thermal barrier coatings for gas-turbine engine applications. Science 2002, 296, 280–284. [Google Scholar] [CrossRef]
- Clarke, D.R.; Phillpot, S.R. Thermal barrier coating materials. Mater. Today 2005, 8, 22–29. [Google Scholar] [CrossRef]
- Vassen, R.; Stuke, A.; Stöver, D. Recent developments in the field of thermal barrier coatings. J. Therm. Spray Technol. 2009, 18, 181–186. [Google Scholar] [CrossRef]
- Stöver, D.; Pracht, G.; Lehmann, H. New material concepts for the next generation of plasma-sprayed thermal barrier coatings. J. Therm. Spray. Technol. 2004, 13, 76–83. [Google Scholar] [CrossRef]
- Cao, X.; Vaßen, R.; Stöver, D. Ceramic materials for thermal barrier coatings. J. Eur. Ceram. Soc. 2004, 24, 1–10. [Google Scholar] [CrossRef]
- Vaßen, R.; Jarligo, M.O.; Steinke, T.D.; Mack, E.; Stöver, D. Overview on advanced thermal barrier coatings. Surf. Coat. Technol. 2010, 205, 938–942. [Google Scholar] [CrossRef]
- Zhong, X.; Zhao, H.; Liu, C.; Wang, L.; Shao, F.; Zhou, X.; Tao, S.; Ding, C. Improvement in thermal shock resistance of gadolinium zirconate coating by addition of nanostructured yttria partially-stabilized zirconia. Ceram. Int. 2015, 41, 7318–7324. [Google Scholar] [CrossRef]
- Tsukada, S.; Kuroda, S.; Nishijima, M.; Araki, H.; Yumoto, A.; Watanabe, M. Effects of amorphous phase on hot corrosion behavior of plasma-sprayed LaMgAl11O19 coating. Surf. Coat. Technol. 2019, 363, 95–105. [Google Scholar] [CrossRef]
- Feuerstein, A.; Knapp, J.; Taylor, T. Technical and economical aspects of current thermal barrier coating systems for gas turbine engines by thermal spray and EBPVD: A review. J. Therm. Spray. Technol. 2008, 17, 199–213. [Google Scholar] [CrossRef]
- Miller, R.A. Current status of thermal barrier coatings—An overview. Surf. Coat. Technol. 1987, 30, 1–11. [Google Scholar] [CrossRef]
- Movchan, B.A. EB-PVD technology in the gas turbine industry: Present and future. JOM 1996, 48, 40–45. [Google Scholar] [CrossRef]
- Gao, L.H.; Guo, H.B.; Wei, L.Q. Microstructure and mechanical properties of yttria stabilized zirconia coatings prepared by plasma spray physical vapor deposition. Ceram. Int. 2015, 41, 8305–8311. [Google Scholar] [CrossRef]
- Hannink, R.H.J.; Kelly, P.M.; Muddle, B.C. Transformation toughening in zirconia-containing ceramics. J. Am. Ceram. Soc. 2000, 83, 416–487. [Google Scholar] [CrossRef]
- Liu, Z.G.; Ouyang, J.H.; Zhou, Y.; Li, J.; Xia, X.L. Influence of ytterbium-and samarium-oxides codoping on structure and thermal conductivity of zirconate ceramics. J. Eur. Ceram. Soc. 2009, 29, 647–652. [Google Scholar] [CrossRef]
- Withey, E.; Petorak, C.; Trice, R.; Dickinson, G.; Taylor, T. Design of 7 wt.% Y2O3–ZrO2/mullite plasma-sprayed composite coatings for increased creep resistance. J. Eur. Ceram. Soc. 2007, 27, 4675–4683. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Gu, L.; Zou, B.; Wang, Y.; Cao, X. Hot corrosion behaviour of plasma sprayed YSZ/LaMgAl11O19 composite coatings in molten sulfate–vanadate salt. Corros. Sci. 2011, 53, 2335–2343. [Google Scholar] [CrossRef]
