The Action Difference of Lasiodiplodia theobromae on Infecting and Dyeing Poplar Wood in Spatial Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Agents
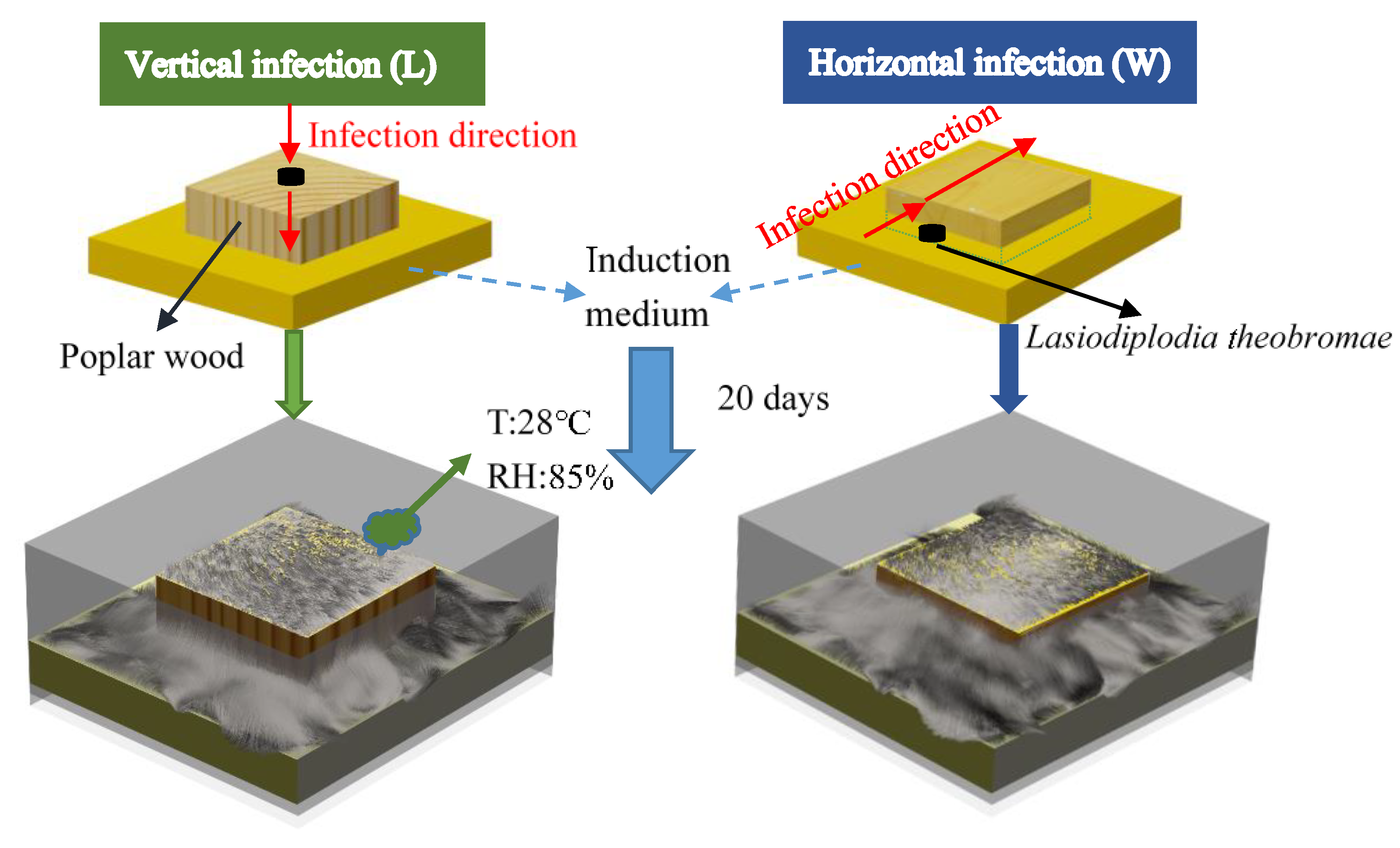
2.2. Biological Dyeing Process
2.3. Instruments and Equipment
2.4. Measurements
2.4.1. Chromaticity Value Test
2.4.2. Microsection Analysis
2.4.3. Chemical Composition Analysis
2.4.4. Surface Characteristics
3. Results and Discussion
3.1. Visual Staining Phenomenon
3.2. Micromorphology
3.3. Infective Characters
3.4. Chemical Composition
3.5. Wettability
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gao, H.G.; Gong, W.J.; Zhao, Y.G. Rapid method for quantification of seven synthetic pigments in colored Chinese steamed buns using UFLC-MS/MS without SPE. Anal. Sci. 2015, 31, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Lomax, S.Q. The application of X-ray powder diffraction for the analysis of synthetic organic pigments. Part 1: Dry pigments. J. Coat. Technol. Res. 2010, 7, 331–346. [Google Scholar] [CrossRef]
- Zahroojian, N.; Moravej, H.; Shivazad, M. Comparison of marine algae (Spirulina platensis) and synthetic pigment in enhancing egg yolk colour of laying hens. Br. Poult. 2011, 52, 584–588. [Google Scholar] [CrossRef]
- Ghelardi, E.; Degano, I.; Colombini, M.P.; Mazurek, J.; Schilling, M.; Khanjian, H.; Learner, T. A multi-analytical study on the photochemical degradation of synthetic organic pigments. Dyes Pigment. 2015, 123, 193–403. [Google Scholar] [CrossRef]
- Ali, H. Biodegradation of synthetic dyes—A Review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Li, X.Q.; Zhang, Q.H.; Ma, K.; Li, H.M.; Guo, Z. Identification and determination of 34 water-soluble synthetic dyes in foodstuff by high performance liquid chromatography-diode array detection-ion trap time-of-flight tandem mass spectrometry. Food Chem. 2015, 182, 316–326. [Google Scholar] [CrossRef]
- Chen, V.J.; Smith, G.D.; Holden, A.; Paydar, N.; Kiefer, K. Chemical analysis of dyes on an Uzbek ceremonial coat: Objective evidence for artifact dating and the chemistry of early synthetic dyes. Dyes Pigment. 2016, 131, 320–332. [Google Scholar] [CrossRef]
- Atav, R.; Güneş, E.; Çifçi, D.İ.; Güneş, Y. Comparison of wool fabric dyeing with natural and synthetic dyes in view of ecology and treatability. AATCC J. Res. 2020, 7, 15–22. [Google Scholar] [CrossRef]
- Deveoglu, O.; Muhammed, A.; Fouad, A.; Torgan, E.; Karaadag, R. Chromatographic analysis of natural pigments produced from Datisca cannabina L. and quercus infectoria Oliv. plants and their antimicrobial activity. J. Chem. Soc. Pak. 2012, 34, 890–895. Available online: https://link.gale.com/apps/doc/A301252551/AONE (accessed on 17 August 2021).
