3.1. Fabrication of Ni60A Alloy on Copper Pipe
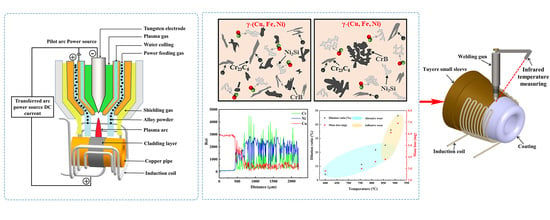
Figure 3a shows the schematic diagram of the cladding system. To reduce the heat loss of the plasma arc on the surface of the copper pipe, an induction preheating system was introduced, which can preheat the copper pipe to the required temperature in a few minutes. The induction preheating system included an induction power supply, an infrared thermometer (CTlaser MT, Optris, Germany) and a proportional integration differentiation (PID) controller. To ensure the accuracy of temperature measurement, the parameter of emissivity needed to be set to the emissivity of copper (the value is 0.8). The surface temperature of the cladding zone measured by the infrared thermometer was fed back to the PID controller, so as to achieve the dynamic adjustment of heating power. Most importantly, the infrared thermometer was fixed on the bracket of the plasma welding gun, which can ensure that the temperature measurement point and the cladding point were always synchronized. The temperature curve of the cladding zone during the cladding process was recorded by a computer.
To improve the efficiency of the plasma cladding, a program of swing arc was used, as shown in
Figure 3b. That is, the copper pipe kept a high-speed rotation, while the plasma welding gun swung back and forth perpendicular to the direction of rotation. The magnified view of the swing arc process is shown in
Figure 3c, where
Y is the direction of the swing arc, and
X is the direction of the rotation direction. The actual cladding path can be seen as a “Z” shape. During the cladding process, the perfect overlap can only be achieved by matching the rotation speed of the copper pipe and the swing speed of the welding gun. The preliminarily optimized cladding process parameters are shown in
Table 2. Among them, the preheating temperature was obtained based on the previous experimental results on the copper plate (
Figure S1 in the Supplementary Materials). The basic principle of process parameter optimization is to ensure that the powder can be completely melted and fused with the copper substrate. Under these process parameters, the cladding efficiency of the coating (
Figure 2b) can reach 32.72 mm
2/s, which is about 2.7 times higher than that of a single-track cladding of copper pipe (12 mm
2/s). The detailed calculation method of cladding efficiency is shown in the
Section 4 of the
Supplementary Material.
3.2. Microstructure Analysis
Figure 4 shows the variation trend of the temperature, height, bath depth, and dilution ratio of the coating from S1 to S6. The dilution ratio of the coating was calculated according to the formula in reference [
23]. As shown in
Figure 4, the surface temperature of the cladding zone of the copper pipe increases continuously with the progress of the cladding. The height of coatings shows a decreasing trend, while the bath depth and dilution ratio gradually increased from S1 to S6. This indicates that the coating appears inconsistent at the head and tail even if the process parameters are constant. The heat balance of the substrate surface can explain this inconsistency. Actually, the surface temperature of the cladding zone of copper pipe depends on the balance of heat input and output. The input heat mainly comes from the induction heating system and plasma arc, and the output heat is mainly the heat dissipation of the copper pipe. Theoretically, the surface temperature is constant under the condition of fixed process parameters. However, the plasma arc continuously heated the copper pipe, while the heat cannot be dissipated in time during the cladding process. Therefore, the original heat balance on the surface of the cladding zone was broken, resulting in a continuous increase in the surface temperature. The higher temperature corresponds to the larger proportion of melted copper, leading to an increase in the bath depth. In thermodynamics, the surface tension of the melt exhibits a negative temperature coefficient [
23]. The higher temperature results in the smaller surface tension, the molten pool can be easier to spread to the edge, thus increasing the dilution ratio of coating.
Figure 5 shows the SEM images of microstructure of the coatings from S1 to S6. It can be seen that the coatings have no defects such as surface cracks or pores and the top, middle and bottom areas of the coating always show different microstructures. A large number of fine and uniform black granular phases and gray acicular phases are distributed on the top and bottom of the coating. Compared with the top and the bottom part, the phases in the middle are significantly larger in size. This phenomenon is related to the temperature gradient of the coating. During the cladding process, the top of the coating was cooled by the airflow and had a large temperature gradient. Meanwhile, the bottom also endured a large temperature gradient due to the high thermal conductivity of the copper substrate. Therefore, the solidification time of the top and bottom of the coating was shorter than that of the middle part. The precipitated phase in the middle of the coating had enough time to grow up, resulting in a larger size.
