The Influence of Organically Modified Derivatives of Silica on the Structure and the Wetting Angle Values of Silica Coatings
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
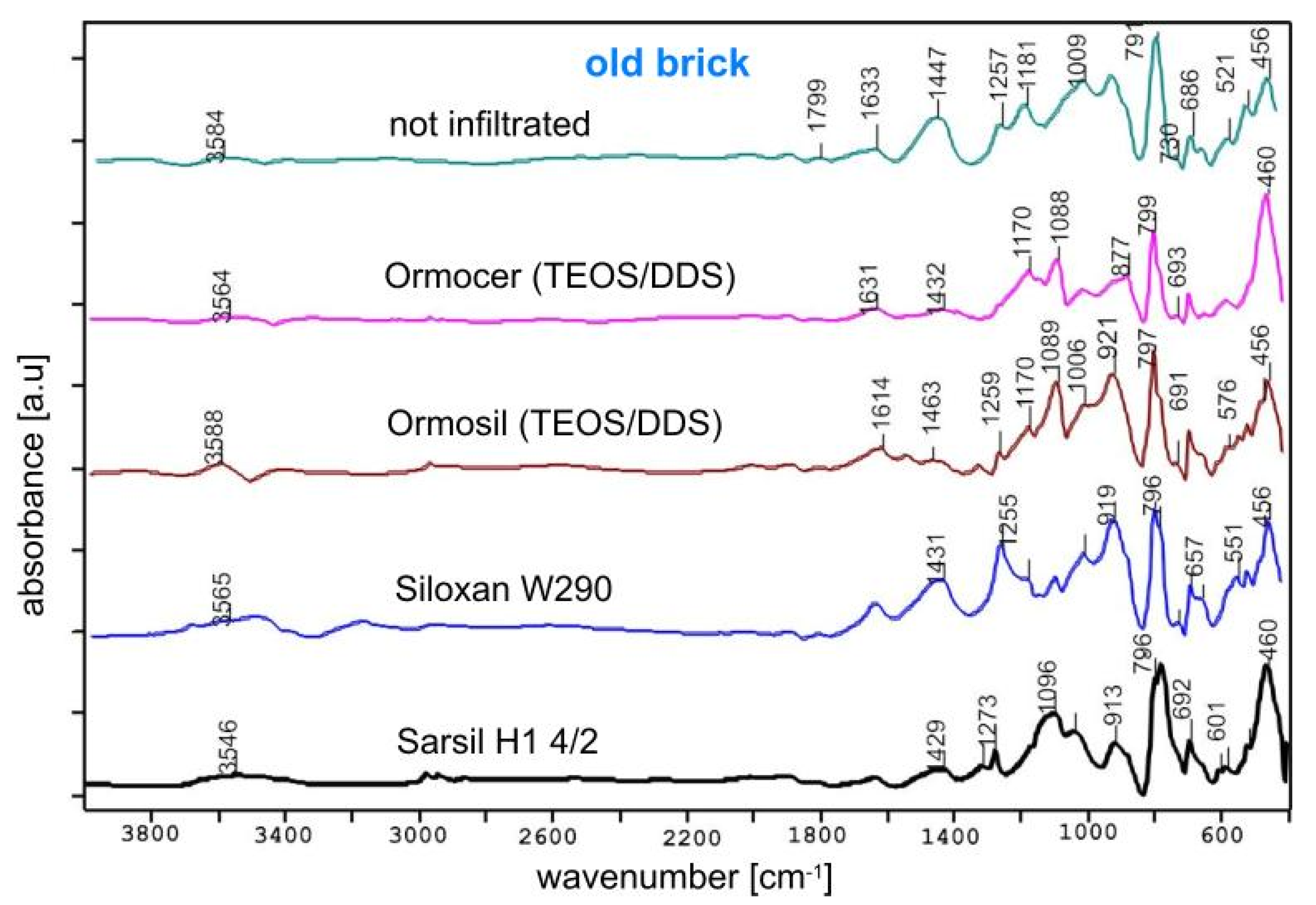
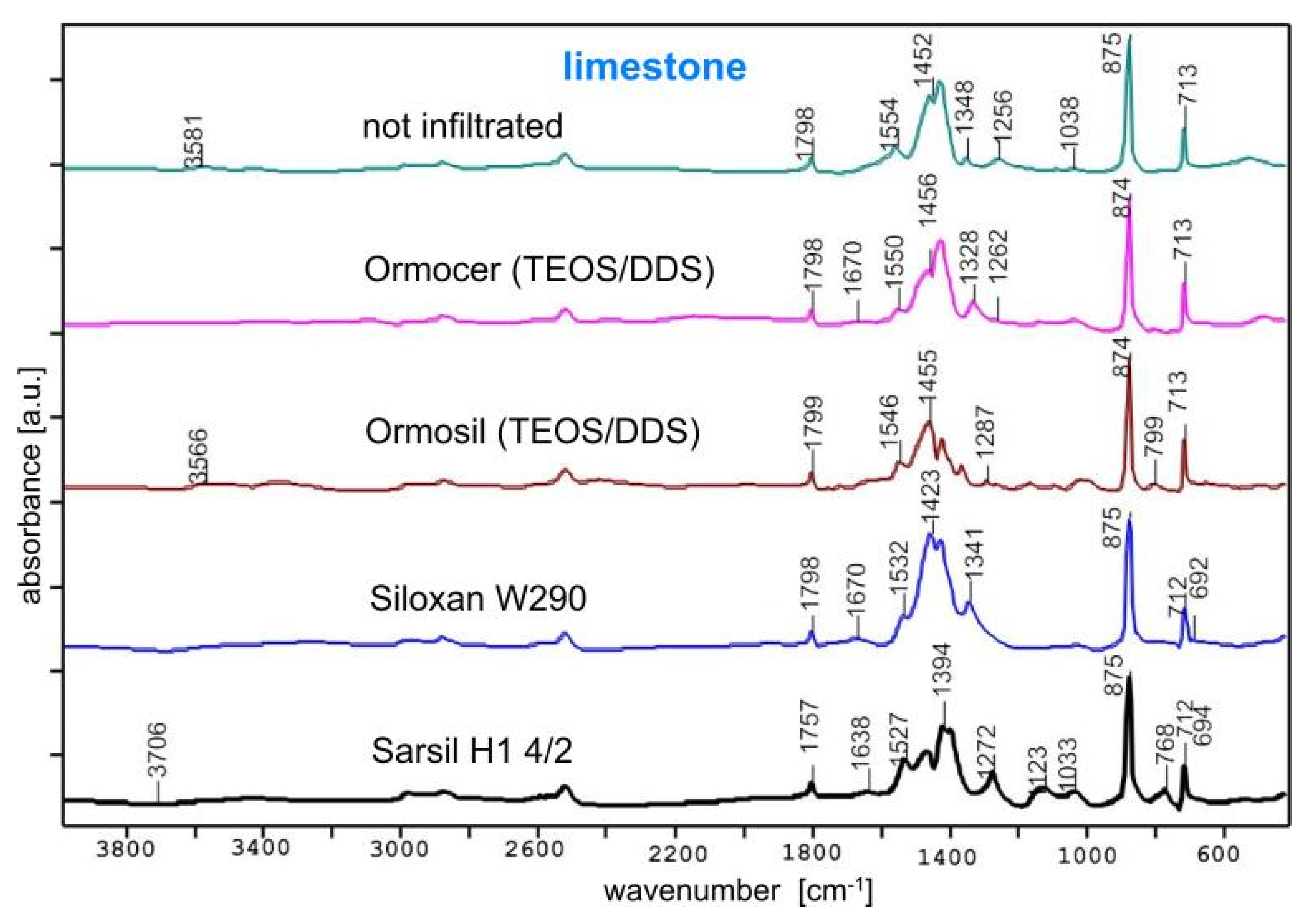
3.1. FTIR Studies of Samples
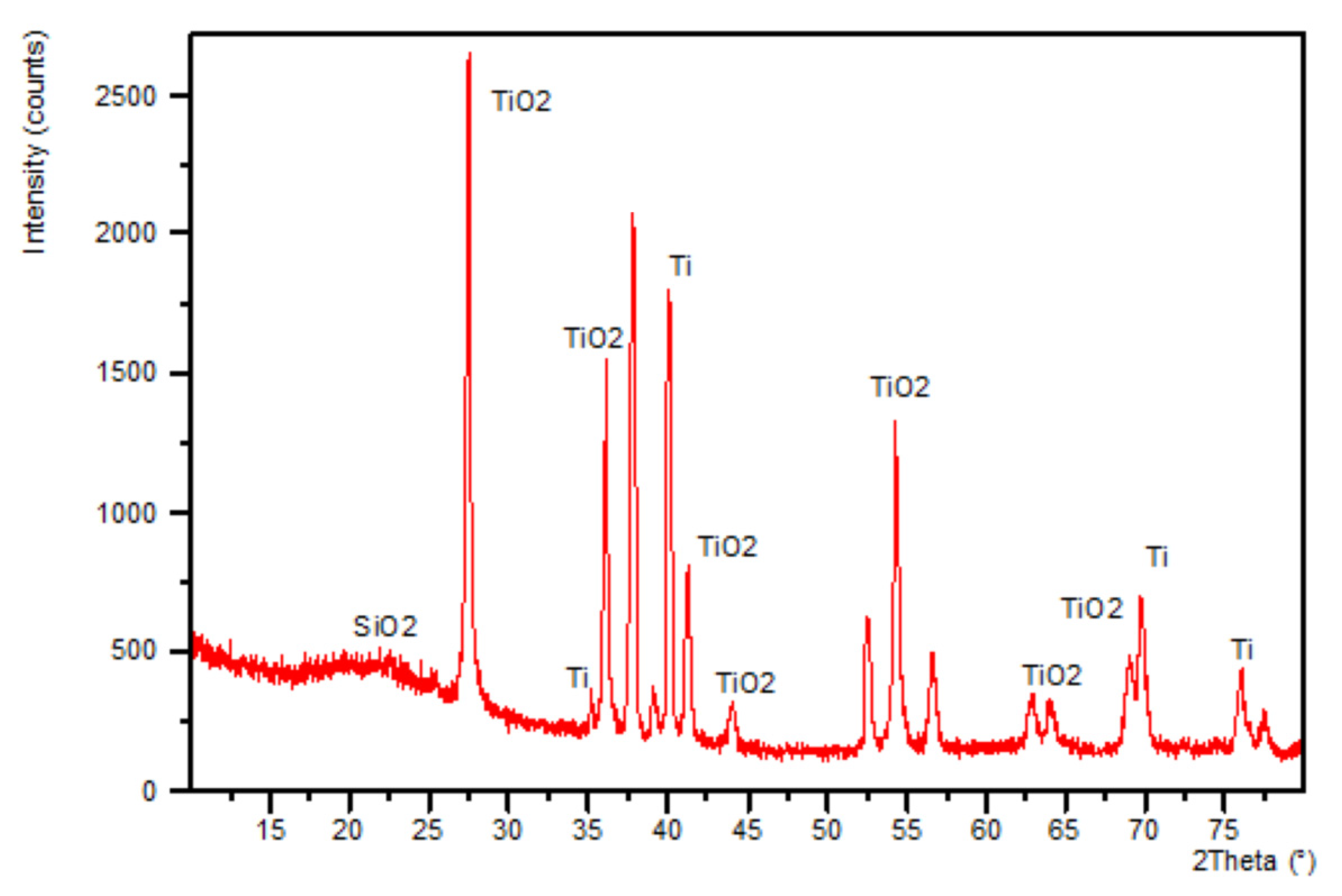
3.2. XRD Studies of Samples
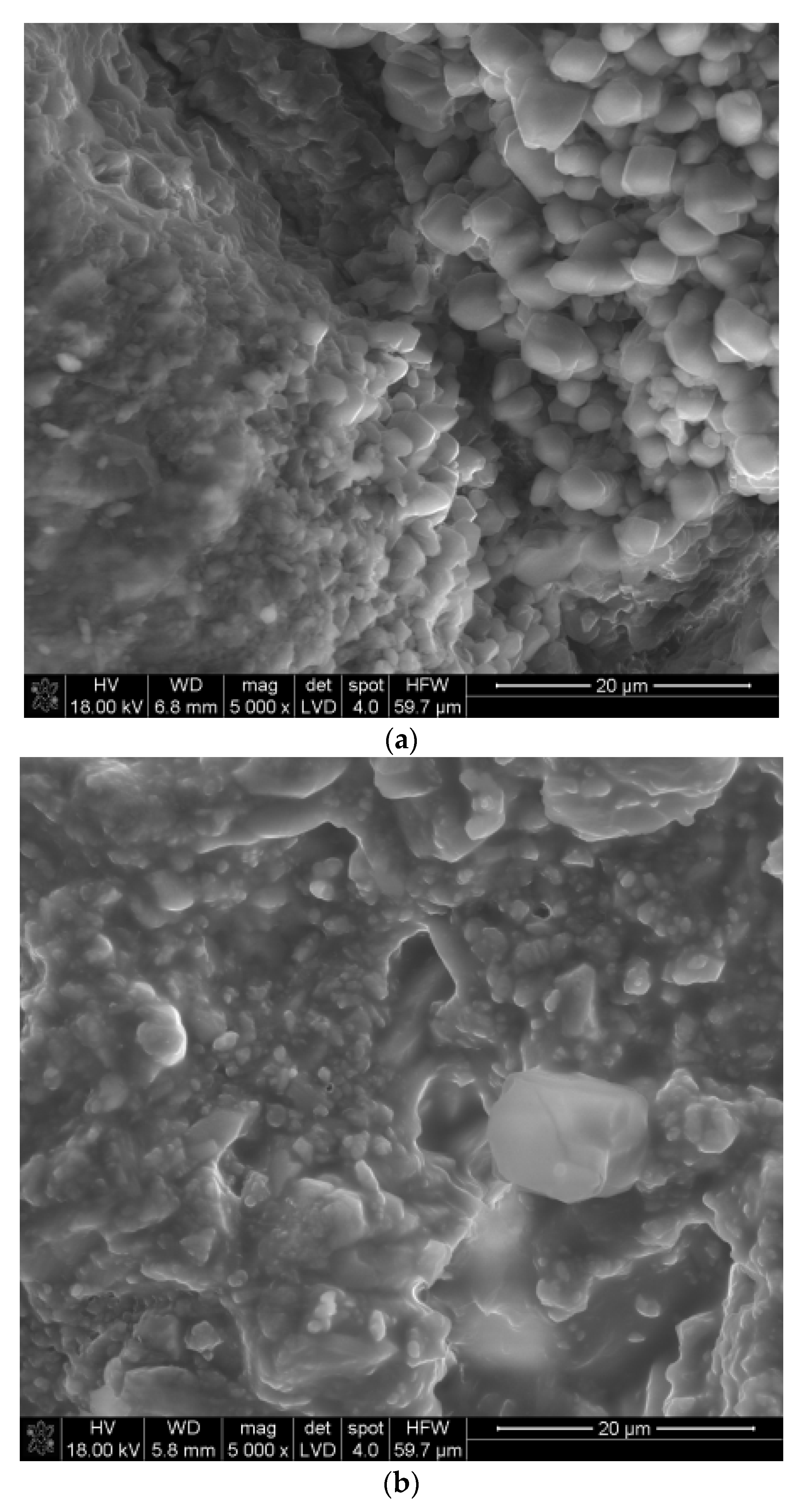
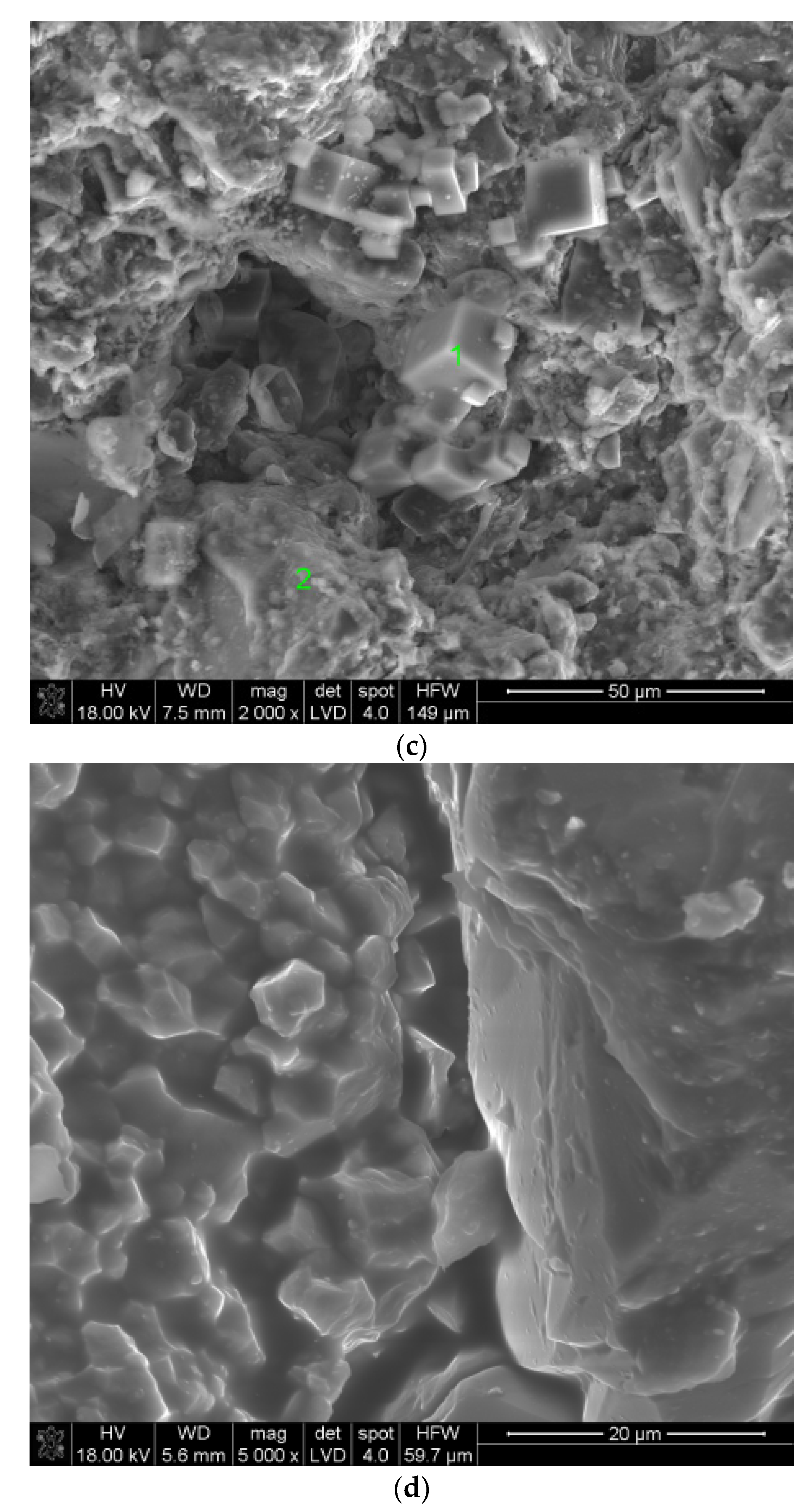
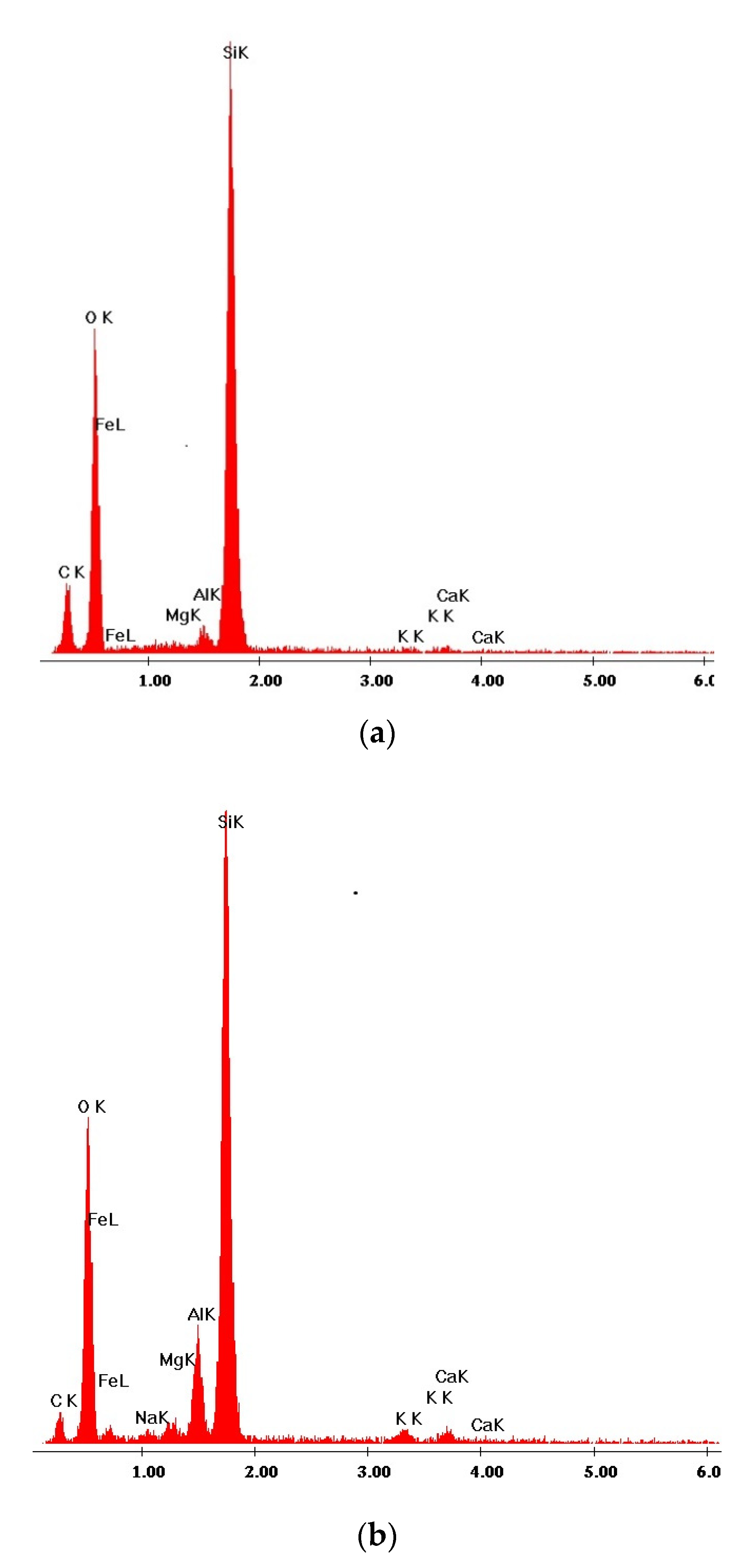
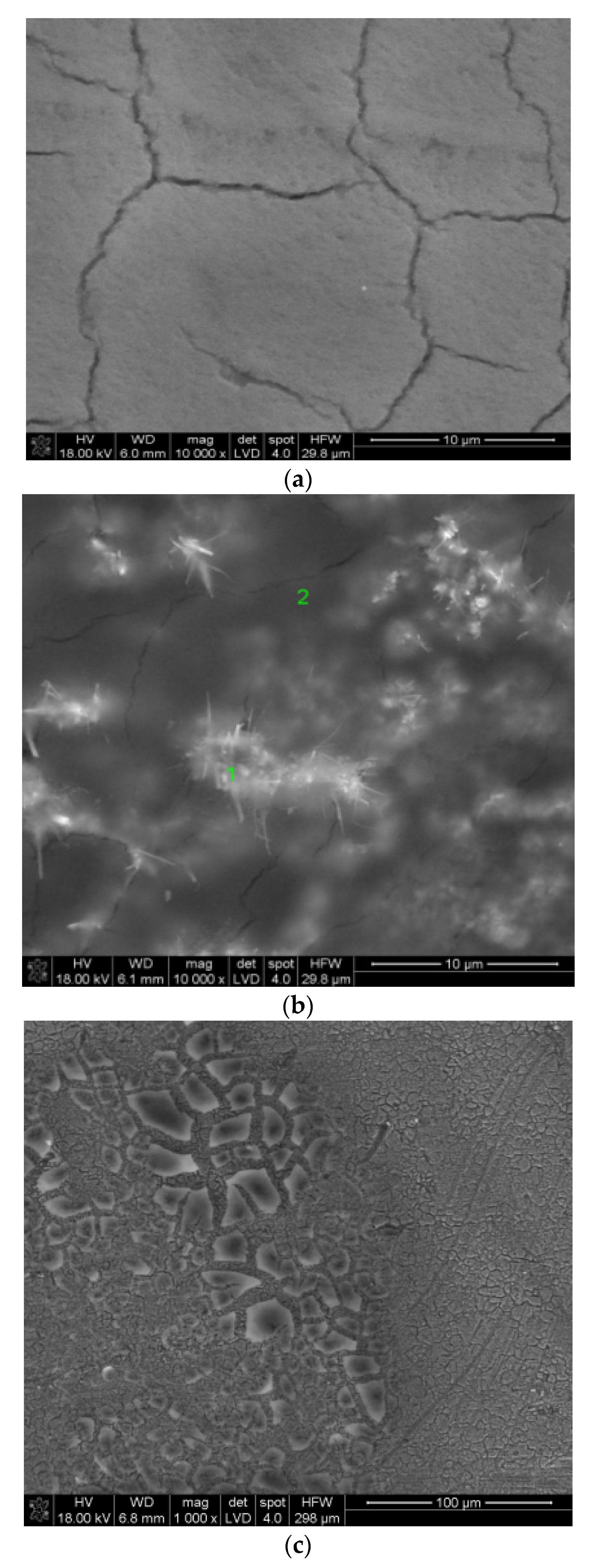
3.3. SEM Studies of Samples
3.4. AFM Studies of Samples
3.5. Wetting Angle Measurements
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Viles, H.A. Acid corrosion (of stone and metal). In Environmental Geology. Encyclopedia of Earth Science; Springer: Dordrecht, The Netherlands, 1999. [Google Scholar] [CrossRef]
- Harris, J.E. Weathering of rock, corrosion of stone and rusting of iron. Meccanica 1992, 27, 233–250. [Google Scholar] [CrossRef]
