Application of Propolis Extract in Gelatin Coatings as Environmentally Friendly Method for Extending the Shelf Life of Pork Loin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Propolis Extract Preparation
2.3. Preparation of Gelatin Coating Solutions
2.4. Meat Coating Procedure
2.5. Quality Evaluation of Pork Meat
2.5.1. pH Measurement
2.5.2. Weight Loss
2.5.3. Color Measurement
2.5.4. Metmyoglobin Content
2.5.5. Lipid Oxidation
2.5.6. Microbiological Analysis
2.5.7. Meat Quality Acceptance
2.6. Statistical Analysis
3. Results and Discussion
3.1. pH Measurement
3.2. Weight Loss
3.3. Color Measurement
3.4. Metmyoglobin Content
3.5. Lipid Oxidation
3.6. Microbiological Analysis
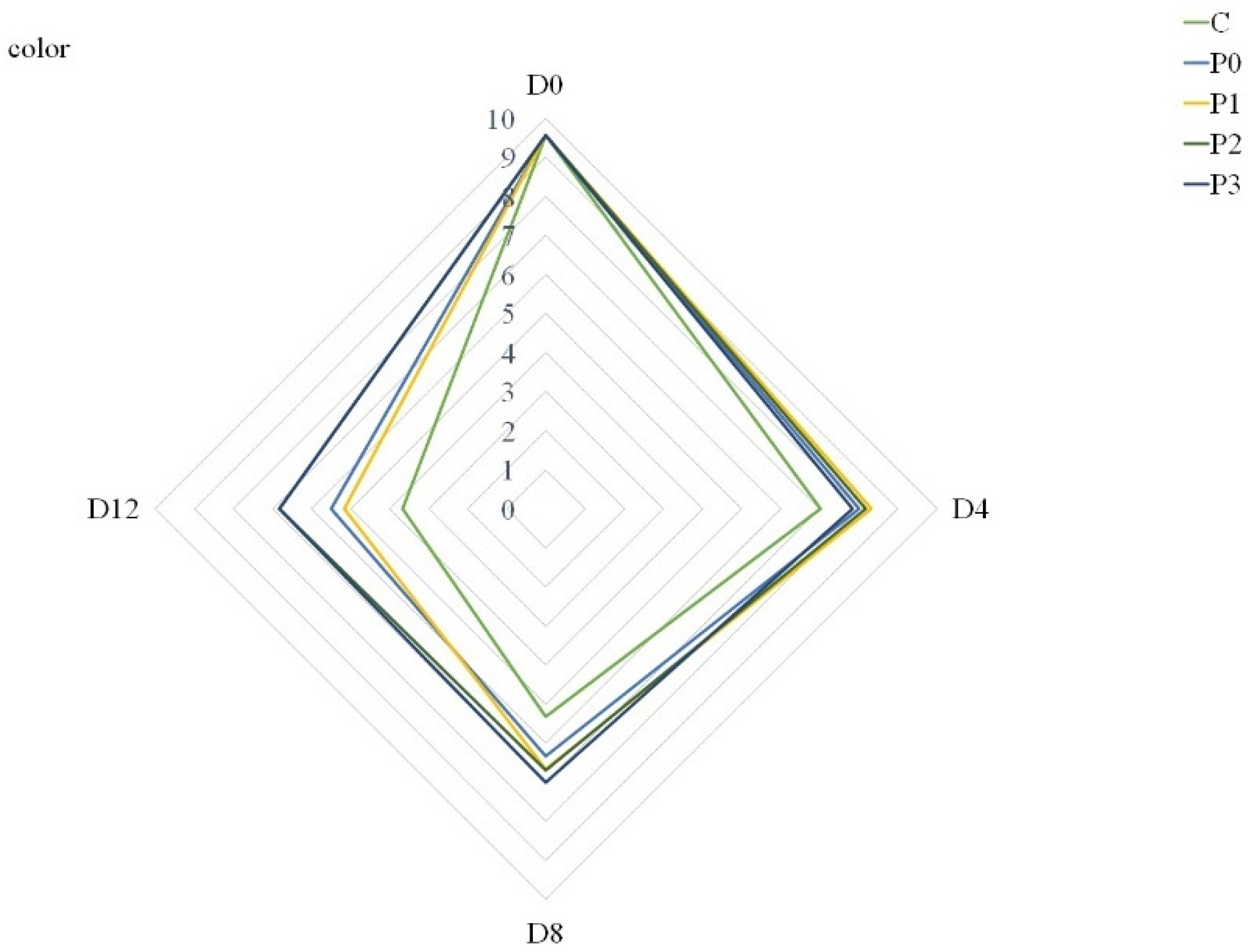
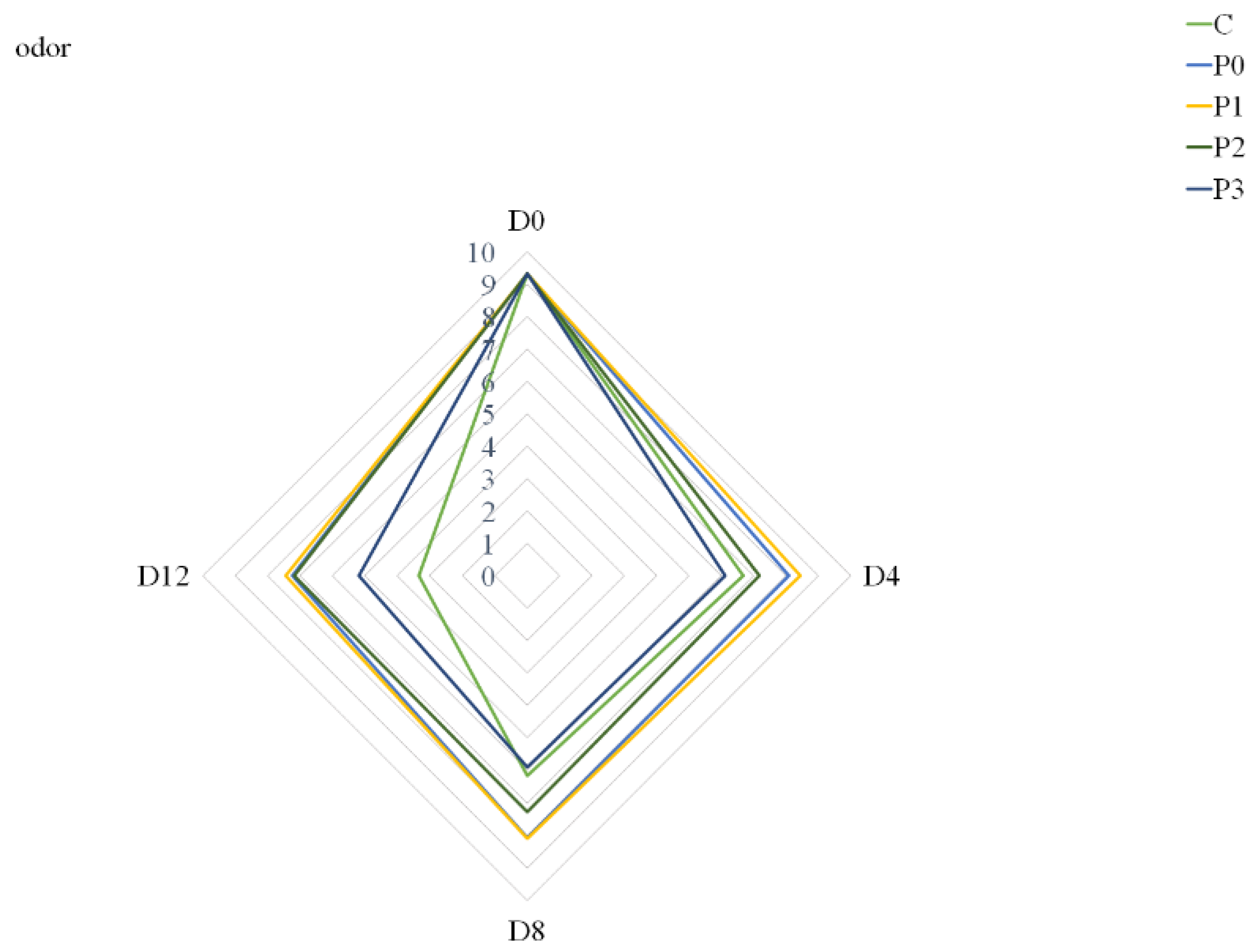
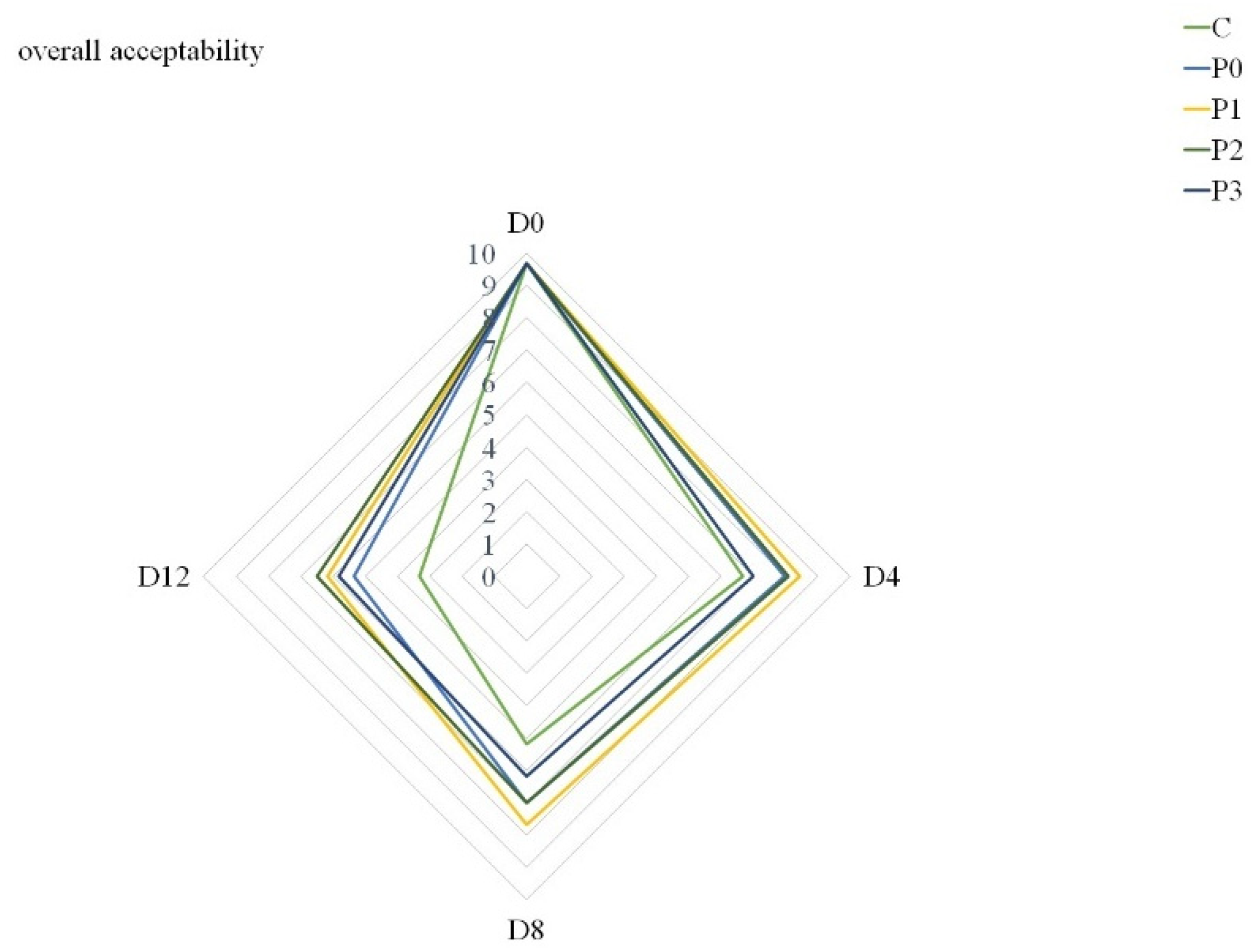
3.7. Meat Quality Acceptance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Ma, S.; Wang, Q.; McClements, D.J.; Liu, X.; Ngai, T.; Liu, F. Fortification of edible films with bioactive agents: A review of their formation, properties, and application in food preservation. Crit. Rev. Food Sci. Nutr. 2021, 1–27. [Google Scholar] [CrossRef]
- Thawien, W. Protein-Based Edible Films: Characteristics and Improvement of Properties. Intech 2012, 43–64. [Google Scholar] [CrossRef] [Green Version]
- Pajak, P.; Fortuna, T.; Przetaczek-Roznowska, I. Opakowania jadalne na bazie białek i polisacharydów—Charakterystyka i zastosowanie. Żywność Nauk. Technol. Jakość 2013, 20, 5–18. [Google Scholar]
- Suput, D.Z.; Lazic, V.L.; Popovic, S.Z.; Hromis, N.M. Edible films and coatings: Sources, properties and application. Food Feed. Res. 2015, 42, 11–22. [Google Scholar] [CrossRef]
- Saberi, B.; Golding, J.; Chockchaisawasdee, S.; Scarlett, C.J.; Stathopoulos, C.E. Effect of Biocomposite Edible Coatings Based on Pea Starch and Guar Gum on Nutritional Quality of “Valencia” Orange During Storage. Starch Stärke 2018, 70, 1700299. [Google Scholar] [CrossRef] [Green Version]
- Falguera, V.; Quintero, J.P.; Jiménez, A.; Muñoz, J.A.; Ibarz, A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011, 22, 292–303. [Google Scholar] [CrossRef]
- Hassan, B.; Chatha, S.A.S.; Hussain, A.I.; Zia, K.M.; Akhtar, N. Recent advances on polysaccharides, lipids and protein based edible films and coatings: A review. Int. J. Biol. Macromol. 2018, 109, 1095–1107. [Google Scholar] [CrossRef]