- Sun, J.B.; Wang, J.S.; Dong, S.J. Effect of heat treatment on microstructure and property of plasma-sprayed lanthanum hexaaluminate coating. J. Compd. 2018, 739, 856–865. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Tao, S. Thermophysical properties of lanthanum zirconate coating prepared by plasma spraying and the influence of post-annealing. J. Alloys Compd. 2009, 486, 391–399. [Google Scholar] [CrossRef]
- Bobzin, K.; Zhao, L.; Öte, M. Deposition and characterization of thermal barrier coatings of ZrO2–4 mol.% Y2O3–1 mol.% Gd2O3–1 mol.% Yb2O3. Surf. Coat. Technol. 2015, 268, 205–208. [Google Scholar] [CrossRef]
- Gadow, R.; Lischka, M. Lanthanum hexaaluminate—Novel thermal barrier coatings for gas turbine applications—Materials and process development. Surf. Coat. Technol. 2002, 151, 392–399. [Google Scholar] [CrossRef]
- Haoran, L.; Chang-An, W.; Chenguang, Z. Thermo-physical properties of rare-earth hexaaluminates LnMgAl11O19 (Ln: La, Pr, Nd, Sm, Eu and Gd) magnetoplumbite for advanced thermal barrier coatings. J. Eur. Ceram. Soc. 2015, 35, 1297–1306. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liu, Z.G.; Ouyang, J.H. Preparation and thermophysical properties of LaMgAl11O19–Yb3Al5O12 ceramic composites. Ceram. Int. 2011, 37, 2489–2493. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhang, J.F.; Zhang, Y.F. Thermal cycling behaviors of the plasma sprayed thermal barrier coatings of hexaluminates with magnetoplumbite structure. J. Eur. Ceram. Soc. 2010, 30, 1649–1657. [Google Scholar] [CrossRef]
- Sun, J.; Hui, Y.; Jiang, J. Crystallization mechanism of plasma-sprayed LaMgAl11O19 coating. Appl. Surf. Sci. 2020, 504, 144509. [Google Scholar] [CrossRef]
- Friedrich, C.; Gadow, R.; Schirmer, T. Lanthanum hexaaluminate—A new material for atmospheric plasma spraying of advanced thermal barrier coatings. J. Therm. Spray Technol. 2001, 10, 592–598. [Google Scholar] [CrossRef]
- Liu, H.Z.; Ouyang, J.H.; Liu, Z.G. Microstructure, thermal shock resistance and thermal emissivity of plasma sprayed LaMAl11O19 (M= Mg, Fe) coatings for metallic thermal protection systems. Appl. Surf. Sci. 2013, 271, 52–59. [Google Scholar] [CrossRef]
- Tarasi, F.; Medraj, M.; Dolatabadi, A. Thermal cycling of suspension plasma sprayed alumina-YSZ coatings containing amorphous phases. J. Am. Ceram. Soc. 2012, 95, 2614–2621. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Sun, J.; Zhang, H.; Yang, X.; Qiu, F.; Zhou, P.; Niu, W.; Dong, S.; Zhou, X.; Cao, X. Lanthanum magnesium hexaluminate thermal barrier coatings with pre-implanted vertical microcracks: Thermal cycling lifetime and CMAS corrosion behaviour. Ceram. Int. 2018, 44, 11472–11485. [Google Scholar] [CrossRef]
- Jana, P.; Jayan, P.S.; Mandal, S.; Biswas, K. Hot corrosion behaviour of rare-earth magnesium hexaaluminate based thermal barrier coatings under molten sulphatevanadate salts. Surf. Coat. Technol. 2017, 332, 108–119. [Google Scholar] [CrossRef]
- Chen, X.; Cao, X.; Zou, B.; Gong, J.; Sun, C. High-temperature corrosion behaviour of plasma sprayed lanthanum magnesium hexaluminate coating by vanadium oxide. J. Eur. Ceram. Soc. 2015, 35, 227–236. [Google Scholar] [CrossRef]
