Wear and Corrosion Resistance of CoCrFeNiSiMoW Medium-Entropy Alloy Coatings on Q235 Steel
Abstract
:1. Introduction
2. Experimental Details
2.1. CoCrFeNiSiMoW Coating Fabrication
2.2. Microstructure Observation and Hardness Measurement
2.3. Wear Measurements
2.4. Electrochemical Corrosion
3. Results and Discussion
3.1. Morphology Observation
3.2. XRD Analysis
3.3. Hardness of Coatings
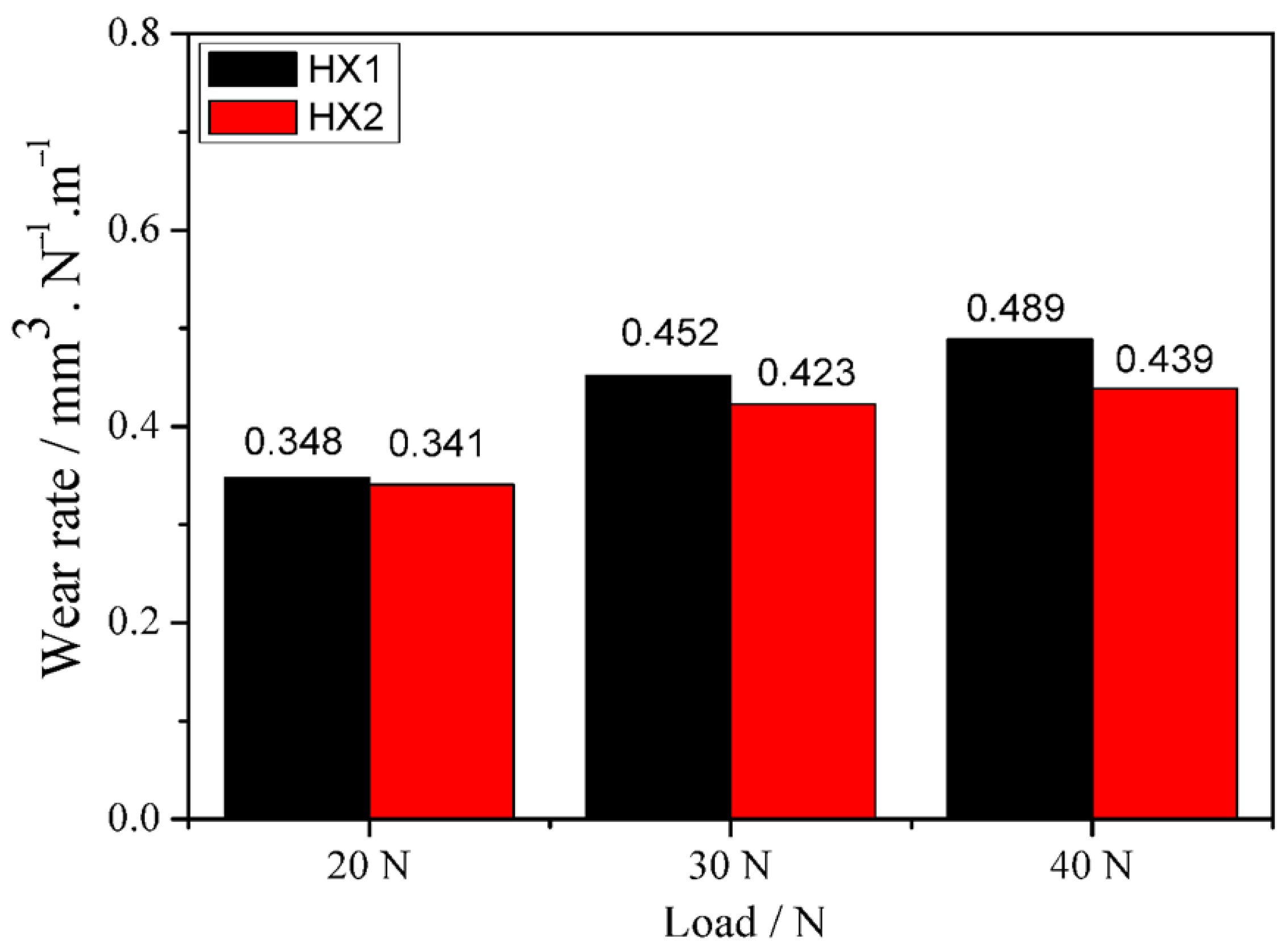
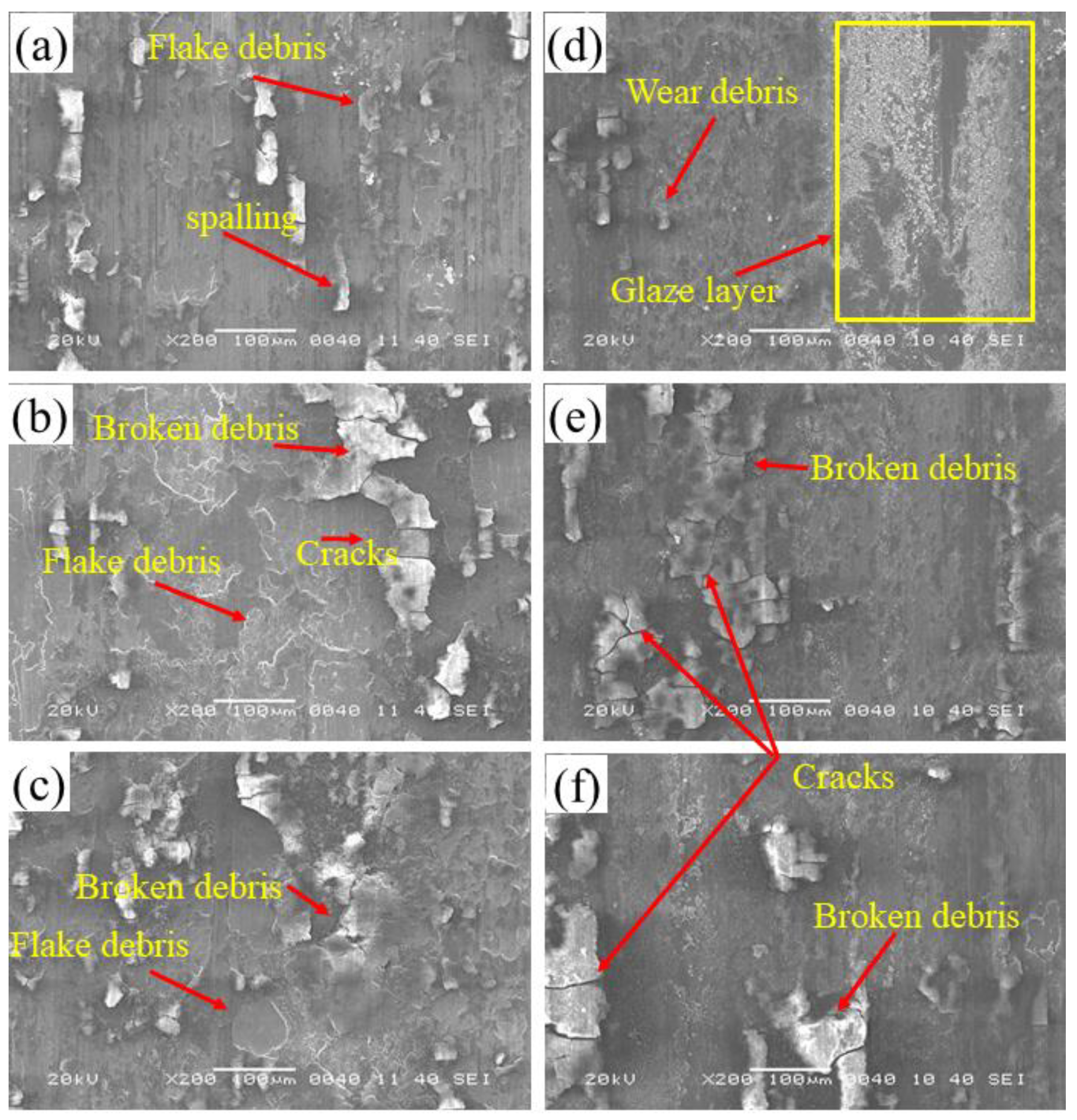
3.4. Wear Analysis
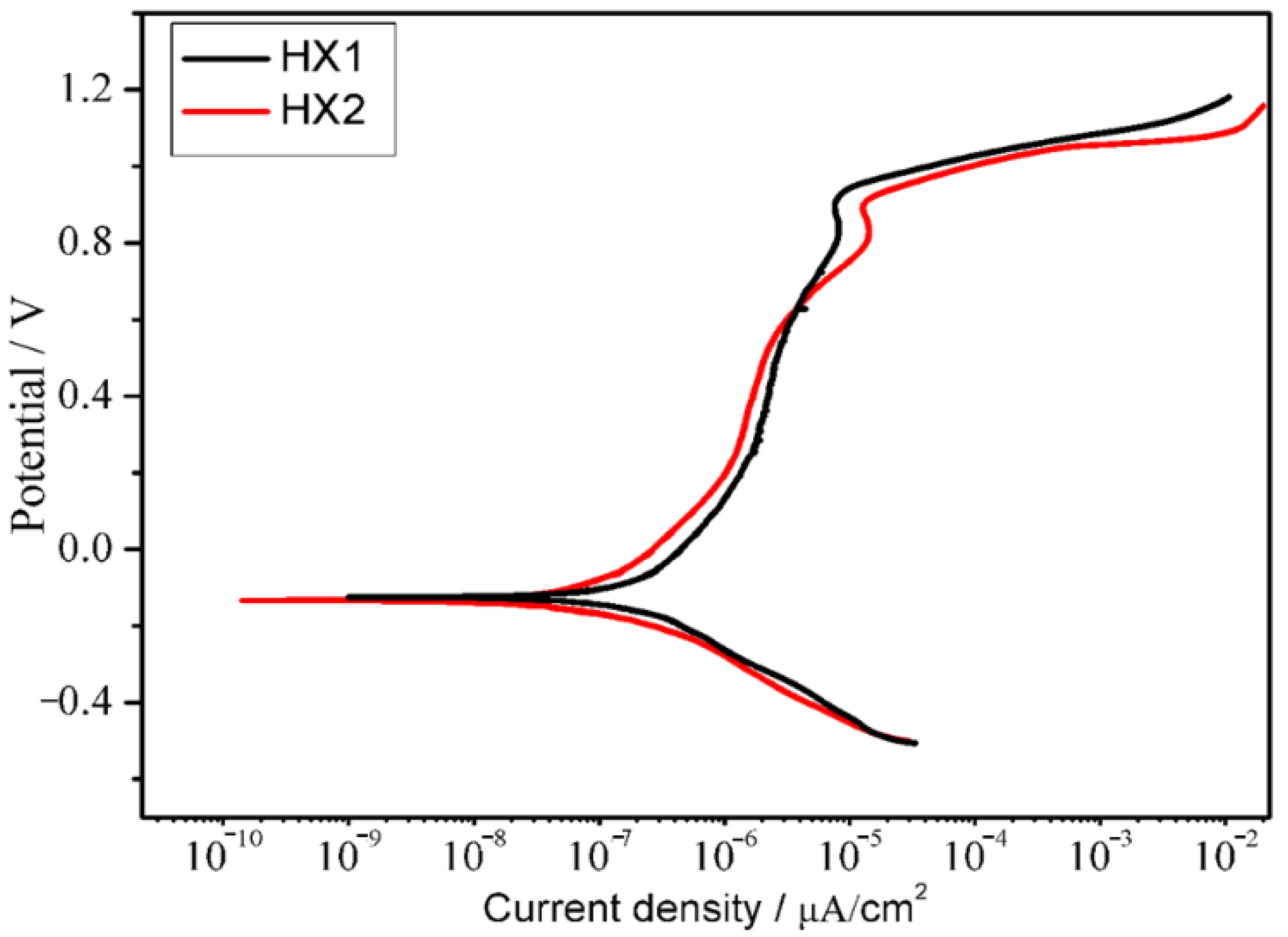
3.5. Electrochemical Corrosion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.T.; Axinte, E.; Ge, W.J.; Shang, C.Y.; Wang, Y. Microstructure, mechanical properties and corrosion resistance of CuZrY/Al, Ti, Hf series high-entropy alloys. Mater. Des. 2016, 108, 106–113. [Google Scholar] [CrossRef]
- Wu, P.H.; Peng, Z.; Liu, N.; Niu, M.Y.; Zhu, Z.X.; Wang, X.J. The effect of Mn content on the microstructure and properties of CoCrCu0.1Fe0.15Mo1.5MnxNi. Mater. Trans. 