- Galus, S.; Lenart, A. Effect f protein concentration on kinetics of water vapour adsorption by coatings prepared on the basis of whey protein isolate. Żywność Nauk. Technol. Jakość 2011, 4, 66–73. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Zavala, J.F.A.; Olivas, G.; González-Aguilar, G.A. Edible coatings as encapsulating matrices for bioactive compounds: A review. J. Food Sci. Technol. 2014, 51, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Maru, V.R.; Gupta, S.; Ranade, V.; Variyar, P.S. Pullulan or chitosan based active coating by incorporating polyphenols from lemon peel in raw poultry meat. J. Food Sci. Technol. 2020, 1–10. [Google Scholar] [CrossRef]
- Lashkari, H.; Halabinejad, M.; Rafati, A.; Namdar, A. Shelf Life Extension of Veal Meat by Edible Coating Incorporated with Zataria multiflora Essential Oil. J. Food Qual. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Liu, J.; Shibata, M.; Ma, Q.; Liu, F.; Lu, Q.; Shan, Q.; Hagiwara, T.; Bao, J. Characterization of fish collagen from blue shark skin and its application for chitosan-collagen composite coating to preserve red porgy (Pagrus major) meat. J. Food Biochem. 2020, 44, e13265. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, Y.; Li, X.; Kang, H. Effect of chitosan-gelatin coating containing nano-encapsulated tarragon essential oil on the preservation of pork slices. Meat Sci. 2020, 166, 108137. [Google Scholar] [CrossRef] [PubMed]
- Gallego, M.; Arnal, M.; Talens, P.; Toldrá, F.; Mora, L. Effect of Gelatin Coating Enriched with Antioxidant Tomato By-Products on the Quality of Pork Meat. Polymers 2020, 12, 1032. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, G.P.; Andrade, M.P.D.; Rodrigues, L.M.; Massingue, A.A.; Fontes, P.R.; Ramos, A.D.L.S.; Ramos, E.M. Retail display of beef steaks coated with monolayer and bilayer chitosan-gelatin composites. Meat Sci. 2019, 152, 20–30. [Google Scholar] [CrossRef]
- Herring, J.L.; Jonnalongadda, S.C.; Narayanan, V.C.; Coleman, S.M. Oxidative stability of gelatin coated pork at refrigerated storage. Meat Sci. 2010, 85, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Marcinkowska-Lesiak, M.; Onopiuk, A.; Wojtasik-Kalinowska, I.; Zalewska, M.; Półtorak, A.; Wierzbicka, A. The influence of sage and hemp oils addition to gelatin-based edible coating on the quality features of pork. CyTA J. Food 2020, 18, 719–727. [Google Scholar] [CrossRef]
- Jridi, M.; Leticia, M.; Nabil, S.; Aristoy, M.C.; Moncef, N.; Toldrá, F. Effects of active gelatin coated with henna (L. inermis) extract on beef meat quality during chilled storage. Food Control. 2018, 84, 238–245. [Google Scholar] [CrossRef]