The magnified SEM micrograph corresponding to the area marked by a small rectangle in
Figure 5c is shown in
Figure 6a. It can be found that the microstructure mainly includes four phases: black blocky phases (indicated by Spot 1), dark gray strip-shaped phases (indicated by Spot 2), light gray dendritic phases (indicated by Spot 3) and a light gray matrix (indicated by Spot 4). The EDS analysis results of these points are shown in
Figure 6b–e, and the atomic percentage of each marked point is listed in
Table 3.
The XRD pattern of the Ni60A coating is shown in
Figure 7a. According to the PDF comparison results of diffraction peaks, the phase constituents of Ni60A coating include CrB, Cr
23C
6, Ni
3Si and γ-(Cu, Fe, Ni). Combined with the results of EDS analysis in
Figure 6, the black blocky phase can be identified as CrB and the dark gray phase as Cr
23C
6. They are the primary reinforcements of the coating. The light gray dendritic phase is Ni
3Si, and the light gray matrix is γ-(Cu, Fe, Ni). Due to the dissolution of Cu, the diffraction peak of γ-(Cu, Fe, Ni) shifts to a small angle and the diffraction peak intensity becomes higher from S1 to S6.
Figure 7b shows the X-ray diffraction spectrum of the S2. There is no difference in the phase types of the top, middle and bottom of the coating. The diffraction peak of bottom is shifted to the left relative to that of the middle and top, which may be due to the higher copper content at the bottom of the coating.
The principle diagram of the coating solidification process is shown in
Figure 8. The melting point of CrB (2760 °C) is higher than that of Cr
23C
6 (1520 °C), so it firstly precipitates form the molten pool during the plasma cladding process. Due to the incompatibility of Cu and Cr, Cu atoms were repelled into the remaining liquid phase (
Figure 8a). As the temperature of the molten pool further decreases, Cr
23C
6 becomes needle-like phase precipitation. Subsequently, the atom of Si and Ni formed Ni
3Si and then the γ-(Cu, Fe, Ni) solid solution formed in the remaining liquid phase (
Figure 8b). However, from
Figure 5, it can also be seen that the CrB phases have a slight tendency to grow up and reunite with the progress of the cladding. Meanwhile, the area of the carbide phase has a significant change during the solid solution process. The statistical results in
Figure 9 prove that the area fraction of Cr
23C
6 has a decreasing trend (The area fraction was studied from 10 randomly selected fields in the middle of each sample). This is mainly related to the longer liquid time under the high surface temperature of cladding zone. In the longer liquid time, the CrB more easily adsorbed a large amount of Cr atom, which not only results in the growth and agglomeration of CrB (
Figure 8c) but also leads to a certain decrease in the area fraction of Cr
23C
6 (
Figure 8d).
Figure 10 shows the EDS line scan results along the copper substrate to the top of the coating (S1–S6). The distribution of several main elements (Cu, Ni and Cr) is specially displayed. It can be seen that there is an interface transition zone between the coating and the substrate. The Cu element presents a gradient decrease at the interface transition zone, while the content of Cu element inside the coating is basically the same. The content change of the Ni element is opposite that of the Cu element. The content of Cr element fluctuates in the transition zone, which is related to the dispersion distribution of CrB and Cr
23C
6 precipitates. Therefore, the coating prepared by plasma cladding can be regarded as an in situ synthesized gradient coating. With the increase of cladding temperature, the copper content in the coating and the range of the interface transition zone has an increasing trend. Especially for S5 and S6, the copper content and its distribution have the most significant changes. Because of the good compatibility of copper and Ni-based alloys, the molten copper and Ni60A alloy can be fully mixed. At higher cladding temperatures, the molten pool has a lower viscosity, which leads to a stronger stirring effect of the molten pool, thus resulting in more copper distribution in the coating [
24]. In addition, due to the dilution effect of copper, the composition of the entire coating is changed. Therefore, the coating prepared by the cladding method cannot be simply regarded as a pure alloy, but a composite material of Ni60A and copper. For a high-quality coating, there should be no large sudden change in composition. Therefore, further optimization of process parameters is required to control the coating at least between S1–S4.