- Maoka, T.; Mochida, K.; Kozuka, M.; Ito, Y.; Fujiwara, Y.; Hashimoto, K.; Enjo, F.; Ogata, M.; Nobukuni, Y.; Tokuda, H.; et al. Cancer chemopreventive activity of carotenoids in the fruits of red paprika Capsicum annuum L. Cancer Lett. 2001, 172, 103–109. [Google Scholar] [CrossRef]
- Hu, Y.L.; Luo, J.Y.; Hu, S.R.; Fu, H.; Yang, S.H.; Yang, M.H. Application of natural plant pigments in enlarged health industry. China J. Chin. Mater. Med. 2017, 42, 2433–2438. [Google Scholar] [CrossRef]
- Castaneda, M.P.; Hirschler, E.M.; Sams, A.R. Skin pigmentation evaluation in broilers fed natural and synthetic pigments. Poult. Sci. 2005, 84, 143. [Google Scholar] [CrossRef]
- Hu, Y.L.; Luo, J.Y.; Zhao, H.Z.; Zhang, S.S.; Yang, S.H.; Yang, M.H. Application of natural plant pigment in hair dyes. China J. Chin. Mater. Med. 2016, 41, 3226–3231. [Google Scholar] [CrossRef]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez de Mejia, E. Natural pigments: Stabilization methods of anthocyanins for food applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Myungsun, Y. Study on the development of plastic color using natural pigments—Focusing on Charcoal, lacquer, loess. J. Digit. Des. 2012, 12, 309–318. [Google Scholar] [CrossRef]
- Rimkiene, S.; Ragazinskiene, O.; Savickiene, N. The cumulation of wild pansy (Viola tricolor L.) accessions: The possibility of species preservation and usage in medicine. Medicina 2003, 39, 411–416. [Google Scholar] [PubMed]
- Fernández-López, J.A.; Fernández-Lledó, V.; Angosto, J.M. New insights into red plant pigments: More than just natural colorants. RSC Adv. 2020, 10, 24669–24682. [Google Scholar] [CrossRef]
- Leong, H.Y.; Show, P.L.; Lim, M.H.; Ooi, C.W.; Ling, T.C. Natural red pigments from plants and their health benefits: A review. Food Rev. Int. 2018, 34, 463–482. [Google Scholar] [CrossRef]
- Scott-Moncrieff, R. Natural anthocyanin pigments: The magenta flower pigment of Primula polyanthus. Biochem. J. 1930, 24, 767–778. [Google Scholar] [CrossRef]
- Boo, H.O.; Hwang, S.J.; Bae, C.S.; Park, S.H.; Heo, B.G.; Gorinstein, S. Extraction and characterization of some natural plant pigments. Ind. Crops Prod. 2012, 40, 129–135. [Google Scholar] [CrossRef]
- Baird, P.; Dan, R.; Hink, S.A. A remote marking device and newly developed permanent dyes for Wildlife research. Wildl. Soc. Bull. 2017, 41, 785–795. [Google Scholar] [CrossRef]
- Hober, R.; Moore, E. Studies concerning the nature of the secretory activity of the isolated ringer-perfused frog liver. J. Gen. Physiol. 1939, 23, 191–202. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Wan, X. Analysis of the spectral reflectance and color of mineral pigments affected by their particle size. Color Res. Appl. 2020, 45, 246–261. [Google Scholar] [CrossRef]