- Osés, S.; Pascual-Maté, A.; Muiño, M.A.F.; López-Díaz, T.; Sancho, M. Bioactive properties of honey with propolis. Food Chem. 2016, 196, 1215–1223. [Google Scholar] [CrossRef]
- Silva, F.R.G.; Matias, T.M.S.; Souza, L.I.O.; Matos-Rocha, T.J.; Fonseca, S.A.; Mousinho, K.; Santos, A.F. Phytochemical screening and in vitro antibacterial, antifungal, antioxidant and antitumor activities of the red propolis Alagoas. Braz. J. Biol. 2019, 79, 452–459. [Google Scholar] [CrossRef]
- Seibert, J.B.; Bautista-Silva, J.P.; Amparo, T.R.; Petit, A.; Pervier, P.; Almeida, J.C.D.S.; Azevedo, M.C.; Silveira, B.M.; Brandão, G.C.; de Souza, G.H.B.; et al. Development of propolis nanoemulsion with antioxidant and antimicrobial activity for use as a potential natural preservative. Food Chem. 2019, 287, 61–67. [Google Scholar] [CrossRef]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Kumazawa, S.; Hamasaka, T.; Nakayama, T. Antioxidant activity of propolis of various geographic origins. Food Chem. 2004, 84, 329–339. [Google Scholar] [CrossRef]
- Shimizu, K.; Ashida, H.; Matsuura, Y.; Kanazawa, K. Antioxidative bioavailability of artepillin C in Brazilian propolis. Arch. Biochem. Biophys. 2004, 424, 181–188. [Google Scholar] [CrossRef]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent progress in pharmacological research of propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Mehdizadeh, T.; Langroodi, A.M. Chitosan coatings incorporated with propolis extract and Zataria multiflora Boiss oil for active packaging of chicken breast meat. Int. J. Biol. Macromol. 2019, 141, 401–409. [Google Scholar] [CrossRef]
- Shavisi, N.; Khanjari, A.; Basti, A.A.; Misaghi, A.; Shahbazi, Y. Effect of PLA films containing propolis ethanolic extract, cellulose nanoparticle and Ziziphora clinopodioides essential oil on chemical, microbial and sensory properties of minced beef. Meat Sci. 2017, 124, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Marquez, D.G.P.; Fuenmayor, C.A.; Mahecha, H.S. Effect of chitosan-propolis edible coatings on stability of refrigerated cachama (Piaractus brachypomus) vacuum-packed fish fillets. Packag. Technol. Sci. 2018, 32, 143–153. [Google Scholar] [CrossRef]
- Ali, A.; Chow, W.L.; Zahid, N.; Ong, M.K. Efficacy of Propolis and Cinnamon Oil Coating in Controlling Post-Harvest Anthracnose and Quality of Chilli (Capsicum annuum L.) during Cold Storage. Food Bioprocess Technol. 2014, 7, 2742–2748. [Google Scholar] [CrossRef]