- Krämer, S.; Yang, J.; Levi, C.G. Thermochemical interaction of thermal barrier coatings with molten CaO–MgO–Al2O3–SiO2 (CMAS) deposits. J. Am. Ceram. Soc. 2006, 89, 3167–3175. [Google Scholar] [CrossRef]
- Cui, J.J.; Ouyang, J.H.; Liu, Z.G. Hot corrosion behavior of LaMgAl11O19 ceramic coated with molten CMAS deposits at temperature of 1250 °C in air. J. Alloys Comp. 2016, 685, 316–321. [Google Scholar] [CrossRef]
- Huang, L.L.; Meng, H.M.; Tand, J. Crystallization behavior of plasma-sprayed lanthanide magnesium hexaaluminate coatings. Int. J. Miner. Metall. Mater. 2014, 21, 1247–1253. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Zhou, J.X.; Dong, S.; Deng, L.; Jiang, J.; Cao, X. Microstructure and thermal cycling behavior of plasma-sprayed LaMgAl11O19 coatings. Ceram. Int. 2018, 44, 5572–5580. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; Hao, Z.; Yuan, J.; Dong, S.; Jiang, J.; Deng, L.; Xin, Z.; Cao, X. Preparation, structure, mechanical properties and thermal cycling behavior of porous LaMgAl11O19 coating. J. Alloys Compd. 2018, 750, 1007–1016. [Google Scholar] [CrossRef]
- Meng, G.H.; Zhang, B.Y.; Liu, H. Vacuum heat treatment mechanisms promoting the adhesion strength of thermally sprayed metallic coatings. Surf. Coat. Technol. 2018, 344, 102–110. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, Y.; Fan, X. Thermal cycling failure of new LaMgAl11O19/YSZ double ceramic top coat thermal barrier coating systems. Surf. Coat. Technol. 2011, 205, 3293–3300. [Google Scholar] [CrossRef]
- Liu, R.D.; Jiang, S.M.; Yu, H.J. Preparation and hot corrosion behaviour of Pt modified AlSiY coating on a Ni-based superalloy. Corros. Sci. 2016, 104, 162–172. [Google Scholar] [CrossRef]
- Chen, X.; Cao, X.; Zou, B.; Gong, J.; Sun, C. Corrosion of lanthanum magnesium hexaaluminate as plasma-sprayed coating and as bulk material when exposed to molten V2O5-containing salt. Corros. Sci. 2015, 91, 185–194. [Google Scholar] [CrossRef]

| Sample | Annealing Temperature (°C) | Time (h) |
|---|---|---|
| S1 | 900 | 2 |
| S2 | 1200 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, G.; Sun, Y.; Xiang, L.; Wang, Z.; Hu, S.; Xie, Z. Effect of Vacuum Annealing on Microstructure and Hot-Salt Corrosion Behavior of CoNiCrAlY/YSZ/LaMgAl11O19 Double-Ceramic Coating. Coatings 2021, 11, 951. https://doi.org/10.3390/coatings11080951
Xue G, Sun Y, Xiang L, Wang Z, Hu S, Xie Z. Effect of Vacuum Annealing on Microstructure and Hot-Salt Corrosion Behavior of CoNiCrAlY/YSZ/LaMgAl11O19 Double-Ceramic Coating. Coatings. 2021; 11(8):951. https://doi.org/10.3390/coatings11080951
Chicago/Turabian StyleXue, Guanming, Yingchao Sun, Ling Xiang, Zhiguo Wang, Suying Hu, and Zhiwen Xie. 2021. "Effect of Vacuum Annealing on Microstructure and Hot-Salt Corrosion Behavior of CoNiCrAlY/YSZ/LaMgAl11O19 Double-Ceramic Coating" Coatings 11, no. 8: 951. https://doi.org/10.3390/coatings11080951
APA StyleXue, G., Sun, Y., Xiang, L., Wang, Z., Hu, S., & Xie, Z. (2021). Effect of Vacuum Annealing on Microstructure and Hot-Salt Corrosion Behavior of CoNiCrAlY/YSZ/LaMgAl11O19 Double-Ceramic Coating. Coatings, 11(8), 951. https://doi.org/10.3390/coatings11080951






