Investigations on the Interaction of EDTA with Calcium Silicate Hydrate and Its Impact on the U(VI) Sorption
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Interaction of EDTA with C-S-H
2.3. The Effect of EDTA on the Sorption of U(VI) by C-S-H
3. Results and Discussion
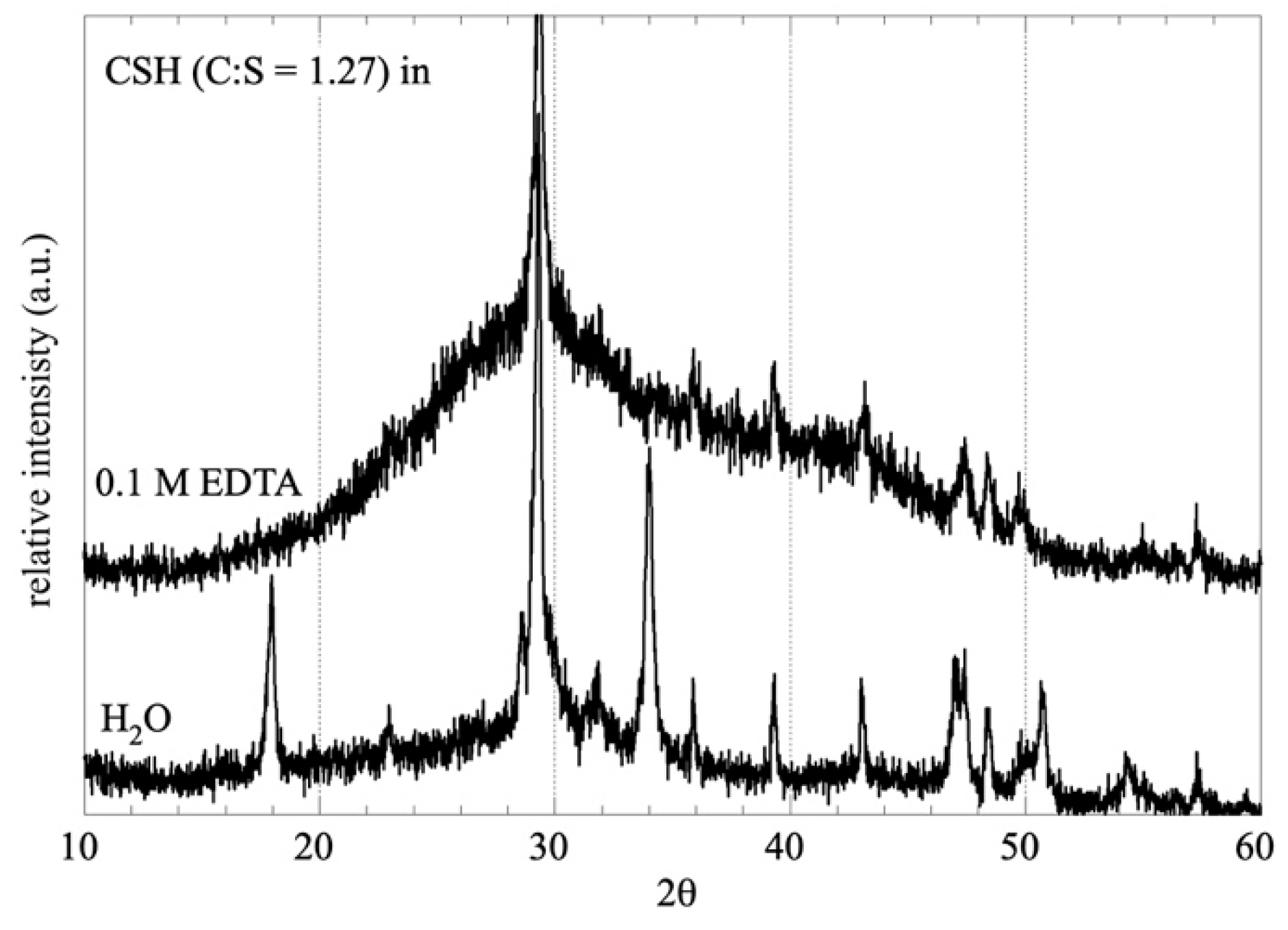
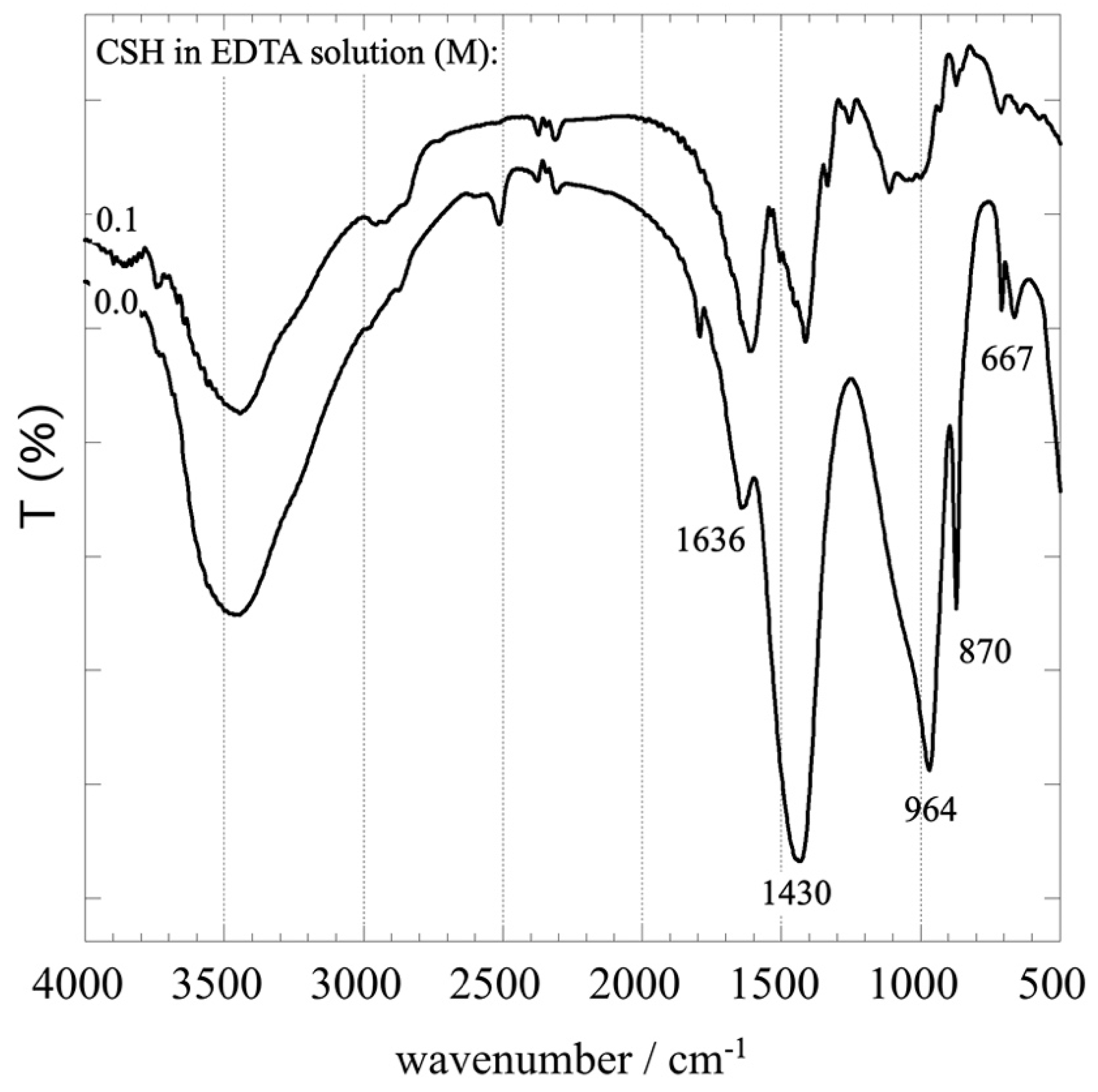
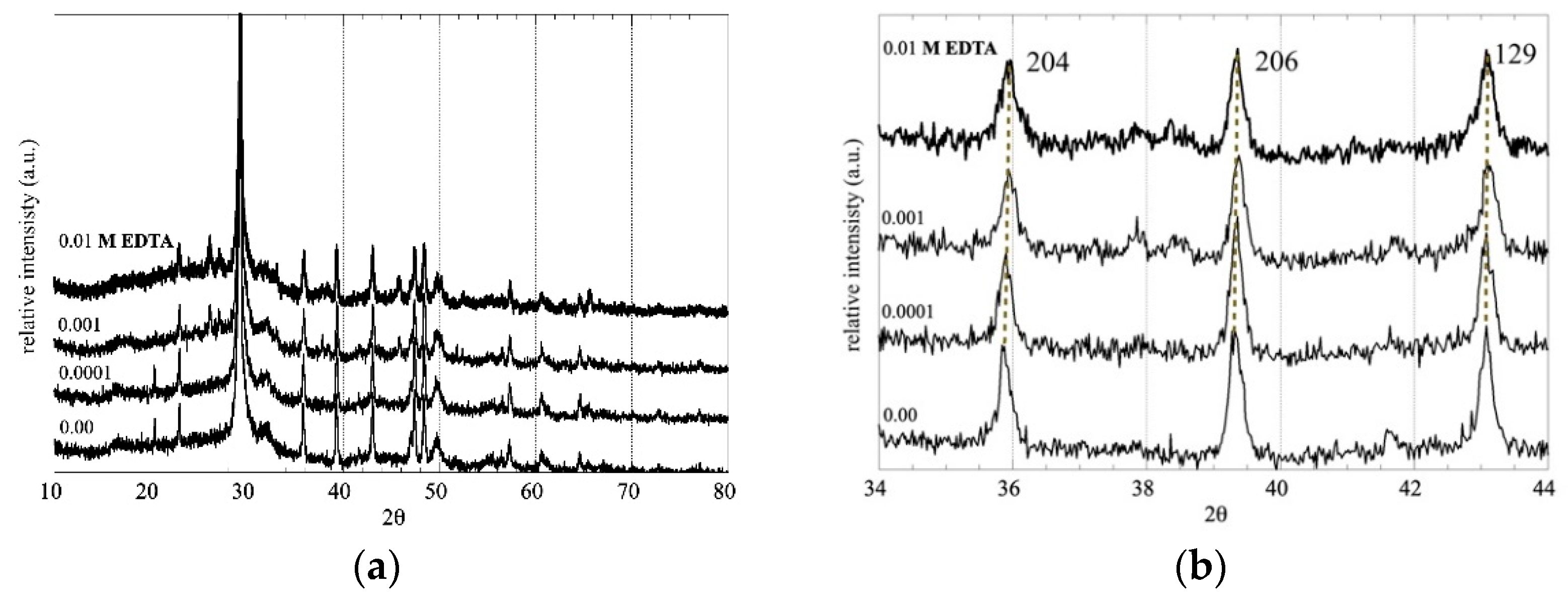
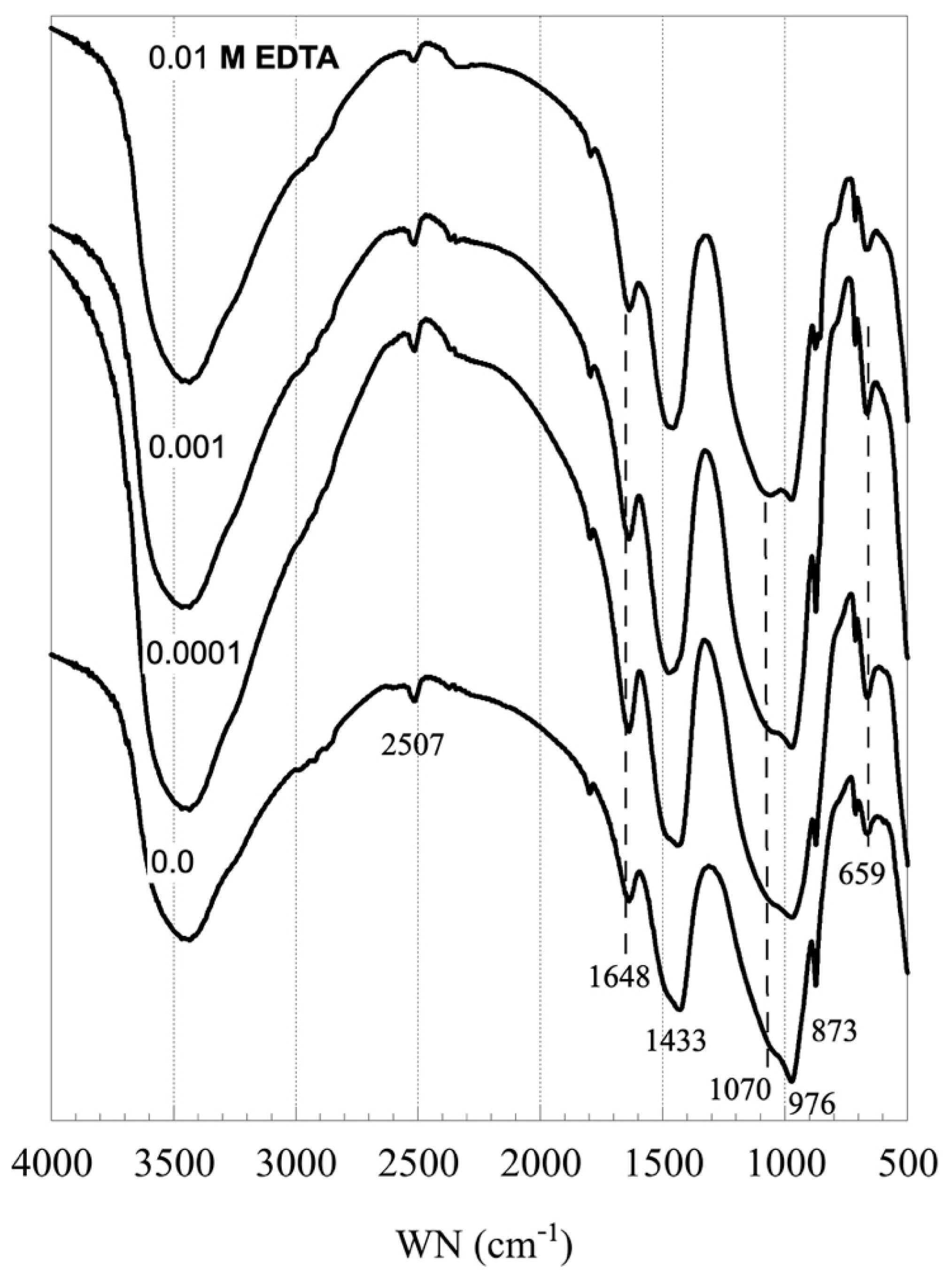
3.1. Impact of EDTA on the C-S-H Stability
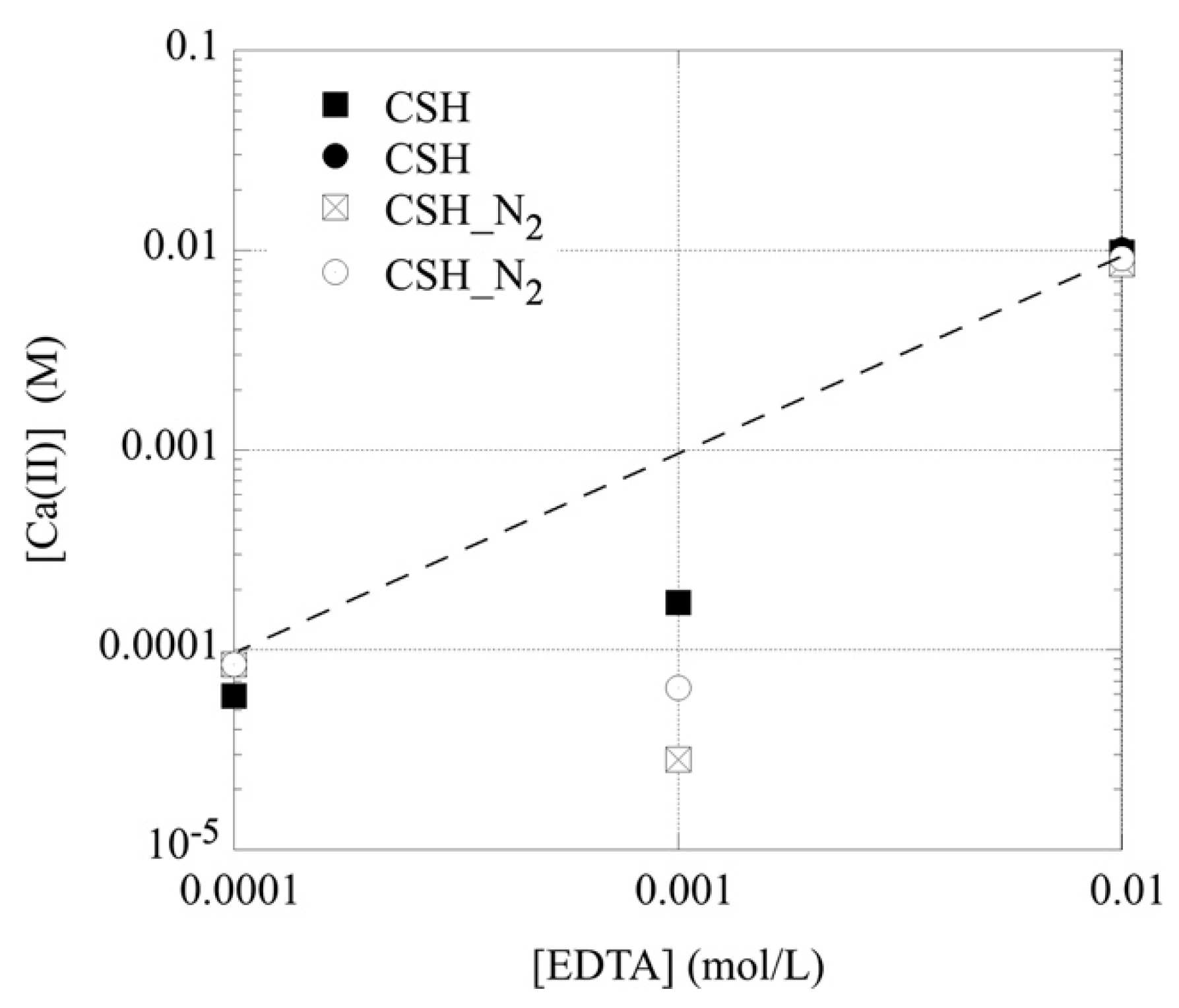
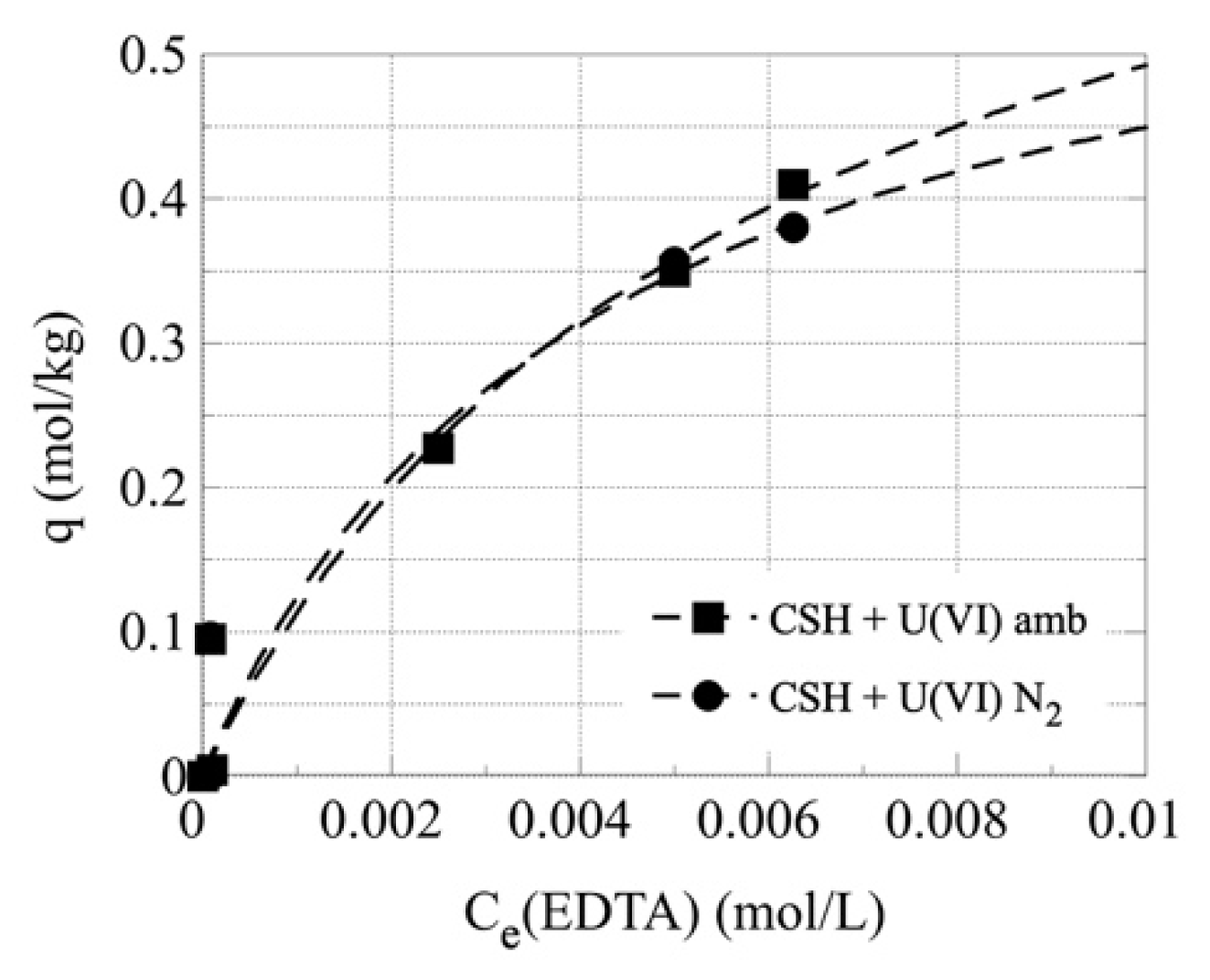
3.2. Sorption of EDTA by C-S-H
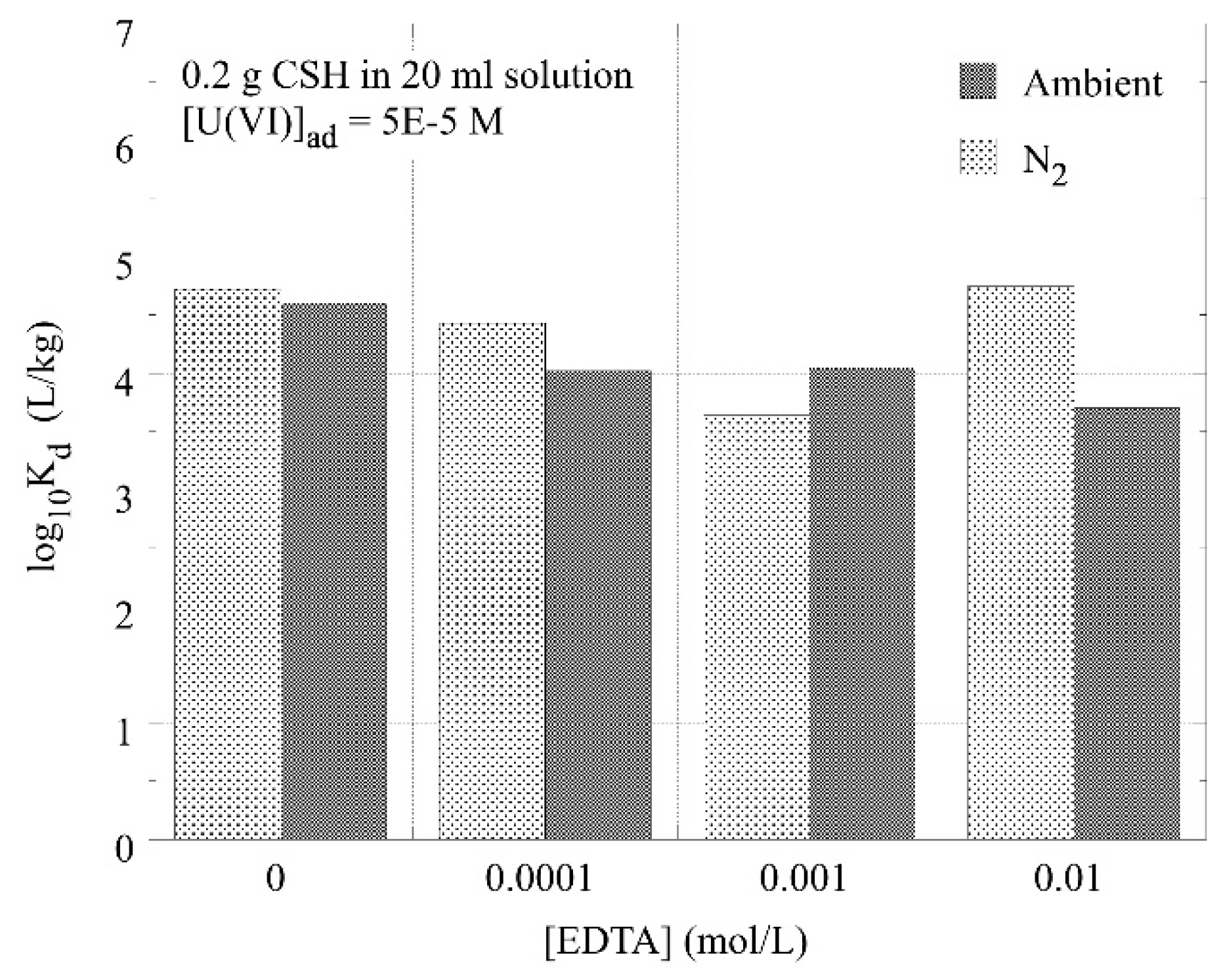
3.3. The Effect of EDTA on the Sorption of U(VI) by C-S-H
4. Conclusions and Future Work
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Grambow, B. Geological disposal of radioactive waste in clay. Elements 2016, 12, 239–245. [Google