- Del Carmen Martínez-González, M.; Bautista-Baños, S.; Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Ventura-Aguilar, R.I.; Del Río-García, J.C.; Ramos-García, M.D.L. Effect of Nanostructured Chitosan/Propolis Coatings on the Quality and Antioxidant Capacity of Strawberries During Storage. Coatings 2020, 10, 90. [Google Scholar] [CrossRef] [Green Version]
- Poltorak, A.; Marcinkowska-Lesiak, M.; Lendzion, K.; Moczkowska, M.; Onopiuk, A.; Wojtasik-Kalinowska, I.; Wierzbicka, A. Evaluation of the antioxidant, anti-inflammatory and antimicrobial effects of catuaba, galangal, roseroot, maca root, guarana and polyfloral honey in sausages during storage. LWT 2018, 96, 364–370. [Google Scholar] [CrossRef]
- Krzywicki, K. Assessment of relative content of myoglobin, oxymyoglobin and metmyoglobin at the surface of beef. Meat Sci. 1979, 3, 1–10. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A.C. Food Chemistry and Toxicology Krzywicki Revisited: Equations for Spectro-photometric Determination of Myoglobin Redox Forms in ABSTRACT: Krzywicki ’ s equations have been widely used for estimating the relative proportions of myoglobin. J. Food Sci. 2004, 69, 717–720. [Google Scholar] [CrossRef]
- Pikul, J.; Leszczynski, D.E.; Kummerow, F.A. Evaluation of three modified TBA methods for measuring lipid oxidation in chicken meat. J. Agric. Food Chem. 1989, 37, 1309–1313. [Google Scholar] [CrossRef]
- Berardo, A.; Devreese, B.; De Maere, H.; Stavropoulou, D.; Van Royen, G.; Leroy, F.; De Smet, S. Actin proteolysis during ripening of dry fermented sausages at different pH values. Food Chem. 2017, 221, 1322–1332. [Google Scholar] [CrossRef] [Green Version]
- Fik, M.; Leszczyńska-Fik, A. Microbiological and Sensory Changes in Minced Beef Treated with Potassium Lactate and Sodium Diacetate during Refrigerated Storage. Int. J. Food Prop. 2007, 10, 589–598. [Google Scholar] [CrossRef]
- Medić, H.; Kušec, I.D.; Pleadin, J.; Kozačinski, L.; Njari, B.; Hengl, B.; Kušec, G. The impact of frozen storage duration on physical, chemical and microbiological properties of pork. Meat Sci. 2018, 140, 119–127. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Active packaging films and edible coatings based on polyphenol-rich propolis extract: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 2106–2145. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Gómez-Guillén, M.C.; Fernández-Martín, F.; Montero, P. Effects of gelatin origin, bovine-hide and tuna-skin, on the properties of compound gelatin–chitosan films. Food Hydrocoll. 2011, 25, 1461–1469. [Google Scholar] [CrossRef] [Green Version]
