Comparison of the Growth and Thermal Properties of Nonwoven Polymers after Atomic Layer Deposition and Vapor Phase Infiltration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Characterization
3. Results and Discussion
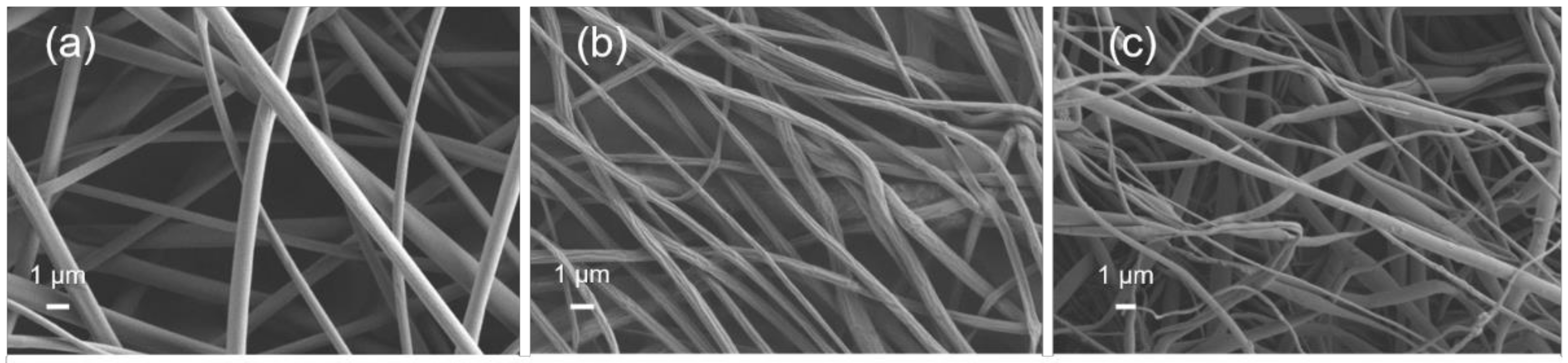
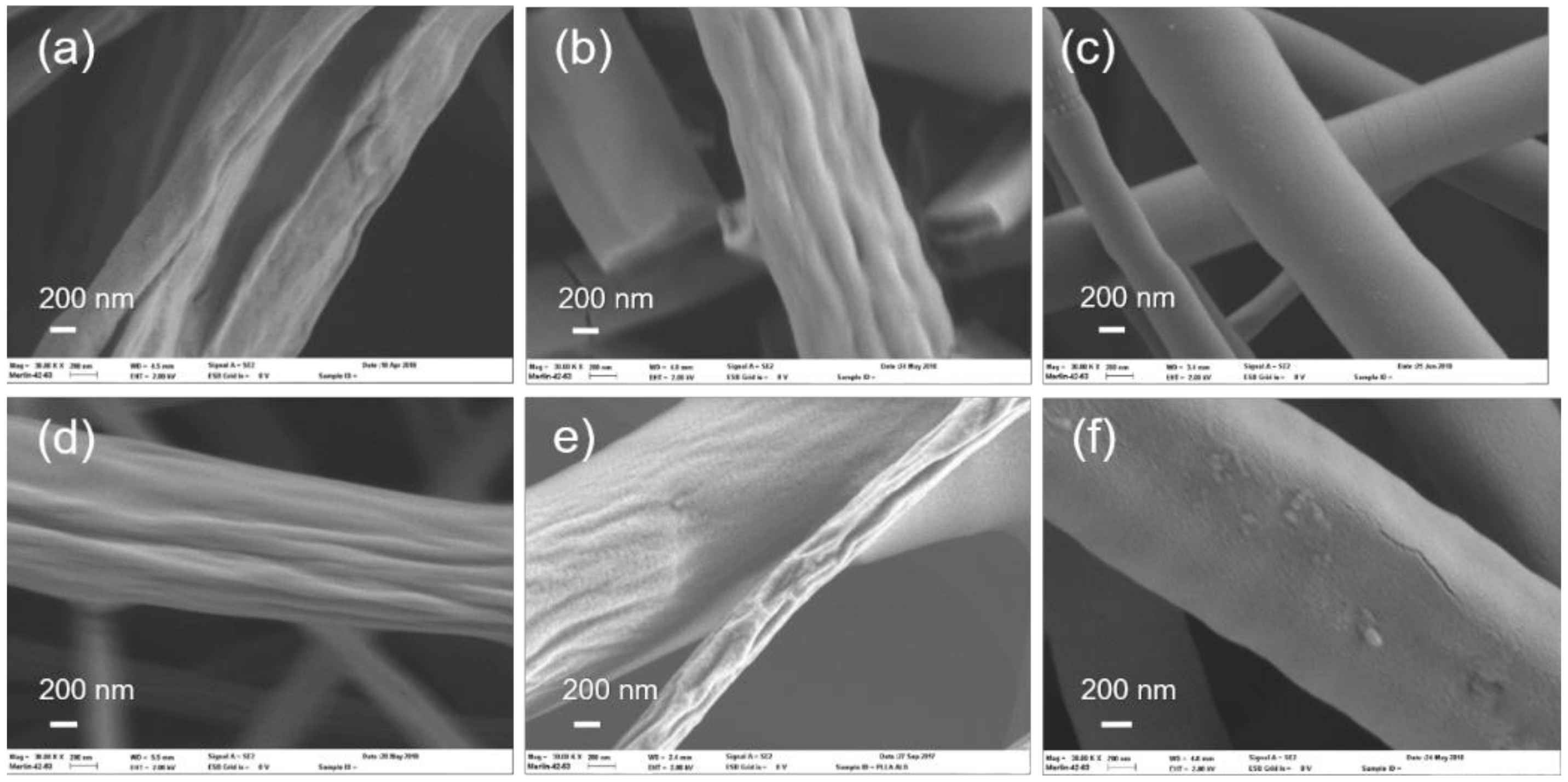
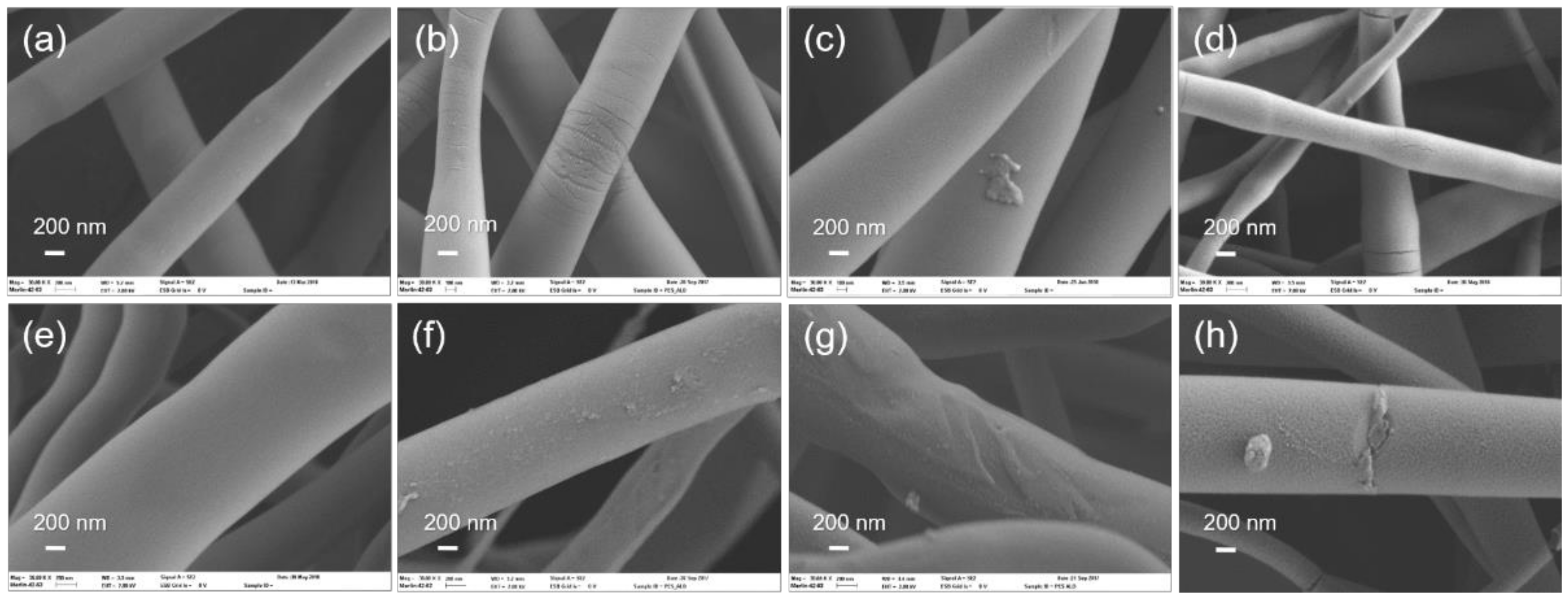
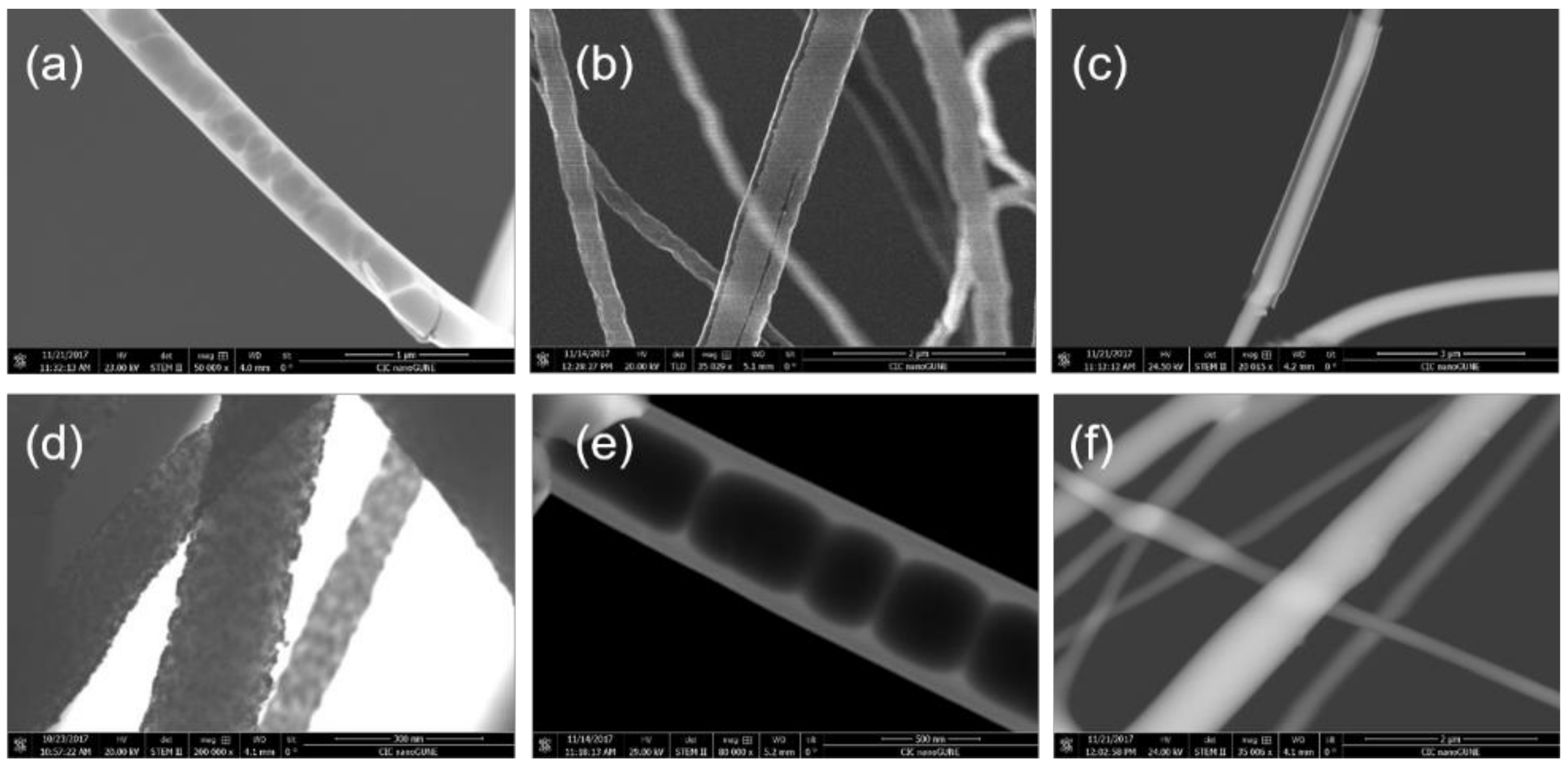
3.1. Al2O3 and ZnO Growth on Polymers
3.2. Study of Film Growth and Interactions
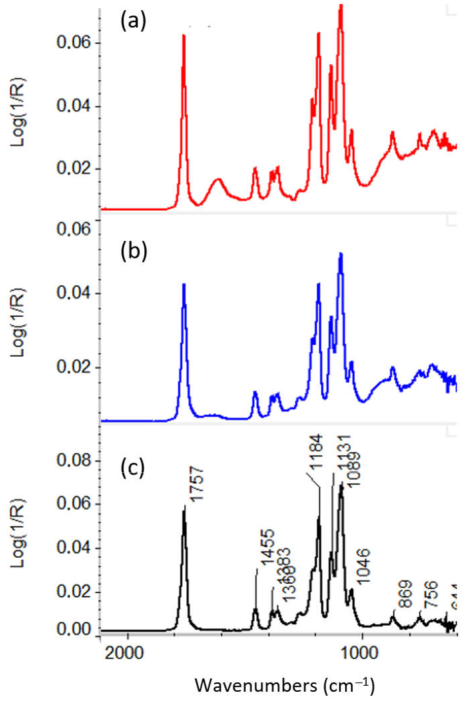
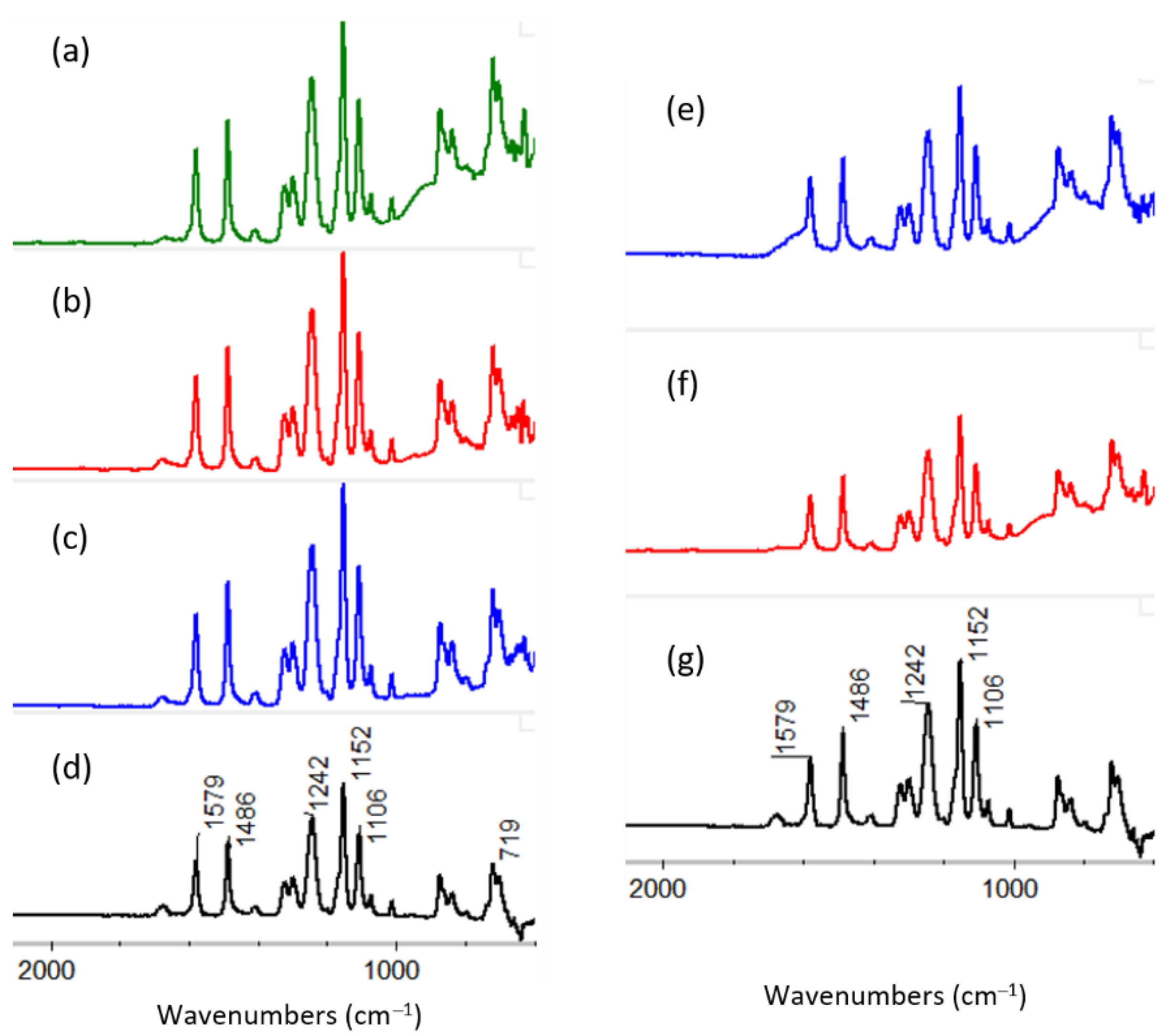
3.2.1. ATR-FTIR of ALD Deposited Materials
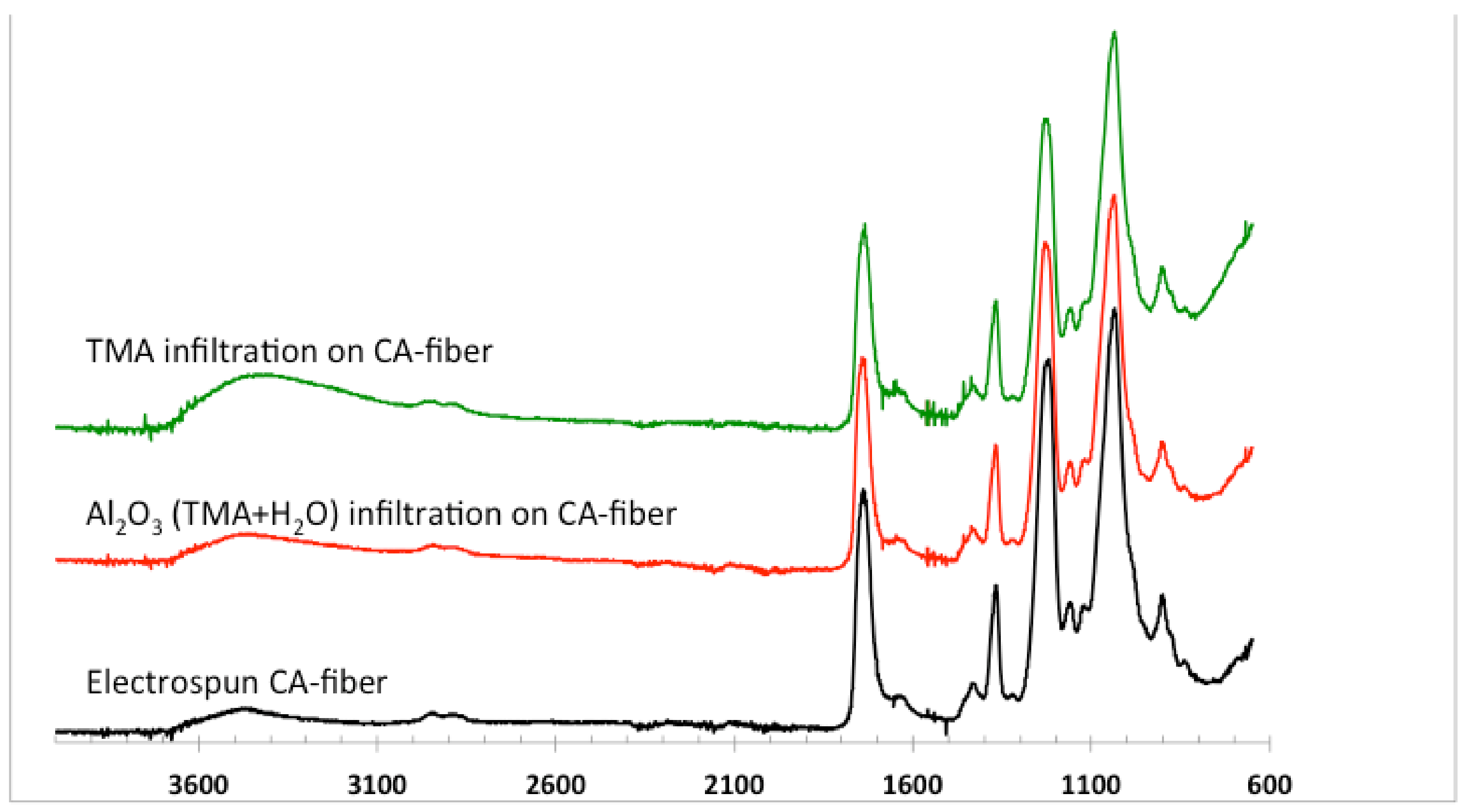
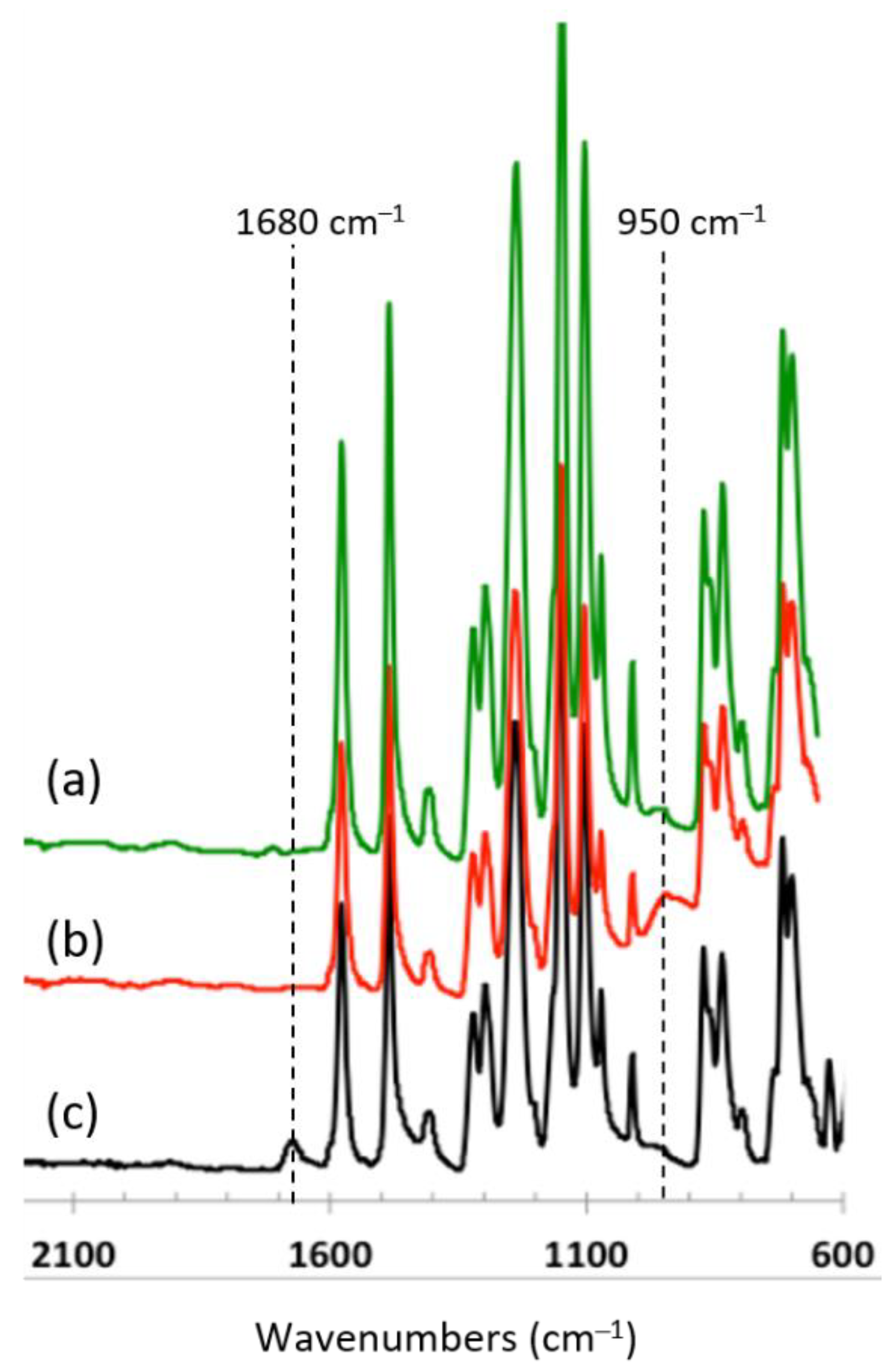
3.2.2. ATR-FTIR of Infiltrated Materials
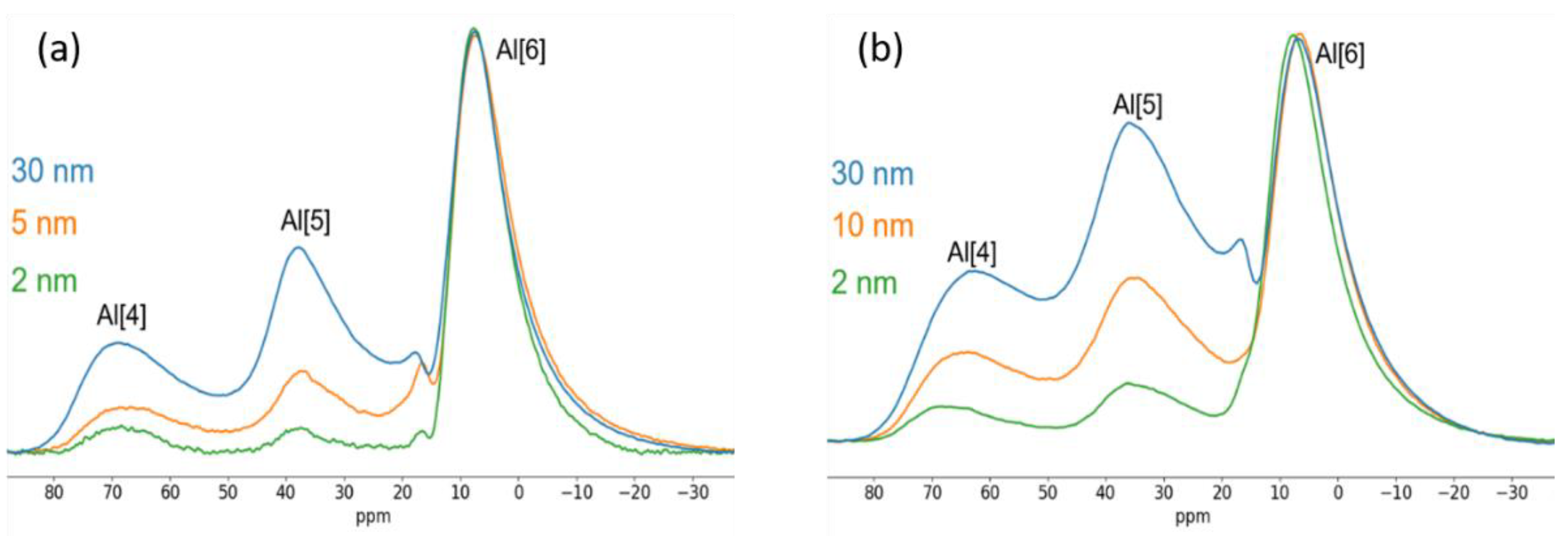
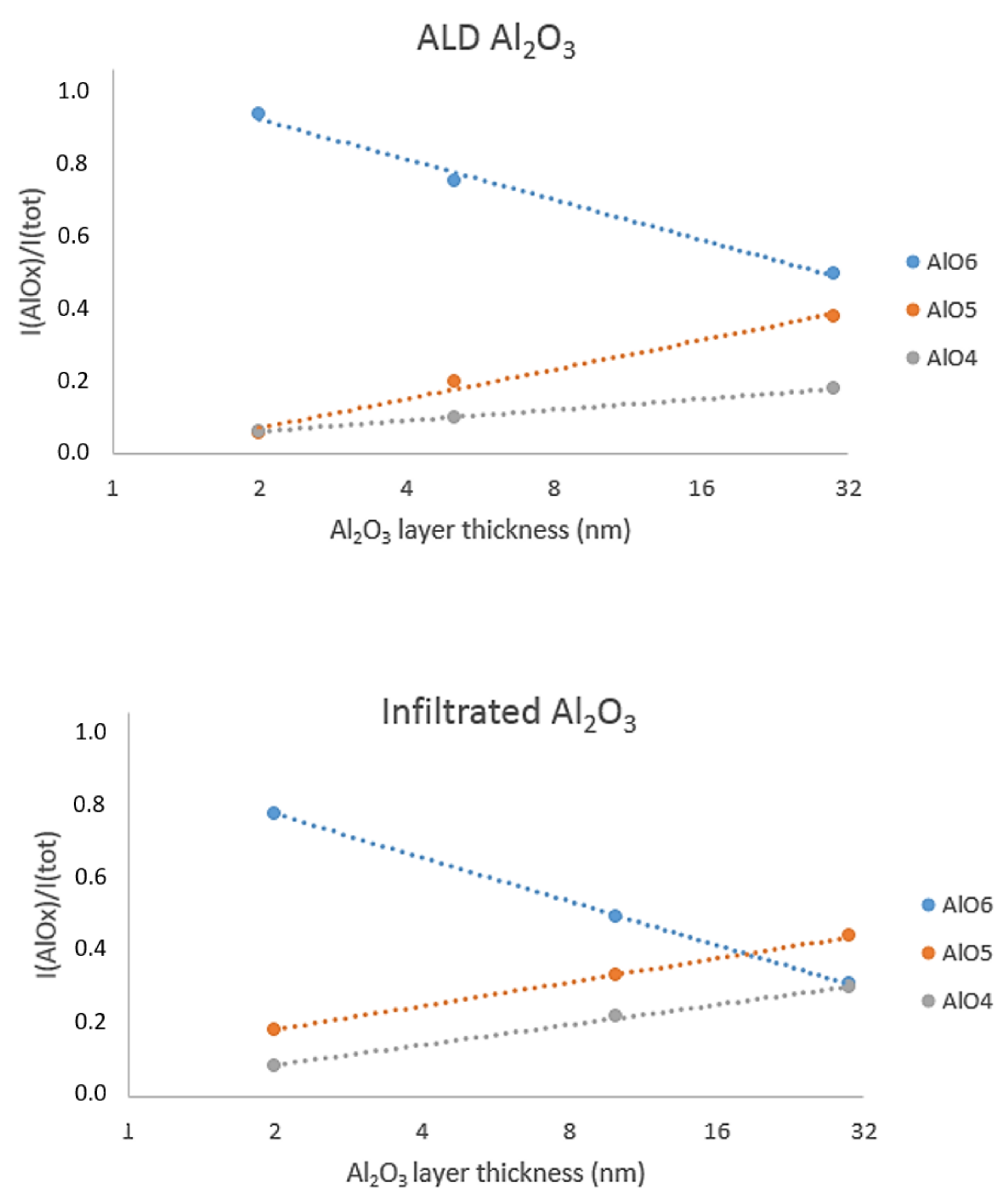
3.2.3. 27Al Solid State NMR
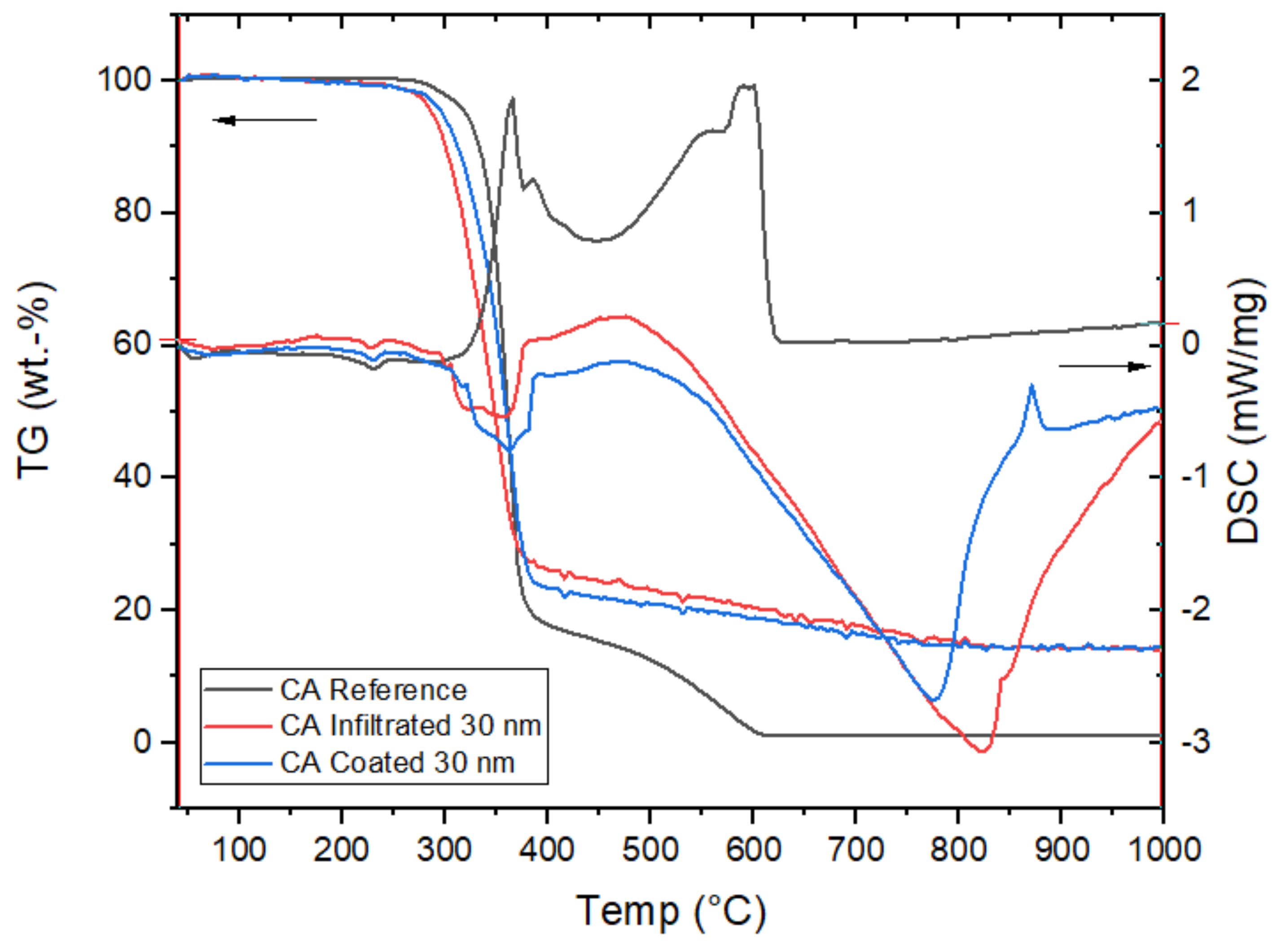
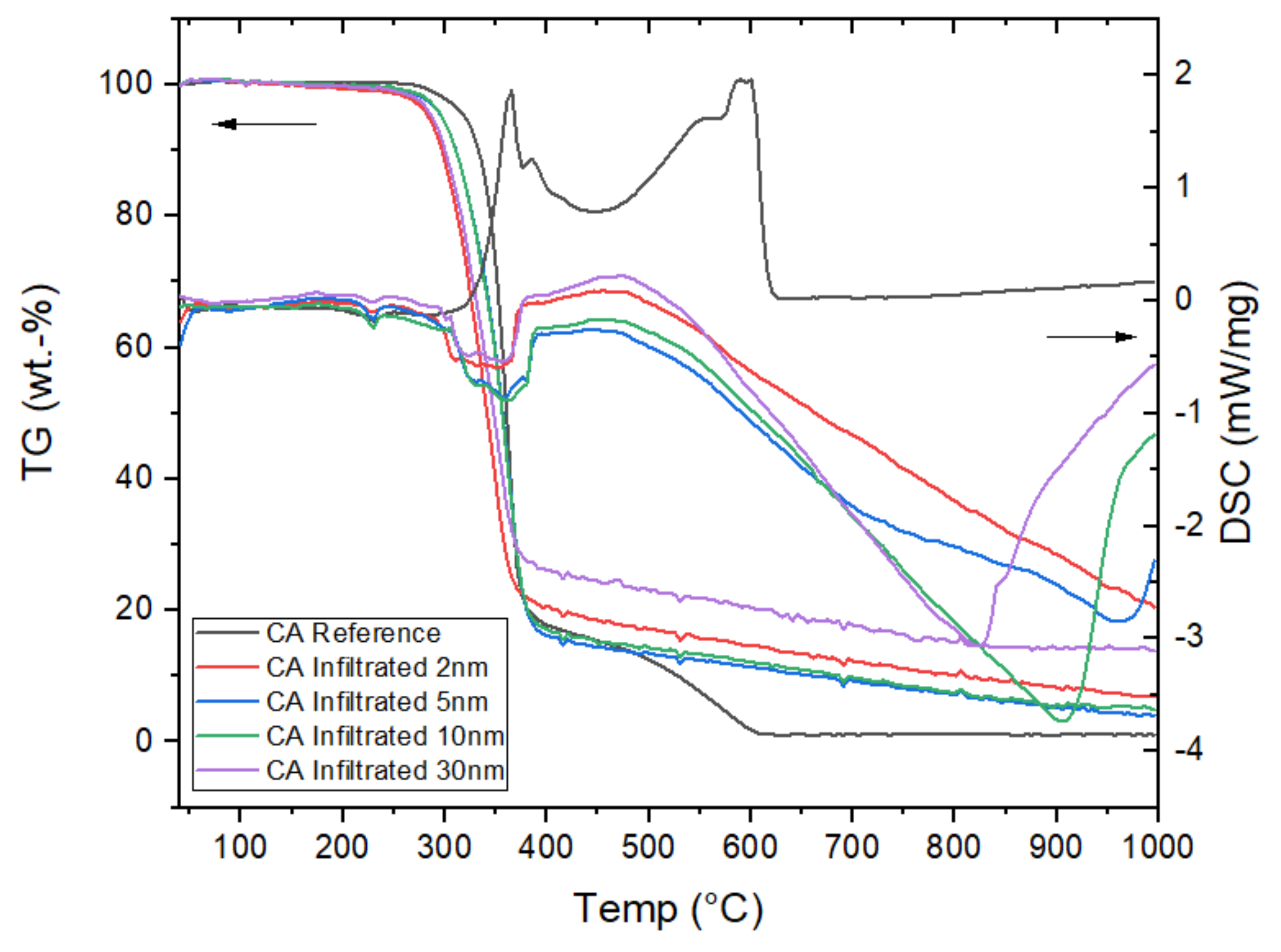
3.2.4. Thermal Behaviour
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leskela, M.; Ritala, M. Atomic layer deposition (ALD): From precursors to thin film structures. Thin Solid Films 2002, 409, 138–146. [Google Scholar] [CrossRef]
- George, S.M. Atomic Layer Deposition: An Overview. Chem. Rev. 2009, 110, 111–131. [Google Scholar] [CrossRef]
- Puurunen, R.L. Surface chemistry of atomic layer deposition: A case study for the trimethylaluminum/water process. J. Appl. Phys. 2005, 97, 121301. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.-C. New development of atomic layer deposition: Processes, methods and applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef] [Green Version]
- Park, H.H. Inorganic Materials by Atomic Layer Deposition for Perovskite Solar Cells. Nanomaterials 2021, 11, 88. [Google Scholar] [CrossRef]
- Lee, J.-H.; You, Y.-J.; Saeed, M.A.; Kim, S.H.; Choi, S.-H.; Kim, S.; Lee, S.Y.; Park, J.-S.; Shim, J.W. Undoped tin dioxide transparent electrodes for efficient and cost-effective indoor organic photovoltaics (SnO2 electrode for indoor organic photovoltaics). NPG Asia Mater. 2021, 13, 1–10. [Google Scholar] [CrossRef]
- Elam, J.W.; Wilson, C.A.; Schuisky, M.; Sechrist, Z.A.; George, S. Improved nucleation of TiN atomic layer deposition films on SiLK low-k polymer dielectric using an Al2O3 atomic layer deposition adhesion layer. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2003, 21, 1099. [Google Scholar] [CrossRef]