- Manning, M. Corrosion of building materials due to atmospheric pollution in the United Kingdom. In Air Pollution, Acid Rain and the Environment; Mellanby, K., Ed.; Watt Committee Report No. 18; Elsevier: London, UK, 1988; pp. 37–66. [Google Scholar]
- Skoulikidis, T.N. Atmospheric corrosion of concrete reinforcements, limestones, and marbles. In Atmospheric Corrosion; Ailor, W.H., Ed.; Wiley Interscience: New York, NY, USA, 1982; pp. 807–825. [Google Scholar]
- Duhan, S.; Tomer, V.K.; Sharma, A.K.; Dehiya, B.S. Development and properties study of microstructure silver-doped silica nanocomposites by chemical process. J. Alloys Compd. 2014, 583, 550–553. [Google Scholar] [CrossRef]
- Günter, S.; Budak, S.; Gibson, B. Optical properties of Ag nanoclusters formed by irradiation and annealing of SiO2/SiO2/Ag thin films. App. Surf. Sci. 2014, 310, 180–183. [Google Scholar] [CrossRef]
- Lien, S.-Y.; Wuu, D.-S. Tri-layer antireflection coatings (SiO2/SiO2-TiO2-TiO2) for silicon solar cells using a sol-gel technique. Energy Mater. Solar Cells 2006, 90, 2710–2719. [Google Scholar] [CrossRef]