Scholar] [CrossRef]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Tits, J.; Stumpf, T.; Rabung, T.; Wieland, E.; Fanghänel, T. Uptake of Cm(III) and Eu(III) by calcium silicate hydrates: A solution chemistry and time-resolved laser fluorescence spectroscopy Study. Environ. Sci. Technol. 2003, 37, 3568–3573. [Google Scholar] [CrossRef]
- Tits, J.; Walther, C.; Stumpf, T.; Mace, N.; Wieland, E. A luminescence line-narrowing spectroscopic study of the uranium(VI) interaction with cementitious materials and titanium dioxide. Dalton Trans. 2015, 44, 966–976. [Google Scholar] [CrossRef] [Green Version]
- Pashalidis, I.; Runde, W.; Kim, I.J. A study of solid-liquid phase equilibria of Pu (VI) and U (VI) in aqueous carbonate systems. Radiochim. Acta 1993, 61, 141–146. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J. Leaching performance of uranium from the cement solidified matrices containing spent radioactive organic solvent. Ann. Nucl. Energy 2017, 101, 31–35. [Google Scholar] [CrossRef]
- Atkins, M.; Glasser, F.P. Application of portland cement-based materials to radioactive waste immobilization. Waste Manage. 1992, 12, 105–131. [Google Scholar] [CrossRef]
- Hongbin, T.; Yuxiang, L. The existence state of uranium(VI) in portland cement matrix material immobilization body. Uranium Min. Metall. 2005, 38, 86–90. [Google Scholar]
- Wang, F.; Chen, G.; Ji, L.; Yuan, Z. Preparation and mechanical properties of cemented uranium tailing backfill based on alkali-activated slag. Adv. Mater. Sci. Eng. 2020, 2020, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ochs, M.; Vriens, B.; Tachi, Y. Retention of uranium in cement systems: Effects of cement degradation and complexing ligands. Prog. Nucl. Sci. Technol. 2018, 5, 208–212. [Google Scholar] [CrossRef]
- Macé, N.; Wieland, E.; Dähn, R.; Tits, J.; Scheinost, A.C. EXAFS investigation on U(VI) immobilization in hardened cement paste: Influence of