- Groner, M.D.; Fabreguette, F.H.; Elam, A.J.W.; George, S. Low-Temperature Al2O3 Atomic Layer Deposition. Chem. Mater. 2004, 16, 639–645. [Google Scholar] [CrossRef]
- Ferguson, J.D.; Weimer, A.W.; George, S.M. Atomic Layer Deposition of Al2O3 Films on Polyethylene Particles. Chem. Mater. 2004, 16, 5602–5609. [Google Scholar] [CrossRef]
- Wilson, C.A.; Grubbs, R.K.; George, S. Nucleation and Growth during Al2O3 Atomic Layer Deposition on Polymers. Chem. Mater. 2005, 17, 5625–5634. [Google Scholar] [CrossRef]
- Spagnola, J.C.; Gong, B.; Arvidson, S.A.; Jur, J.S.; Khan, S.; Parsons, G. Surface and sub-surface reactions during low temperature aluminium oxide atomic layer deposition on fiber-forming polymers. J. Mater. Chem. 2010, 20, 4213–4222. [Google Scholar] [CrossRef]
- Jur, J.S.; Spagnola, J.C.; Lee, K.; Gong, B.; Peng, Q.; Parsons, G. Temperature-Dependent Subsurface Growth during Atomic Layer Deposition on Polypropylene and Cellulose Fibers. Langmuir 2010, 26, 8239–8244. [Google Scholar] [CrossRef] [PubMed]
- Hyde, G.K.; Scarel, G.; Spagnola, J.C.; Peng, Q.; Lee, K.; Gong, B.; Roberts, K.G.; Roth, K.M.; Hanson, C.A.; Devine, C.K.; et al. Atomic Layer Deposition and Abrupt Wetting Transitions on Nonwoven Polypropylene and Woven Cotton Fabrics. Langmuir 2009, 26, 2550–2558. [Google Scholar] [CrossRef] [PubMed]
- Jur, J.S.; Sweet, W.J.; Oldham, C.J.; Parsons, G. Atomic Layer Deposition of Conductive Coatings on Cotton, Paper, and Synthetic Fibers: Conductivity Analysis and Functional Chemical Sensing Using “All-Fiber” Capacitors. Adv. Funct. Mater. 2011, 21, 1993–2002. [Google Scholar] [CrossRef]
- Oldham, C.J.; Gong, B.; Spagnola, J.C.; Jur, J.S.; Senecal, K.J.; Godfrey, T.A.; Parsons, G.N. Encapsulation and Chemical Resistance of Electrospun Nylon Nanofibers Coated Using Integrated Atomic and Molecular Layer Deposition. J. Electrochem. Soc. 2011, 158, D549–D556. [Google Scholar] [CrossRef]
- Knez, M.; Sumser, M.; Bittner, A.; Wege, C.; Jeske, H.; Hoffmann, D.M.P.; Kuhnke, K.; Kern, K. Binding the Tobacco Mosaic Virus to Inorganic Surfaces. Langmuir 2003, 20, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Balci, S.; Leinberger, D.M.; Knez, M.; Bittner, A.M.; Boes, F.; Kadri, A.; Wege, C.; Jeske, H.; Kern, K. Printing and Aligning Mesoscale Patterns of Tobacco mosaic virus on Surfaces. Adv. Mater. 2008, 20, 2195–2200. [Google Scholar] [CrossRef]