2016, 57, 5–8. [Google Scholar] [CrossRef] [Green Version]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Ye, Q.F.; Feng, K.; Li, Z.G.; Lu, F.G.; Li, R.F.; Huang, J.; Wu, Y.X. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Nair, R.B.; Arora, H.S.; Mukherjee, S.; Singh, S.; Singh, H.; Grewal, H.S. Exceptionally high cavitation erosion and corrosion resistance of a high entropy alloy. Ultrason. Sonochem. 2018, 41, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.M.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength-ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Thomas, S.; Gibson, M.A.; Fraser, H.L.; Pohl, K.; Birbilis, N. Microstructure and corrosion properties of the low-density single- phase compositionally complex alloy AlTiVCr. Corros. Sci. 2018, 133, 386–396. [Google Scholar] [CrossRef]
- Feng, K.; Zhang, Y.; Li, Z.G.; Yao, C.W.; Yao, L.; Fan, C.Y. Corrosion properties of laser cladded CrCoNi medium entropy alloy coating. Surf. Coat. Technol. 2020, 397, 126004. [Google Scholar] [CrossRef]
- Zhao, D.C.; Yamaguchi, T.; Tusbasa, D.J.; Wang, W.Q. Fabrication and friction properties of the AlFeCrCo medium-entropy alloy coating on magnesium alloy. Mater. Des. 2020, 193, 108872. [Google Scholar] [CrossRef]