- Hoque, S.; Benjakul, S.; Prodpran, T.; Songtipya, P. Properties of blend film based on cuttlefish (Sepia pharaonis) skin gelatin and mungbean protein isolate. Int. J. Biol. Macromol. 2011, 49, 663–673. [Google Scholar] [CrossRef]
- Antoniewski, M.N.; Barringer, S.A. Meat Shelf-life and Extension using Collagen/Gelatin Coatings: A Review. Crit. Rev. Food Sci. Nutr. 2010, 50, 644–653. [Google Scholar] [CrossRef]
- Avena-Bustillos, R.; Olsen, C.; Olson, D.; Chiou, B.; Yee, E.; Bechtel, P.; McHugh, T. Water Vapor Permeability of Mammalian and Fish Gelatin Films. J. Food Sci. 2006, 71, E202–E207. [Google Scholar] [CrossRef]
- Mustafa, P.; Niazi, M.B.K.; Jahan, Z.; Samin, G.; Hussain, A.; Ahmed, T.; Naqvi, S.R. PVA/starch/propolis/anthocyanins rosemary extract composite films as active and intelligent food packaging materials. J. Food Saf. 2019, 40, 1–11. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Vidal, J.; Dicastillo, C.; Rodriguez, F.; Guarda, A.; Cruz, R.M.S.; Galotto, M.J. Development of poly(lactic acid) films with propolis as a source of active compounds: Biodegradability, physical, and functional properties. J. Appl. Polym. Sci. 2018, 136, 1–11. [Google Scholar] [CrossRef]
- Ebadi, Z.; Khodanazary, A.; Hosseini, S.M.; Zanguee, N. The shelf life extension of refrigerated Nemipterus japonicus fillets by chitosan coating incorporated with propolis extract. Int. J. Biol. Macromol. 2019, 139, 94–102. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, G.K.P.; De Souza, S.J.; Da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. Technol. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- Verma, A.K.; Chatli, M.K.; Kumar, P.; Mehta, N. Antioxidant and Antimicrobial Activity of Porcine Liver Hydrolysate in Meat Emulsion and Their Influence on Physico-Chemical and Color Deterioration during Refrigeration Storage. J. Food Sci. 2019, 84, 1844–1853. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.A.; Vallejo, A.M.; Ballester, A.-R.; Zampini, C.; Isla, M.I.; López-Rubio, A.; Fabra, M.J. Antifungal edible coatings containing Argentinian propolis extract and their application in raspberries. Food Hydrocoll. 2020, 107, 105973. [Google Scholar] [CrossRef]
- Lis, B.; Woźniak, M.; Kiystofiak, T.; Ratajczak, I. Effect of accelerated aging on the color changes of wood treated with eco-friendly formulations based on propolis and silicon compounds. BioResources 2020, 15, 3667–3677. [Google Scholar] [CrossRef]