- Abd Aziz, R.; Sopyan, I. Synthesis of TiO2-SiO2 powder and thin film photocatalysts by sol-gel method. Indian J. Chem. 2009, 48A, 951–957. [Google Scholar]
- Suciu, R.C.; Indrea, E.; Silipas, T.D.; Dreve, S.; Rosu, M.C.; Popescu, V.; Popescu, G.; Nascu, H.I. TiO2 thin films prepared by sol-gel method. J. Phys. Conf. Ser. 2009, 182, 012080. [Google Scholar] [CrossRef]
- Somoghi, R.; Pulcar, V.; Alexandrescu, E.; Gifu, I.C.; Ninciuleanu, C.M.; Cotrut, C.M.; Oancea, F.; Stroescu, H. Synthesis of zinc oxide nanomaterials via sol-gel process with anti-corrosive effect for Cu, Al and Zn metallic substrates. Coatings 2021, 11, 444. [Google Scholar] [CrossRef]
- Gomez, M.; Palza, H.; Quijada, R. Influence of organically-modified montmorillonite and synthesized layered silica nanoparticles on the properties of polypropylene and polyamide-6 nanocomposites. Polymers 2016, 8, 386. [Google Scholar] [CrossRef] [PubMed]
- Nadachowski, F.; Jonas, S.; Ptak, W. Wstęp do Projektowania Technologii Ceramicznych; Uczelniane Wydawnictwa Naukowo-Dydaktyczne: Kraków, Poland, 1999. [Google Scholar]
- Ahmad, D.; van den Boogaert, I.; Miller, J.; Presswell, R.; Jouhara, H. Hydrophilic and hydrophobic materials and their applications. Energy Sources Part A Recover. Util. Environ. Eff. 2018, 40, 2686–2725. [Google Scholar] [CrossRef]
- Pandey, P.C.; Upadhyay, S.; Upadhyay, B.C.; Tripathi, V.S. Studies on new ormosils derived from reactive alkoxysilane precursors as a function of hydrophobicity/hydrophilicity. J. Sol-Gel Sci. Technol. 2005, 33, 25–32. [Google Scholar] [CrossRef]
- Długoń, E.; Adamczyk, A. Personal Communication; AGH University of Science and Technology: Kraków, Poland, 2014. [Google Scholar]
- Zhang, Y.; Wu, D.; Sun, Y. Sol-gel of methyl modified optical silica coatings and gels from DDS and TEOS. J. Sol-Gel Sci. Technol. 2005, 33, 19–24. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Zhang, Y.; Shen, S.; Ge, S.; Ling, L. Synthesis of nanometre silicon carbide whiskers from binary carbonaceous silica aerogels. Carbon 2001, 39, 159–165. [Google Scholar] [CrossRef]