- Tian, Y.; Lu, C.Y.; Shen, Y.F.; Feng, X.M. Microstructure and corrosion property of CrMnFeCoNi high entropy alloy coating on Q235 base metal via mechanical alloying method. Surf. Interfaces. 2019, 15, 135–140. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, L.; Jiang, H.; Pan, X.M.; Cao, Z.Q. Phase evolution and properties of Al2CrFeNiMox high-entropy alloys coatings by laser cladding. J. Therm. Spray Technol. 2015, 24, 1333–1340. [Google Scholar] [CrossRef]
- Xu, J.; Peng, S.; Li, Z.Y.; Jiang, S.Y.; Xie, Z.H.; Munroe, P.; Lu, H. Remarkable cavitation erosion-corrosion resistance of CoCrFeNiTiMo high-entropy alloy coatings. Corros. Sci. 2021, 190, 109663. [Google Scholar] [CrossRef]
- Lloyd, A.C.; Noël, J.J.; Mclntyre, S.; Shoesmith, D.W. Cr, Mo and W alloying additions in Ni and their effect on passivity. Electrochim. Acta 2004, 49, 3015–3027. [Google Scholar] [CrossRef]
- Shun, T.T.; Chang, L.Y.; Shiu, M.H. Microstructure and mechanical properties of multiprincipal component CoCrFeNiMox alloys. Mater. Charact. 2012, 70, 63–67. [Google Scholar] [CrossRef]