- Lee, S.-M.; Pippel, E.; Moutanabbir, O.; Gunkel, I.; Thurn-Albrecht, T.; Knez, M. Improved Mechanical Stability of Dried Collagen Membrane after Metal Infiltration. ACS Appl. Mater. Interfaces 2010, 2, 2436–2441. [Google Scholar] [CrossRef]
- Munzert, P.; Schulz, U.; Kaiser, N. Transparent thermoplastic polymers in plasma-assisted coating processes. Surf. Coatings Technol. 2003, 174–175, 1048–1052. [Google Scholar] [CrossRef]
- Gong, B.; Parsons, G. Quantitative in situ infrared analysis of reactions between trimethylaluminum and polymers during Al2O3 atomic layer deposition. J. Mater. Chem. 2012, 22, 15672–15682. [Google Scholar] [CrossRef]
- Obuchovsky, S.; Frankenstein, H.; Vinokur, J.; Hailey, A.K.; Loo, Y.-L.; Frey, G.L. Mechanism of Metal Oxide Deposition from Atomic Layer Deposition inside Nonreactive Polymer Matrices: Effects of Polymer Crystallinity and Temperature. Chem. Mater. 2016, 28, 2668–2676. [Google Scholar] [CrossRef]
- Lee, S.-M.; Pippel, E.; Gösele, U.; Dresbach, C.; Qin, Y.; Chandran, C.V.; Bräuniger, T.; Hause, G.; Knez, M. Greatly Increased Toughness of Infiltrated Spider Silk. Science 2009, 324, 488–492. [Google Scholar] [CrossRef]
- Lee, S.-M.; Pippel, E.; Moutanabbir, O.; Kim, J.-H.; Lee, H.-J.; Knez, M. In Situ Raman Spectroscopic Study of Al-Infiltrated Spider Dragline Silk under Tensile Deformation. ACS Appl. Mater. Interfaces 2014, 6, 16827–16834. [Google Scholar] [CrossRef] [PubMed]
- Vähä-Nissi, M.; Sundberg, P.; Kauppi, E.; Hirvikorpi, T.; Sievänen, J.; Sood, A.; Karppinen, M.; Harlin, A. Barrier properties of Al2O3 and alucone coatings and nanolaminates on flexible biopolymer films. Thin Solid Films 2012, 520, 6780–6785. [Google Scholar] [CrossRef]
- Grigoras, K.; Franssila, S.; Airaksinen, V.-M. Investigation of sub-nm ALD aluminum oxide films by plasma assisted etch-through. Thin Solid Films 2008, 516, 5551–5556. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Tsai, F.-Y.; Jhuo, S.-J.; Chen, M.-J. Enhanced OLED performance upon photolithographic patterning by using an atomic-layer-deposited buffer layer. Org. Electron. 2008, 9, 667–672. [Google Scholar] [CrossRef]