experimental conditions on speciation. Radiochim. Acta. 2013, 101, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Tits, J.; Geipel, G.; Macé, N.; Eilzer, M.; Wieland, E. Determination of uranium(VI) sorbed species in calcium silicate hydrate phases: A laser-induced luminescence spectroscopy and batch sorption study. J. Colloid Interface Sci. 2011, 359, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Androniuk, I.; Landesman, C.; Henocq, P.; Kalinichev, G.A. Adsorption of gluconate and uranyl on C-S-H phases: Combination of wet chemistry experiments and molecular dynamics simulations for the binary systems. Phys. Chem. Earth. 2017, 99, 194–203. [Google Scholar] [CrossRef] [Green Version]
- du Bois de Maquillé, L.; Renaudin, L.; Goutelard, F.; Jardy, A.; Vial, J.; Thiébaut, D. Determination of ethylenediaminetetraacetic acid in nuclear waste by high-performance liquid chromatography coupled with electrospray mass spectrometry. J. Chromatogr. A. 2013, 1276, 20–25. [Google Scholar] [CrossRef]
- Maddalena, R.; Li, K.; Chater, A.P.; Michalik, S.; Hamilton, A. Direct synthesis of a solid calcium-silicate-hydrate (C-S-H). Constr. Build. Mater. 2019, 223, 554–565. [Google Scholar] [CrossRef]
- Kiliari, T.; Pashalidis, I. Simplified alpha-spectroscopic analysis of uranium in natural waters after its separation by cation-exchange. Radiat. Meas. 2010, 45, 966–968. [Google Scholar] [CrossRef]
- Grangeon, S.; Claret, F.; Linard, Y.; Chiaberge, C. X-ray diffraction: A powerful tool to probe and understand the structure of nanocrystalline calcium silicate hydrates. Acta Crystallogr. 2013, B69, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Crea, F.; De Stefano, C.; Gianguzza, A.; Piazzese, D.; Sammartano, S. Speciation of poly-amino carboxylic compounds in seawater. Chem. Speciat. Bioavailab. 