- Siddall, R. Mineral pigments in archaeology: Their analysis and the range of available materials. Minerals 2018, 8, 201. [Google Scholar] [CrossRef] [Green Version]
- Dehnavi, E.; Shams-Nateri, A.; Khalili, H. Wool dyeing with binary mixture of natural dyes. Pigment. Resin Technol. 2016, 45, 52–61. [Google Scholar] [CrossRef]
- Ding, Y.; Freeman, H.S. Mordant dye application on cotton: Optimisation and combination with natural dyes. Color. Technol. 2017, 133, 369–375. [Google Scholar] [CrossRef]
- Sheikh, J.; Jagtap, P.S.; Teli, M.D. Ultrasound assisted extraction of natural dyes and natural mordants vis a vis dyeing. Fibers Polym. 2016, 17, 738–742. [Google Scholar] [CrossRef]
- Sanli, H.S.; Kayabasi, N.; Lmez, F.N. Dyeing techniques and mordanting methods applied in natural dyeing of wool in Turkey. Asian J. Chem. 2011, 23, 3313–3316. [Google Scholar]
- Robinson, S.C.; Tudor, D.; Cooper, P.A. Wood preference of spalting fungi in urban hardwood species. Int. Biodeterior. Biodegrad. 2011, 65, 1145–1149. [Google Scholar] [CrossRef]
- Robinson, S.C.; Gutierrez, S.M.V.; Garcia, R.A.C.; Iroume, N.; Vorland, N.R.; McClelland, A.; Huber, M.; Stanton, S. Potential for carrying dyes derived from spalting fungi in natural oils. J. Coat. Technol. Res. 2017, 14, 1107–1113. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yu, Z.M.; Zhang, Y.; Wang, H.W. Microbial dyeing for inoculation and pigment used in wood processing: Opportunities and challenges. Dyes Pigment. 2021, 186, 109021. [Google Scholar] [CrossRef]
- Ramesh, C.; Vinithkumar, N.V.; Kirubagaran, R.; Venil, C.K.; Dufossé, L. Multifaceted applications of microbial pigments: Current knowledge, challenges and future directions for public health implications. Microorganisms 2019, 7, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial pigments in the food industry—challenges and the way forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, S.C.; Tudor, D.; Cooper, P.A. Feasibility of using red pigment producing fungi to stain wood for decorative applications. Can. J. For. Res. 2011, 41, 1722–1728. [Google Scholar] [CrossRef]
- Robinson, S.C.; Tudor, D.; Hipson, S.; Snider, H.; Ng, S.; Korshikov, E.; Cooper, P.A. Methods of inoculating Acer spp. Populus tremuloides, and Fagus grandifolia logs for commercial spalting applications. J. Wood Sci. 2013, 59, 351–357. [Google Scholar] [CrossRef]
- Robinson, S.C.; Tudor, D.; Cooper, P.A. Promoting fungal pigment formation in wood by utilizing a modified decay jar method. Wood Sci. Technol. 2012, 46, 841–849. [Google Scholar] [CrossRef]