- Pinna, N.; Hochepied, J.-F.; Niederberger, M.; Gregg, M. Chemistry and physics of metal oxide nanostructures. Phys. Chem. Chem. Phys. 2009, 11, 3607. [Google Scholar] [CrossRef]
- Zhang, L.; Patil, A.J.; Li, L.; Schierhorn, A.; Mann, S.; Gösele, U.; Knez, M. Chemical Infiltration during Atomic Layer Deposition: Metalation of Porphyrins as Model Substrates. Angew. Chem. Int. Ed. 2009, 48, 4982–4985. [Google Scholar] [CrossRef]
- Xu, Y.; Musgrave, C.B. A DFT Study of the Al2O3 Atomic Layer Deposition on SAMs: Effect of SAM Termination. Chem. Mater. 2004, 16, 646–653. [Google Scholar] [CrossRef]
- Vähä-Nissi, M.; Sievänen, J.; Salo, E.; Heikkilä, P.; Kenttä, E.; Johansson, L.-S.; Koskinen, J.T.; Harlin, A. Atomic and molecular layer deposition for surface modification. J. Solid State Chem. 2014, 214, 7–11. [Google Scholar] [CrossRef]
- Lee, K.; Jur, J.S.; Kim, H.; Parsons, G.N. Mechanisms for hydrophilic/hydrophobic wetting transitions on cellulose cotton fibers coated using Al2O3 atomic layer deposition. J. Vac. Sci. Technol. Vac. Surf. Film 2012, 30, 01A163. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vähä-Nissi, M.; Harlin, A.; Marles, J.; Miikkulainen, V.; Karppinen, M. Effect of corona pre-treatment on the performance of gas barrier layers applied by atomic layer deposition onto polymer-coated paperboard. Appl. Surf. Sci. 2010, 257, 736–740. [Google Scholar] [CrossRef]
- Hirvikorpi, T.; Vähä-Nissi, M.; Vartiainen, J.; Penttilä, P.; Nikkola, J.; Harlin, A.; Serimaa, R.; Karppinen, M. Effect of heat-treatment on the performance of gas barrier layers applied by atomic layer deposition onto polymer-coated paperboard. J. Appl. Polym. Sci. 2011, 122, 2221–2227. [Google Scholar] [CrossRef]
- Choi, H.; Lee, S.; Jung, H.; Shin, S.; Ham, G.; Seo, H.; Jeon, H. Moisture Barrier Properties of Al2O3 Films deposited by Remote Plasma Atomic Layer Deposition at Low Temperatures. Jpn. J. Appl. Phys. 2013, 52, 35502. [Google Scholar] [CrossRef]
- Cooper, R.; Upadhyaya, H.; Minton, T.K.; Berman, M.R.; Du, X.; George, S. Protection of polymer from atomic-oxygen erosion using Al2O3 atomic layer deposition coatings. Thin Solid Films 2008, 516, 4036–4039. [Google Scholar] [CrossRef]
- Sweet, W.J.; Jur, J.S.; Parsons, G.N. Bi-layer Al2O3/ZnO atomic layer deposition for controllable conductive coatings on polypropylene nonwoven fiber mats. J. Appl. Phys. 2013, 113, 194303. [Google Scholar] [CrossRef]