2003, 15, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Guan, W.; Ji, F.; Chen, Q.; Peng, Y.; Ling, P. Synthesis and Enhanced Phosphate Recovery Property of Porous Calcium Silicate Hydrate Using Polyethyleneglycol as Pore-Generation Agent. Materials 2013, 6, 2846–2861. [Google Scholar] [CrossRef] [PubMed]
- Nalet, C.; Nonat, A. Ionic complexation and adsorption of small organic molecules on calcium silicate hydrate: Relation with their retarding effect on the hydration. Cem. Concr. Res. 2016, 89, 97–108. [Google Scholar] [CrossRef]
- Song, Z.; Cai, H.; Liu, Q.; Liu, X.; Pu, Q.; Zang, Y.; Xu, N. Numerical Simulation of Adsorption of Organic Inhibitors on C-S-H Gel. Crystals 2020, 10, 742. [Google Scholar] [CrossRef]
- García Tomás, F.; Kojdecki, M.A.; Pardo, P.; Ibańez, R.; Álvarez Larena, A.; Bastida, J. X-ray diffraction microstructural analysis of swelling by ethylene glycolin two reference clay minerals. Acta Phys. Pol. A 2016, 130, 876–879. [Google Scholar] [CrossRef]
- Mosser-Ruck, R.; Devineau, K.; Charpentier, D.; Cathelineau, M. Effects of ethylene glycol saturation protocols on XRD patterns: A critical review and discussion. Clays Clay Miner. 2005, 53, 631–638. [Google Scholar] [CrossRef]
- Mishra, K.V.; Bhattacharjee, N.B.; Kumar, D.; Rai, B.S.; Parkash, O. Effect of a chelating agent at different pH on the spectroscopic and structural properties of microwave derived hydroxyapatite nanoparticles: A bone mimetic material. New J. Chem. 2016, 40, 5432–5441. [Google Scholar] [CrossRef]
- Black, L.; Garbev, K.; Gee, I. Surface carbonation of synthetic C-S-H samples: A Comparison between Fresh and Aged C-S-H Using X-ray Photoelectron Spectroscopy. Cem. Concr. Res. 2008, 38, 745–750. [Google Scholar] [CrossRef] [Green Version]
- Bhat, T.R.; Krishnamurthy, M. Studies on EDTA complexes—II uranyl-EDTA system. J. Inorg. Nucl. Chem. 1964, 26, 587–594. [Google Scholar] [CrossRef]
- Pashalidis, I.; Czerwinski, K.R.; Fanghaenel, T.; Kim, I.J. Solid-Liguid Phase Equilibria of Pu(VI) and U(VI) in Aqueous Carbonate Systems. Determination of the Carbonate Stability Constants. Radiochim. Acta 1997, 76, 55–62. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maragkou, E.; Pashalidis, I. Investigations on the Interaction of EDTA with Calcium Silicate Hydrate and Its Impact on the U(VI) Sorption. Coatings 2021, 11, 1037. https://doi.org/10.3390/coatings11091037
Maragkou E, Pashalidis I. Investigations on the Interaction of EDTA with Calcium Silicate Hydrate and Its Impact on the U(VI) Sorption. Coatings. 2021; 11(9):1037. https://doi.org/10.3390/coatings11091037
Chicago/Turabian StyleMaragkou, Eleni, and Ioannis Pashalidis. 2021. "Investigations on the Interaction of EDTA with Calcium Silicate Hydrate and Its Impact on the U(VI) Sorption" Coatings 11, no. 9: 1037. https://doi.org/10.3390/coatings11091037