- Hsu, Y.J.; Chiang, W.C.; Wu, J.K. Corrosion behavior of FeCoNiCrCux high-entropy alloys in 3.5% sodium chloride solution. Mater. Chem. Phys. 2005, 92, 112–117. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, Y. Microhardness, wear resistance, and corrosion resistance of Al0.8CrFeCoNiCu high-entropy alloy coatings on aluminum by laser cladding. Mater. Res. Express. 2021, 7, 026504. [Google Scholar]
- Chen, T.K.; Shun, T.T.; Yeh, J.W.; Wong, M.S. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188–189, 193–200. [Google Scholar] [CrossRef]
- Zeng, Q.F.; Xu, Y.T. A comparative study on the tribocorrosion behaviors of AlFeCrNiMo high entropy alloy coating and 304 stainless steel. Mater. Today Commun. 2020, 24, 101261. [Google Scholar] [CrossRef]
- Wang, F. Mechanical property study on rapid additive layer manufacture Hastelloy X alloy by selective laser melting technology. Int. J. Adv. Manuf. Technol. 2011, 58, 545–551. [Google Scholar] [CrossRef]
- Zhao, J.C.; Larsen, M.; Ravikumar, V. Phase precipitation and time temperature-transformation diagram of Hastelloy X. Mater. Sci. Eng. A. 2000, 293, 112–119. [Google Scholar] [CrossRef]
- Blue, C.A.; Blue, R.A.; Lin, R.Y.; Lei, J.F.; Williams, W.D. Joining of Hastelloy X to Inconel 718 using an infrared process. J. Mater. Process. Technol. 1996, 58, 32–38. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Sheng, S.L.; Zhang, L.M.; Chen, J.; Zheng, Z.B. Friction and wear behaviors of a high nitrogen austenitic stainless steel Fe-19Cr-15Mn-0.66N. J. Min. Metall. Sect. B Metall. 2021, 57, 285–293. [Google Scholar]
- Qiao, Y.X.; Xu, D.K.; Wang, S.; Ma, Y.J.; Chen, J.; Wang, Y.X.; Zhou, H.L. Effect of hydrogen charging on microstructural evolution and corrosion behavior of Ti-4Al-2V-1Mo-1Fe alloy. J. Mater. Sci. Technol. 2021, 60, 168–176. [Google Scholar] [CrossRef]
- Sharma, P.; Dwivedi, V.K.; Dwivedi, S.P. Development of high entropy alloys: A review. Mater. Today Proc. 2021, 43, 502–509. [Google Scholar] [CrossRef]