- Sakka, S.; Kammiya, K. The sol-gel transition in the hydrolysis of metal alkoxides in relation to the formation of glass fibers and films. J. Non-Cryst. Solids 1982, 48, 31–46. [Google Scholar] [CrossRef]
- Sakka, S.; Yoko, Y. Fibers from gels. J. Non-Cryst. Solids 1992, 147–148, 394–402. [Google Scholar] [CrossRef]
- Available online: https://silikonowe.com.pl/pl/SARSIL-H-142-koncentrat-1-5-0-8-kg (accessed on 1 August 2021).
- Available online: https://www.impregnaty4you.pl/sarsil-h142-5l-impregnat-do-betonu-kamienia-i-ceramiki-koncentrat-p-46.html (accessed on 1 August 2021).
- Available online: http://www.invex.de/Fassadenschutz/siloxan_w_290.htm (accessed on 1 August 2021).
- Available online: http://www.mgdistribution.pl/produkty_coverax_impregnaty.html (accessed on 1 August 2021).
- Lenza, R.F.S.; Nunesb, E.H.M.; Vasconcelosb, D.C.L.; Vasconcelos, W.L. Preparation of sol- gel silica samples modified with drying control chemical additives. J. Non-Cryst. Solids 2015, 423–424, 35–40. [Google Scholar] [CrossRef]
- Adamczyk, A.; Rokita, M. The structural studies of Ag containing TiO2-SiO2 gels and thin films deposited on steel. J. Mol. Str. 2016, 1114, 171–180. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Theory and Applications in Inorganic Chemistry; Willey & Sons, Inc.: New York, NY, USA; London, UK, 2008. [Google Scholar]
- Darmawan, A.; Eka Saputra, R.; Astuti, Y. Structural, thermal and surface properties of sticky hydrophobic silica films: Effect of hydrophilic and hydrophobic precursor compositions. Chem. Phys. Lett. 2020, 761, 138076. [Google Scholar] [CrossRef]
- Monroe, J.; Barry, M.; DeStefano, A.; Gokturk, P.A.; Jiao, S.; Robinson-Brown, D.; Webber, T.; Crumlin, E.J.; Han, S.; Shell, M.S. Water structure and properties at hydrophilic and hydrophobic surfaces. Annu. Rev. Chem. Biomol. Eng. 2020, 11, 523–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]