- Sweet, W.J.; Parsons, G.N. In Situ Conductance Analysis of Zinc Oxide Nucleation and Coalescence during Atomic Layer Deposition on Metal Oxides and Polymers. Langmuir 2015, 31, 7274–7282. [Google Scholar] [CrossRef]
- Napari, M.; Lahtinen, M.; Veselov, A.; Julin, J.; Østreng, E.; Sajavaara, T. Room-temperature plasma-enhanced atomic layer deposition of ZnO: Film growth dependence on the PEALD reactor configuration. Surf. Coat. Technol. 2017, 326, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Xiong, S.; Kong, L.; Huang, J.; Chen, X.; Wang, Y. Atomic-layer-deposition-enabled nonwoven membranes with hierarchical ZnO nanostructures for switchable water/oil separations. J. Membr. Sci. 2015, 493, 478–485. [Google Scholar] [CrossRef]
- Vähä-Nissi, M.; Pitkänen, M.; Salo, E.; Kenttä, E.; Tanskanen, A.; Sajavaara, T.; Putkonen, M.; Sievänen, J.; Sneck, A.; Rättö, M.; et al. Antibacterial and barrier properties of oriented polymer films with ZnO thin films applied with atomic layer deposition at low temperatures. Thin Solid Films 2014, 562, 331–337. [Google Scholar] [CrossRef]
- Lahtinen, K.; Kääriäinen, T.; Johansson, P.T.; Kotkamo, S.; Maydannik, P.; Seppänen, T.; Kuusipalo, J.; Cameron, D.C. UV protective zinc oxide coating for biaxially oriented polypropylene packaging film by atomic layer deposition. Thin Solid Films 2014, 570, 33–37. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Tian, Y.; Zhang, J.; Zhan, W.; Zhao, J.; Ding, Y.; Zuo, W. Hydrophilic modification of polyvinylidene fluoride membranes by ZnO atomic layer deposition using nitrogen dioxide/diethylzinc functionalization. J. Membr. Sci. 2016, 514, 241–249. [Google Scholar] [CrossRef]
- Hyung, G.W.; Park, J.; Koo, J.R.; Choi, K.M.; Kwon, S.J.; Cho, E.S.; Kim, Y.S.; Kim, Y.K. ZnO thin-film transistors with a polymeric gate insulator built on a polyethersulfone substrate. Solid-State Electron. 2012, 69, 27–30. [Google Scholar] [CrossRef]
- Heo, J.H.; Ryu, H.; Lee, W.-J. Effect of O2 plasma pretreatment on structural and optical properties of ZnO films on PES substrate by atomic layer deposition. J. Ind. Eng. Chem. 2013, 19, 1638–1641. [Google Scholar] [CrossRef]