- Liu, N.; Ding, W.; Wang, X.J.; Du, J.J.; Liu, L.X. Microstructure evolution and phase formation of Fe25Ni25CoxMoy Multi-principal component alloys. Metall. Mater. Trans. A 2020, 51, 2990–2997. [Google Scholar] [CrossRef]
- Ding, W.; Liu, N.; Fan, J.C.; Cao, J.; Wang, X.J. Diffusion bonding of copper and titanium with an interlayer of CoCrFeMnNi high-entropy alloy. Intermetallics 2021, 129, 107027. [Google Scholar] [CrossRef]
- Lin, K.H.; Tseng, C.M.; Chueh, C.C.; Chang, S.Y.; Lo, Y.C.; Wang, C.C.; Lin, S.J.; Yeh, J.W. Different lattice distortion effects on the tensile properties of Ni-W dilute solutions and CrFeNi and CoCrFeMnNi concentrated solutions. SSRN Electron. J. 2021. [Google Scholar] [CrossRef]
- Xi, Y.T.; Bai, Y.Y.; Gao, K.W.; Pang, X.L.; Yang, H.S.; Yan, L.C.; Volinsky, A.A. Residual stress and microstructure effects on mechanical, tribological and electrical properties of TiN coating on 304 stainless steel. Ceram. Int. 2018, 44, 15851–15858. [Google Scholar] [CrossRef]
- Li, Y.Z.; Shi, Y. Microhardness, wear resistance, and corrosion resistance of AlxCrFeCoNiCu high-entropy alloy coatings on aluminum y laser cladding. Opt. Laser Technol. 