- Reis, A.; Diedrich, C.; de Moura, C.; Pereira, D.; Almeida, J.F.; da Silva, L.D.; Plata-Oviedo, M.S.V.; Tavares, R.A.W.; Carpes, S.T. Physico-chemical characteristics of microencapsulated propolis co-product extract and its effect on storage stability of burger meat during storage at −15 °C. LWT 2017, 76, 306–313. [Google Scholar] [CrossRef]
- Vargas-Sánchez, R.D.; Torrescano-Urrutia, G.R.; Acedo-Félix, E.; Carvajal-Millan, E.; González-Córdova, A.F.; Vallejo-Galland, B.; Torres-Llanez, M.J.; Sánchez-Escalante, A. Antioxidant and Antimicrobial Activity of Commercial Propolis Extract in Beef Patties. J. Food Sci. 2014, 79, C1499–C1504. [Google Scholar] [CrossRef] [PubMed]
- Rizzolo, A.; Bianchi, G.; Povolo, M.; Migliori, C.A.; Contarini, G.; Pelizzola, V.; Cattaneo, T.M. Volatile compound composition and antioxidant activity of cooked ham slices packed in propolis-based active packaging. Food Packag. Shelf Life 2016, 8, 41–49. [Google Scholar] [CrossRef]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, C.; Rachtanapun, P. Biodegradable Rice Starch/Carboxymethyl Chitosan Films with Added Propolis Extract for Potential Use as Active Food Packaging. Polymers 2018, 10, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafari, N.J.; Kargozari, M.; Ranjbar, R.; Rostami, H.; Hamedi, H. The effect of chitosan coating incorporated with ethanolic extract of propolis on the quality of refrigerated chicken fillet. J. Food Process. Preserv. 2017, 42, e13336. [Google Scholar] [CrossRef]
- Ercolini, D.; Russo, F.; Torrieri, E.; Masi, P.; Villani, F. Changes in the Spoilage-Related Microbiota of Beef during Refrigerated Storage under Different Packaging Conditions. Appl. Environ. Microbiol. 2006, 72, 4663–4671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M.D.C. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- Nessianpour, E.; Khodanazary, A.; Hosseini, S.M. Shelf Life of Saurida tumbil during Storage at Refrigeration Condition as affected by Gelatin-Based Edible Coatings Incorporated with propolis Extract. Int. J. Food Prop. 2019, 22, 1749–1759. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Vitchayakitti, W. Improving functional properties of chitosan films as active food packaging by incorporating with propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
| Item | Effect | InteractionT × ST | |
|---|---|---|---|
| Treatment (T) | Storage Time (ST) | ||
| pH | ***1 | *** | *** |