- Sinha, A.; Hess, D.W.; Henderson, C.L. Transport behavior of atomic layer deposition precursors through polymer masking layers: Influence on area selective atomic layer deposition. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2007, 25, 1721. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.-M.; Pippel, E.; Knez, M. Metal Infiltration into Biomaterials by ALD and CVD: A Comparative Study. ChemPhysChem 2011, 12, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Azpitarte, I.; Zuzuarregui, A.; Ablat, H.; Ruiz-Rubio, L.; López-Ortega, A.; Elliott, S.D.; Knez, M. Suppressing the Thermal and Ultraviolet Sensitivity of Kevlar by Infiltration and Hybridization with ZnO. Chem. Mater. 2017, 29, 10068–10074. [Google Scholar] [CrossRef]
- McClure, C.D.; Oldham, C.J.; Parsons, G. Effect of Al2O3 ALD coating and vapor infusion on the bulk mechanical response of elastic and viscoelastic polymers. Surf. Coat. Technol. 2015, 261, 411–417. [Google Scholar] [CrossRef]
- Wang, W.; Chen, C.; Tollan, C.; Yang, F.; Qin, Y.; Knez, M. Efficient and controllable vapor to solid doping of the polythiophene P3HT by low temperature vapor phase infiltration. J. Mater. Chem. C 2017, 5, 2686–2694. [Google Scholar] [CrossRef]
- Wang, W.; Yang, F.; Chen, C.; Zhang, L.; Qin, Y.; Knez, M. Tuning the Conductivity of Polyaniline through Doping by Means of Single Precursor Vapor Phase Infiltration. Adv. Mater. Interfaces 2016, 4. [Google Scholar] [CrossRef]
- Lee, J.; Shin, C.; Heo, J.; Kim, C.; Park, J.; Lee, T.; Ryu, H.; Son, C.; Shin, B.; Lee, W. Effects of O2 plasma pre-treatment on ZnO thin films grown on polyethersulfone substrates at various deposition temperatures by atomic layer deposition. Curr. Appl. Phys. 2010, 10, S290–S293. [Google Scholar] [CrossRef]
- Choi, D.-W.; Kim, S.-J.; Lee, J.H.; Chung, K.-B.; Park, J.-S. A study of thin film encapsulation on polymer substrate using low temperature hybrid ZnO/Al2O3 layers atomic layer deposition. Curr. Appl. Phys. 2012, 12, S19–S23. [Google Scholar] [CrossRef]
- Yun, S.J.; Lim, J.W.; Lee, J.-H. Low-Temperature Deposition of Aluminum Oxide on Polyethersulfone Substrate Using Plasma-Enhanced Atomic Layer Deposition. Electrochem. Solid-State Lett. 2004, 7, C13–C15. [Google Scholar] [CrossRef]