- Robinson, S.C.; Tudor, D.; MacDonald, G.; Mansourian, Y.; Cooper, P.A. Repurposing mountain pine beetle blue wood for art through additional fungal colonization. Int. Biodeterior. Biodegrad. 2013, 85, 372–374. [Google Scholar] [CrossRef]
- Vega Gutierrez, S.M.; Vega Gutierrez, P.T.; Godinez, A.; Pittis, L.; Huber, M.; Stanton, S.; Robinson, S.C. Feasibility of coloring bamboo with the application of natural and extracted fungal pigments. Coatings 2016, 6, 37. [Google Scholar] [CrossRef] [Green Version]
- Kamperidou, V. The biological durability of thermally-and chemically-modified black pine and poplar wood against basidiomycetes and mold action. Forests 2019, 10, 1111. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.S.; Zhang, Y.; Yu, Z.M.; Qi, C.S.; Tang, R.L.; Zhao, B.S.; Wang, H.W.; Han, Y.Y. Microbial dyes: Dyeing of poplar veneer with melanin secreted by Lasiodiplodia theobromae isolated from wood. Appl. Microbiol. Biotechnol. 2020, 104, 3367–3377. [Google Scholar] [CrossRef]
- Liu, Y.S.; Yu, Z.M.; Zhang, Y.; Qi, C.S.; Tang, R.L.; Zhao, B.S.; Wang, H.W.; Han, Y.Y. Microbial dyeing—infection behavior and influence of Lasiodiplodia theobromae in poplar veneer. Dyes Pigment. 2019, 173, 107988. [Google Scholar] [CrossRef]
- Rassabina, A.E.; Gurjanov, O.P.; Beckett, R.P.; Minibayeva, F.V. Melanin from the lichens Cetraria islandica and Pseudevernia furfuracea: Structural features and physicochemical properties. Biochemistry 2020, 85, 623–628. [Google Scholar] [CrossRef]
- Girdthep, S.; Sirirak, J.; Daranarong, D.; Daengngern, R.; Chayabutra, S. Physico-chemical characterization of natural lake pigments obtained from Caesalpinia Sappan Linn. and their composite films for poly (lactic acid)-based packaging materials. Dyes Pigment. 2018, 157, 27–39. [Google Scholar] [CrossRef]
- Segal, L.G.J.M.A.; Creely, J.J.; Martin, A.E., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Gao, Y.; Yu, Z.; Zhang, Y. The Action Difference of Lasiodiplodia theobromae on Infecting and Dyeing Poplar Wood in Spatial Growth. Coatings 2021, 11, 985. https://doi.org/10.3390/coatings11080985
Liu Y, Gao Y, Yu Z, Zhang Y. The Action Difference of Lasiodiplodia theobromae on Infecting and Dyeing Poplar Wood in Spatial Growth. Coatings. 2021; 11(8):985. https://doi.org/10.3390/coatings11080985
Chicago/Turabian StyleLiu, Yuansong, Yunxiao Gao, Zhiming Yu, and Yang Zhang. 2021. "The Action Difference of Lasiodiplodia theobromae on Infecting and Dyeing Poplar Wood in Spatial Growth" Coatings 11, no. 8: 985. https://doi.org/10.3390/coatings11080985
APA StyleLiu, Y., Gao, Y., Yu, Z., & Zhang, Y. (2021). The Action Difference of Lasiodiplodia theobromae on Infecting and Dyeing Poplar Wood in Spatial Growth. Coatings, 11(8), 985. https://doi.org/10.3390/coatings11080985