| Weight loss | *** | *** | *** |
| L* | *** | *** | *** |
| a* | ** | *** | *** |
| b* | NS | *** | *** |
| ΔE | * | *** | *** |
| TBARS | *** | *** | *** |
| MetMb | NS | *** | *** |
| TAPC | NS | *** | *** |
| color | *** | * | *** |
| odor | *** | *** | *** |
| overall acceptance | *** | ** | *** |
| Parameters | Treatments | Storage Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | ||
| pH | C | 5.56 aA1 ± 0.01 | 5.60 abA ± 0.02 | 5.64 bB ± 0.01 | 5.75 cC ± 0.03 |
| P0 | 5.56 aA ± 0.01 | 5.56 aA ± 0.01 | 5.58 aA ± 0.02 | 5.63 bB ± 0.01 | |
| P1 | 5.56 aA ± 0.01 | 5.55 aA ± 0.03 | 5.55 aA ± 0.04 | 5.63 bAB ± 0.04 | |
| P2 | 5.56 aA ± 0.01 | 5.57 aA ± 0.02 | 5.56 aA ± 0.02 | 5.60 aAB ± 0.03 | |
| P3 | 5.56 aA ± 0.01 | 5.55 aA ±0.04 | 5.58 aA ± 0.02 | 5.58 aA ± 0.02 | |
| Weight Loss (%) | C | - | 3.43 aB ± 0.35 | 5.15 bC ± 0.24 | 7.92 cC ± 0.38 |
| P0 | - | 2.33 aA ± 0.2 | 3.47 bB ± 0.37 | 4.56 cB ± 0.28 | |
| P1 | - | 2.23 aA ± 0.21 | 2.98 bAB ± 0.17 | 4.34 cAB ± 0.29 | |
| P2 | - | 2.31 aA ± 0.50 | 3.01 bAB ± 0.17 | 4.21 cAB ± 0.26 | |
| P3 | - | 2.16 aA ± 0.11 | 2.96 bA ± 0.20 | 4.00 cA ± 0.18 | |
| L* (%) | C | 57.10 cA ± 0.98 | 55.59 cA ± 0.28 | 52.35 bA ± 0.51 | 49.68 aA ± 0.61 |
| P0 | 57.10 cA ± 0.98 | 56.08 cAB ± 0.77 | 53.95 bB ± 0.85 | 51.80 aB ± 0.76 | |
| P1 | 57.10 bA ± 0.98 | 57.17 bB ± 0.66 | 55.20 aB ± 0.56 | 53.84 aC ± 0.42 | |
| P2 | 57.10 bA ± 0.98 | 57.19 bB ± 0.64 | 54.69 aB ± 0.78 | 54.18 aC ± 0.36 | |
| P3 | 57.10 bA ± 0.98 | 57.14 bB ± 0.44 | 54.57 aB ± 0.79 | 54.14 aC ± 0.71 | |
| a* | C | 7.27 bA ± 0.45 | 6.79 bA ± 0.13 | 6.01 aA ± 0.46 | 5.75 aA ± 0.12 |
| P0 | 7.27 bA ± 0.45 | 7.03 bA ± 0.43 | 6.18 aA ± 0.42 | 6.26 aAB ± 0.33 | |
| P1 | 7.27 bA ± 0.45 | 7.14 bA ± 0.24 | 6.32 aA ± 0.26 | 6.42 aAB ± 0.28 | |
| P2 | 7.27 bA ± 0.45 | 7.17 bA ± 0.25 | 6.61 abAB ± 0.37 | 6.31 aAB ± 0.20 | |
| P3 | 7.27 aA ± 0.45 | 7.11 aA ± 0.29 | 7.14 aB ± 0.41 | 6.88 aB ± 0.17 | |
| b* | C | 3.78 aA ± 0.20 | 7.93 bC ± 0.27 | 8.59 bcB ± 0.40 | 9.02 cB ± 0.25 |
| P0 | 3.78 aA ± 0.20 | 6.35 bA ± 0.21 | 7.62 c ± 0.34 | 8.11 cA ± 0.14 | |
| P1 | 3.78 aA ± 0.20 | 6.39 bA ± 0.14 | 7.34 cA ± 0.27 | 7.64 cA ± 0.40 | |
| P2 | 3.78 aA ± 0.20 | 6.83 bAB ± 0.29 | 7.27 bcA ± 0.25 | 7.70 cA ± 0.29 | |
| P3 | 3.78 aA ± 0.20 | 7.01 bB ± 0.13 | 7.30 bA ± 0.34 | 7.60 bA ± 0.30 | |
| ΔE | C | - | 4.62 aB ± 0.33 | 7.12 bC ± 0.42 | 9.41 cC ± 0.60 |
| P0 | - | 3.06 aA ± 0.31 | 5.38 bA ± 0.58 | 7.13 cB ± 0.58 | |
| P1 | - | 2.88 aA ± 0.28 | 4.44 bA ± 0.32 | 5.35 bA ± 0.49 | |