- Park, S.-H.K.; Oh, J.; Hwang, C.-S.; Lee, J.-I.; Yang, Y.S.; Chu, H.Y. Ultrathin Film Encapsulation of an OLED by ALD. Electrochem. Solid-State Lett. 2005, 8, H21–H23. [Google Scholar] [CrossRef]
- Heikkilä, P.; Rautkoski, H.; Kenttä, E.; Svärd, L.; Vähä-Nissi, M.; Virtanen, T.; Putkonen, M. Electrospun Sheet Materials from CA, PES and PLLA as Supports for ALD Coating. In Proceedings of the International Conference on Electrospinning: From Design and Processing to Advanced Nanomaterials and Applications, ElectrospinCY_2017, Nicosia, Cyprus, 19–21 April 2017. [Google Scholar]
- Freitas, W.; Merkle, H.P.; Gander, B. Microencapsulation by solvent extraction/evaporation: Reviewing the state of the art of microsphere preparation process technology. J. Control. Release 2005, 102, 313–332. [Google Scholar] [CrossRef]
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef]
- Van Meerten, S.; Franssen, W.; Kentgens, A. ssNake: A cross-platform open-source NMR data processing and fitting application. J. Magn. Reson. 2019, 301, 56–66. [Google Scholar] [CrossRef]
- Goldstein, D.N.; McCormick, J.A.; George, S. Al2O3 Atomic Layer Deposition with Trimethylaluminum and Ozone Studied by in Situ Transmission FTIR Spectroscopy and Quadrupole Mass Spectrometry. J. Phys. Chem. C 2008, 112, 19530–19539. [Google Scholar] [CrossRef]
- Klauk, H. Oxide dielectrics: A change of direction. Nat. Mater. 2009, 8, 853–854. [Google Scholar] [CrossRef]
- Lee, S.B.; Park, S.Y.; Yi, Y.S.; Ahn, C.W. Structure of Amorphous Aluminum Oxide. Phys. Rev. Lett. 2009, 103, 095501. [Google Scholar] [CrossRef] [PubMed]
- Sarou-Kanian, V.; Gleizes, A.N.; Florian, P.; Samélor, D.; Massiot, D.; Vahlas, C. Temperature-Dependent 4-, 5- and 6-Fold Coordination of Aluminum of MOCVD-Grown Amorphous Alumina Films: A Very High Field 27Al-NMR Study. J. Phys. Chem. C 2013, 117, 21965–21971. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.K.; Ahn, C.W. Probing of 2 dimensional confinement-induced structural transitions in amorphous oxide thin film. Sci. Rep. 2014, 4, 4200. [Google Scholar] [CrossRef]
- Baggetto, L.; Sarou-Kanian, V.; Florian, P.; Gleizes, A.N.; Massiot, D.; Vahlas, C. Atomic scale structure of amorphous aluminum oxyhydroxide, oxide and oxycarbide films probed by very high field 27 Al nuclear magnetic resonance. Phys. Chem. Chem. Phys. 2017, 19, 8101–8110. [Google Scholar] [CrossRef] [Green Version]