2021, 134, 106632. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Chen, Y.; Li, L.L.; Chen, J.; Emori, W.; Wang, X.J.; Yang, L.; Zhou, H.L.; Song, G.; Naik, N.; et al. Corrosion behavior of a nickel-free high-nitrogen stainless steel with hydrogen charging. JOM 2021, 73, 1165–1172. [Google Scholar] [CrossRef]
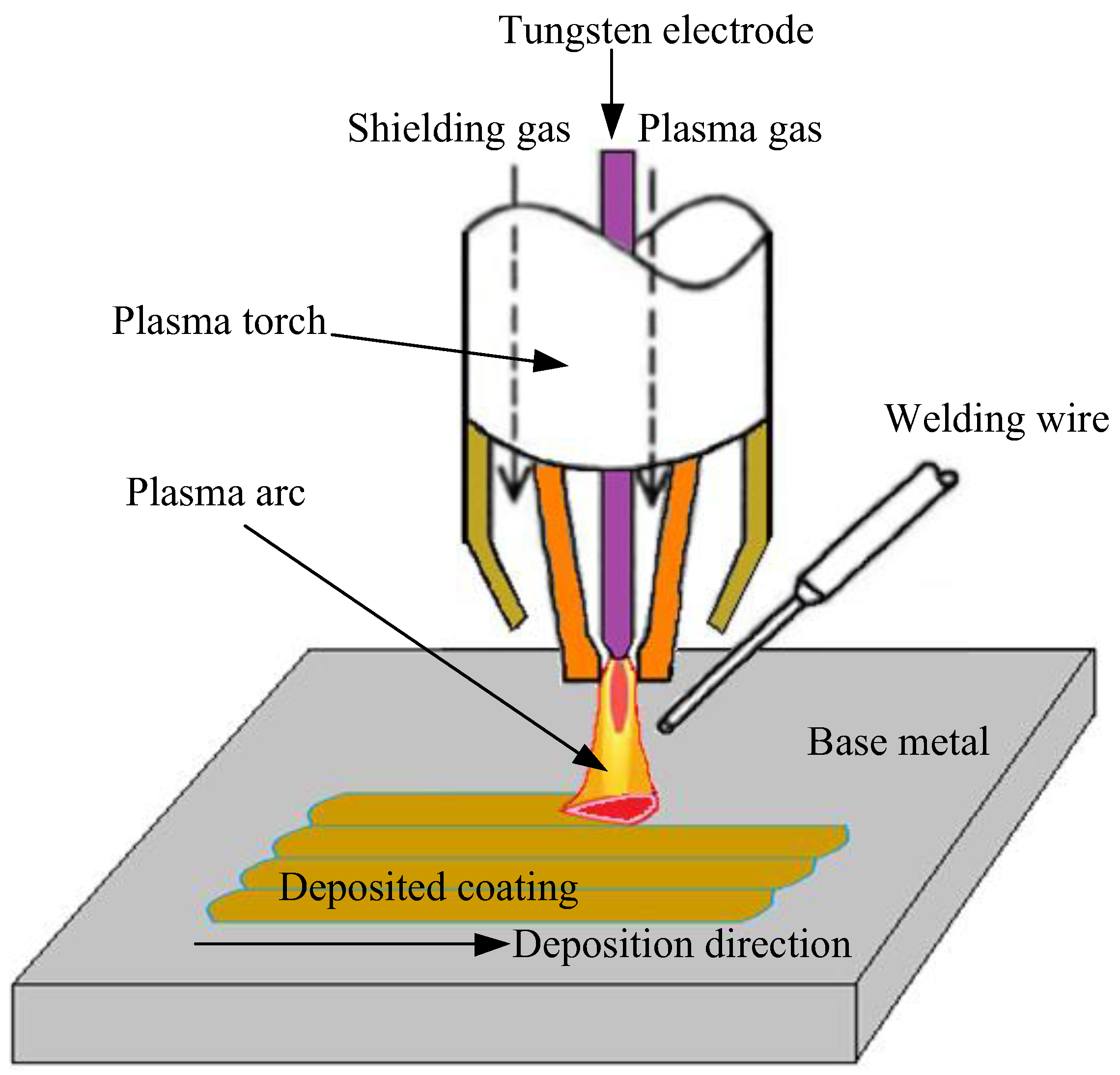

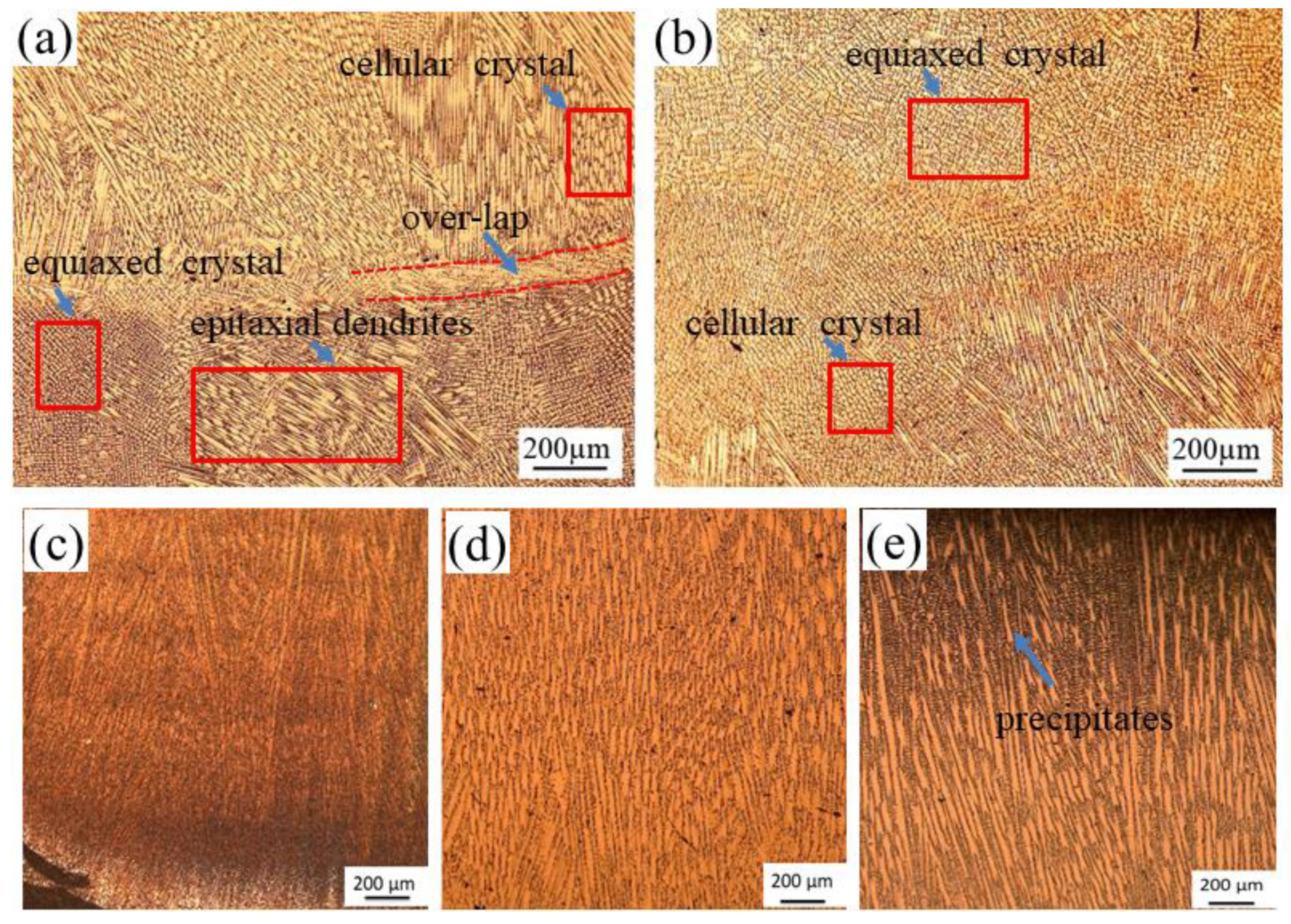
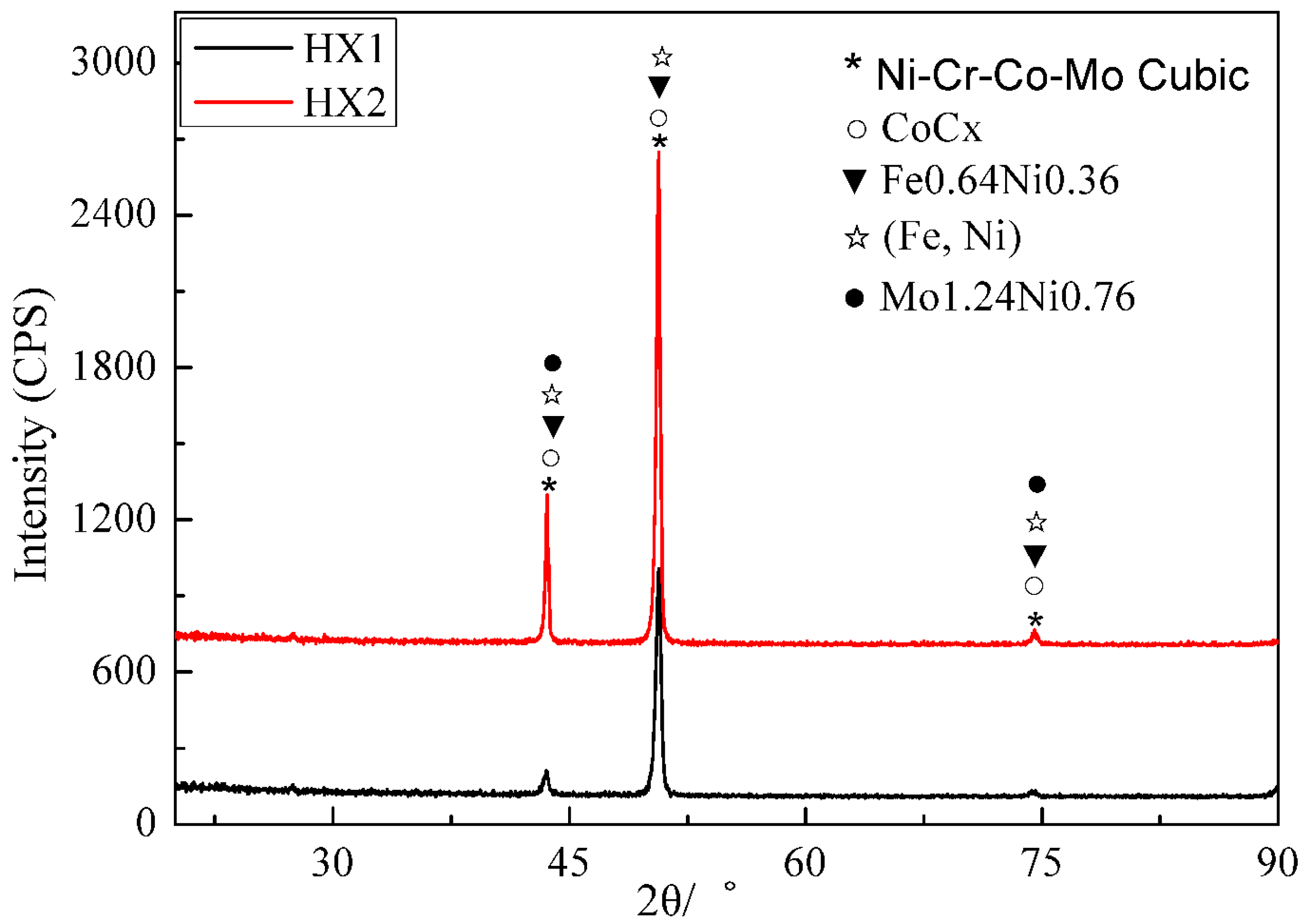
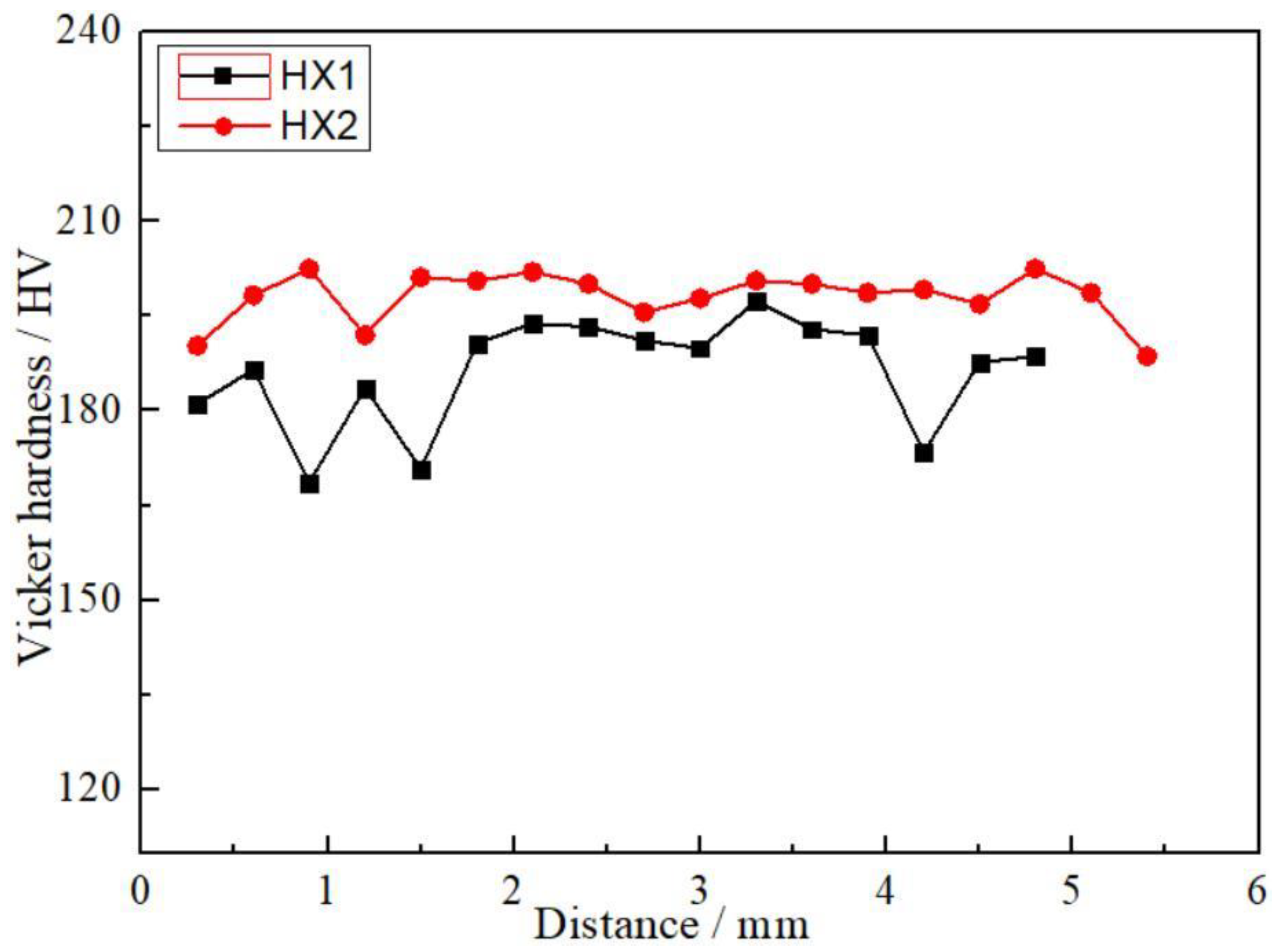




| Elements | Mn | Si | W | Co | Mo | Cr | Fe | Ni |
|---|---|---|---|---|---|---|---|---|
| Q235 | 0.3 | 0.15 | / | / | / | / | Bal. | / |
| HX | 0.247 | 0.264 | 0.201 | 0.875 | 9.16 | 22.34 | 18.15 | Bal. |
| Parameters | Welding Voltage (V) | Welding Current (A) | Welding Speed (cm/min) | Welding Torch Height (mm) | The Plasma Gas Flow (L/min) |
|---|---|---|---|---|---|
| Value | 20.1 | 120 | 24 | 7 | 2.5 |
| Elements | Ni | Cr | Fe | Mo | Co | W | Si |
|---|---|---|---|---|---|---|---|
| Coating HX1 | 42.21 | 18.85 | 31.85 | 4.43 | 1.28 | 0.15 | 1.23 |