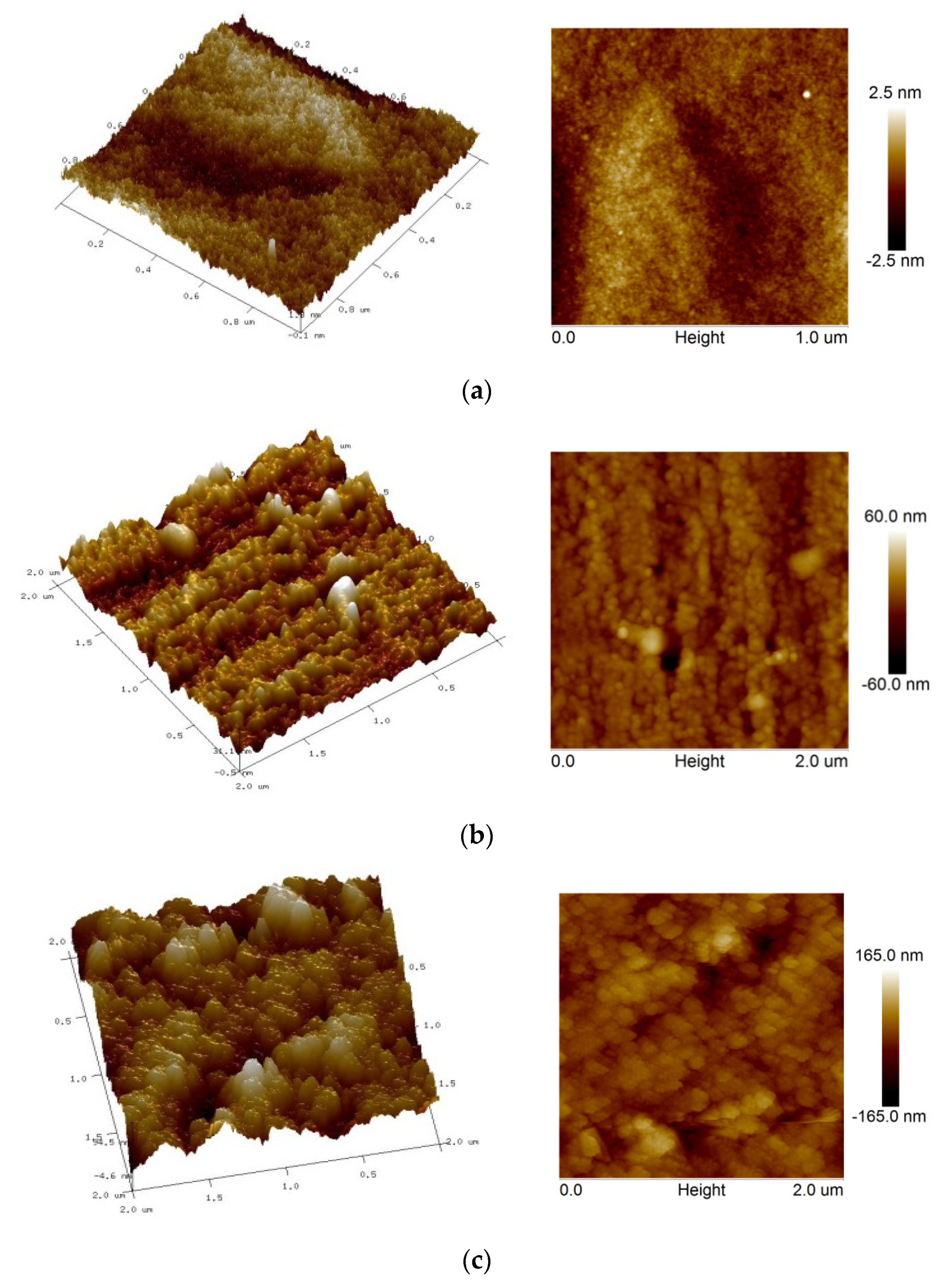
| No. | Sample/SiO2 Precursor | Substrate | Rmax (nm) | Ra (nm) |
|---|---|---|---|---|
| 1 | TEOS (14% SiO2) | steel | 279 | 21.1 |
| 2 | TEOS (10% SiO2) | steel | 152 | 20.3 |
| 3 | TEOS (5% SiO2) | steel | 98 | 7.96 |
| 4 | TEOS/DDS | steel | 268 | 20.7 |
| 5 | AerosilTM | steel | 226 | 19.1 |
| 6 | AerosilTM | titanium | 161 | 17 |
| 7 | - | steel | 53.4 | 6.15 |
| 8 | - | steel | 70.9 | 10.2 |
| Sol/Preparation | Substrate | Average Value of Wetting Angle (°) |
|---|---|---|
| SILOXAN W 290 | limestone | 118.1 |
| SARSIL H/14/2 | limestone | 108.9 |
| ORMOCER V | limestone | 112.2 |
| ORMOCER IV | limestone | 115.8 |
| Sol/Preparation | Substrate | Average Value of Wetting Angle (°) |
|---|---|---|
| SILOXAN W 290 | sandstone I | 107.4 |
| SARSIL H/14/2 | sandstone I | 112.5 |
| ORMOCER V | sandstone I | 120.2 |
| ORMOCER IV | sandstone I | 118.0 |
| Sol/Preparation | Substrate | Average Value of Wetting Angle (°) |
|---|---|---|
| SILOXAN W 290 | old brick | 137.4 |
| SARSIL H/14/2 | old brick | 125.2 |
| ORMOCER V | old brick | 131.9 |
| ORMOCER IV | old brick | 144.6 |
| Sol | Substrate | Average Value of Wetting Angle (°) |
|---|---|---|
| - | pure steel | 89.94 |
| - | pure titanium | 65.74 |
| TEOS (5% SiO2) | steel | 53.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk, A. The Influence of Organically Modified Derivatives of Silica on the Structure and the Wetting Angle Values of Silica Coatings. Coatings 2021, 11, 1058. https://doi.org/10.3390/coatings11091058
Adamczyk A. The Influence of Organically Modified Derivatives of Silica on the Structure and the Wetting Angle Values of Silica Coatings. Coatings. 2021; 11(9):1058. https://doi.org/10.3390/coatings11091058
Chicago/Turabian StyleAdamczyk, Anna. 2021. "The Influence of Organically Modified Derivatives of Silica on the Structure and the Wetting Angle Values of Silica Coatings" Coatings 11, no. 9: 1058. https://doi.org/10.3390/coatings11091058