- Lucena, M.D.C.C.; de Alencar, A.E.V.; Mazzeto, S.E.; Soares, S. The effect of additives on the thermal degradation of cellulose acetate. Polym. Degrad. Stab. 2003, 80, 149–155. [Google Scholar] [CrossRef]
- Cimalla, V.; Baeumler, M.; Kirste, L.; Prescher, M.; Christian, B.; Passow, T.; Benkhelifa, F.; Bernhardt, F.; Eichapfel, G.; Himmerlich, M.; et al. Densification of Thin Aluminum Oxide Films by Thermal Treatments. Mater. Sci. Appl. 2014, 05, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Broas, M.; Kanninen, O.; Vuorinen, V.; Tilli, M.; Paulasto-Kröckel, M. Chemically Stable Atomic-Layer-Deposited Al2O3 Films for Processability. ACS Omega 2017, 2, 3390–3398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broas, M.; Lemettinen, J.; Sajavaara, T.; Tilli, M.; Vuorinen, V.; Suihkonen, S.; Paulasto-Kröckel, M. In-situ annealing characterization of atomic-layer-deposited Al2O3 in N2, H2 and vacuum atmospheres. Thin Solid Films 2019, 682, 147–155. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keskiväli, L.; Heikkilä, P.; Kenttä, E.; Virtanen, T.; Rautkoski, H.; Pasanen, A.; Vähä-Nissi, M.; Putkonen, M. Comparison of the Growth and Thermal Properties of Nonwoven Polymers after Atomic Layer Deposition and Vapor Phase Infiltration. Coatings 2021, 11, 1028. https://doi.org/10.3390/coatings11091028
Keskiväli L, Heikkilä P, Kenttä E, Virtanen T, Rautkoski H, Pasanen A, Vähä-Nissi M, Putkonen M. Comparison of the Growth and Thermal Properties of Nonwoven Polymers after Atomic Layer Deposition and Vapor Phase Infiltration. Coatings. 2021; 11(9):1028. https://doi.org/10.3390/coatings11091028
Chicago/Turabian StyleKeskiväli, Laura, Pirjo Heikkilä, Eija Kenttä, Tommi Virtanen, Hille Rautkoski, Antti Pasanen, Mika Vähä-Nissi, and Matti Putkonen. 2021. "Comparison of the Growth and Thermal Properties of Nonwoven Polymers after Atomic Layer Deposition and Vapor Phase Infiltration" Coatings 11, no. 9: 1028. https://doi.org/10.3390/coatings11091028
APA StyleKeskiväli, L., Heikkilä, P., Kenttä, E., Virtanen, T., Rautkoski, H., Pasanen, A., Vähä-Nissi, M., & Putkonen, M. (2021). Comparison of the Growth and Thermal Properties of Nonwoven Polymers after Atomic Layer Deposition and Vapor Phase Infiltration. Coatings, 11(9), 1028. https://doi.org/10.3390/coatings11091028