| Coating HX2 | 44.37 | 19.95 | 28.53 | 4.81 | 2.02 | 0.16 | 0.90 |
| Coating | Ecorr (VSCE) | Icorr (μA/cm2) |
|---|---|---|
| HX1 | −0.12468 | 4.0998 × 10−7 |
| HX2 | −0.13313 | 9.3562 × 10−8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Wang, X.; Shen, X.; Fu, F.; Tan, Z. Wear and Corrosion Resistance of CoCrFeNiSiMoW Medium-Entropy Alloy Coatings on Q235 Steel. Coatings 2021, 11, 1053. https://doi.org/10.3390/coatings11091053
Hu Q, Wang X, Shen X, Fu F, Tan Z. Wear and Corrosion Resistance of CoCrFeNiSiMoW Medium-Entropy Alloy Coatings on Q235 Steel. Coatings. 2021; 11(9):1053. https://doi.org/10.3390/coatings11091053
Chicago/Turabian StyleHu, Qingxian, Xiaoli Wang, Xinwang Shen, Fanglian Fu, and Zemin Tan. 2021. "Wear and Corrosion Resistance of CoCrFeNiSiMoW Medium-Entropy Alloy Coatings on Q235 Steel" Coatings 11, no. 9: 1053. https://doi.org/10.3390/coatings11091053
APA StyleHu, Q., Wang, X., Shen, X., Fu, F., & Tan, Z. (2021). Wear and Corrosion Resistance of CoCrFeNiSiMoW Medium-Entropy Alloy Coatings on Q235 Steel. Coatings, 11(9), 1053. https://doi.org/10.3390/coatings11091053