| P2 | - | 3.24 aA ± 0.25 | 4.55 bA ± 0.47 | 5.21 bA ± 0.30 | |
| P3 | - | 3.40 aA ± 0.17 | 4.54 bA ± 0.35 | 5.03 bA ± 0.52 | |
| Parameter | Treatments | Storage Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | ||
| MetMb (%) | C | 20.87 aA1 ± 0.67 | 33.51 bC ± 2.63 | 51.77 cC ± 1.63 | 62.07 dD ± 1.88 |
| P0 | 20.87 aA ± 0.67 | 30.22 bBC ± 1.39 | 46.20 cB ± 2.63 | 56.23 dC ± 1.41 | |
| P1 | 20.87 aA ± 0.67 | 29.12 bAB ± 1.71 | 44.78 cAB ± 2.07 | 51.93 dB ± 1.69 | |
| P2 | 20.87 aA ± 0.67 | 27.19 bAB ± 2.66 | 43.94 cAB ± 0.87 | 49.26 dAB ± 1.50 | |
| P3 | 20.87 aA ± 0.67 | 26.48 bA ± 1.46 | 42.12 cA ± 1.48 | 46.92 dA ± 2.21 | |
| TBARS (mg/100 g) | C | 0.12 aA ± 0.01 | 0.61 bD ± 0.03 | 0.96 cD ± 0.06 | 1.68 dD ± 0.06 |
| P0 | 0.12 aA ± 0.01 | 0.47 bC ± 0.03 | 0.83 cC ± 0.01 | 1.43 dC ± 0.05 | |
| P1 | 0.12 aA ± 0.01 | 0.42 bBC ± 0.02 | 0.77 cC ± 0.03 | 1.44 dC ± 0.05 | |
| P2 | 0.12 aA ± 0.01 | 0.37 bAB ± 0.03 | 0.55 cB ± 0.03 | 1.25 dB ± 0.08 | |
| P3 | 0.12 aA ± 0.01 | 0.30 bA ± 0.04 | 0.46 cA ± 0.03 | 1.05 dA ± 0.05 | |
| Parameters | Treatments | Storage Time (Days) | |||
|---|---|---|---|---|---|
| 0 | 4 | 8 | 12 | ||
| TAPC (log CFU/g) | C | 2.99 aA1 ± 0.24 | 4.37 bD ± 0.17 | 5.58 cD ± 0.11 | 6.96 dD ± 0.16 |
| P0 | 2.99 aA ± 0.24 | 3.71 bC ± 0.20 | 5.17 cC ± 0.13 | 5.53 dC ± 0.08 | |
| P1 | 2.99 aA ± 0.24 | 3.50 bBC ± 0.09 | 4.44 cC ± 0.15 | 4.59 dC ± 0.13 | |
| P2 | 2.99 aA ± 0.24 | 3.39 bAB ± 0.06 | 4.27 cB ± 0.17 | 4.35 dB ± 0.10 | |
| P3 | 2.99 aA ± 0.24 | 3.26 bA ± 0.08 | 3.95 cA ± 0.15 | 4.03 dA ± 0.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcinkowska-Lesiak, M.; Wojtasik-Kalinowska, I.; Onopiuk, A.; Zalewska, M.; Poltorak, A. Application of Propolis Extract in Gelatin Coatings as Environmentally Friendly Method for Extending the Shelf Life of Pork Loin. Coatings 2021, 11, 979. https://doi.org/10.3390/coatings11080979
Marcinkowska-Lesiak M, Wojtasik-Kalinowska I, Onopiuk A, Zalewska M, Poltorak A. Application of Propolis Extract in Gelatin Coatings as Environmentally Friendly Method for Extending the Shelf Life of Pork Loin. Coatings. 2021; 11(8):979. https://doi.org/10.3390/coatings11080979
Chicago/Turabian StyleMarcinkowska-Lesiak, Monika, Iwona Wojtasik-Kalinowska, Anna Onopiuk, Magdalena Zalewska, and Andrzej Poltorak. 2021. "Application of Propolis Extract in Gelatin Coatings as Environmentally Friendly Method for Extending the Shelf Life of Pork Loin" Coatings 11, no. 8: 979. https://doi.org/10.3390/coatings11080979