3.3. Microhardness
Figure 11 gives the microhardness variation tendency of the coatings (S1–S6) along the direction of cross-section depth. The hardness value inside the coating (about 840 HV–450 HV) is significantly higher than that of the copper substrate (about 65 HV), which mainly attributed to the presence of precipitated phases such as CrB, Cr
23C
6, Ni
3Si and γ-(Cu, Fe, Ni) phase in the coating (
Figure 6). They increase the hardness of the coating under the influence of dispersion strengthening of CrB and Cr
23C
6 phases and the solid-solution strengthening of γ-(Cu, Fe, Ni) phase. Moreover, Ni
3Si also has the effect by hindering the dislocation motion [
25]. The hardness value near the copper substrate shows a gradient decline trend, which is basically consistent with the change trend of copper content. From S1 to S6, the hardness of the coating gradually decreased. Compared with the S1, the average hardness of the S6 coating is reduced by about 1.5 times, attributed to the increase of the dilution ratio. In addition, due to the low dilution ratio of S1, the hardness transition zone is not obvious. From S2 to S6, the range of the hardness transition zone gradually increases, which corresponds to the gradient change distance of the Cu element in each sample (
Figure 10).
3.4. Wear Resistance
Figure 12a shows the friction coefficient of the pure copper and Ni60A coatings (S1–S6). Obviously, the average friction coefficient of pure copper (0.264) is higher than that of the coatings. Among all samples, S5 and S6 show higher friction coefficients, while the friction coefficients of other samples are basically the same. Generally, a material with a low coefficient of friction indicates better wear resistance. The wear resistance of the coating is better than that of pure copper mainly due to the good bearing capacity of carbide- and boride-reinforced particles in the coating. These hard particles can reduce the contact area between the friction surface and its surface, thus reducing the smearing effect [
26].
Figure 12b shows the mass loss of the pure copper and Ni60A coatings (S1–S6). It can be seen intuitively that the mass loss of the coatings is greatly reduced compared to the copper substrate. Among all samples, the wear resistance of S1 is about 4.5 times that of the copper substrate. As the cladding progresses, the mass loss is slightly reduced from S1 to S4, while of the S5 and S6 has a significant in the mass loss. Generally, under the same surface roughness, the small amount of wear of the coating is mainly due to its higher hardness. The mass loss of material is directly proportional to the coefficient friction and inversely proportional to the hardness of the material [
27]. From the head to the tail of coating, the higher the dilution ratio, the closer the performance of the coating is to pure copper.
Figure 13 shows the SEM morphologies from worn surfaces of Ni60 cladding (S1–S6). It can be seen from
Figure 13a–c that there is no spalling or adhesion phenomenon on the surfaces of the S1–S3 coatings, only some grooves formed by plowing, which is a typical abrasive wear. As shown in
Figure 13d, some spalling pits appear on the surface of the S4 coating, and grooves can still be found on its surface. This indicates that the wear mechanism of S4 coating is abrasive wear and adhesive wear. However, the S5 and S6 coatings suffer a severe adhesive wear due to the large number of spalling pits on the surface. Combined with
Figure 11, the increase in mass loss of coating mainly depends on the severity of adhesive wear. From the analysis of the hard phase of the coating, the change of the wear mechanism is related to the decrease of Cr
23C
6.
3.5. Regulation Mechanism of Plasma Cladding Process
As shown in
Figure 14, the fundamental reason for the evolution of dilution ratio and properties is the change in the surface temperature of the cladding zone on copper pipe. A surface temperature of 850 °C can be regarded as the critical temperature for a sudden change of properties. Before and after this temperature, the wear mechanism of the coating completes the transition from abrasive wear to abrasive wear and adhesive wear to adhesive wear, thus significantly reducing the wear resistance. Therefore, controlling the surface temperature of the cladding zone below 850 °C can basically maintain the good quality of the coating.
In addition, as discussed in
Section 3.1, the temperature of the cladding zone (
T) is directly related to the heat balance on the surface of the copper pipe, which can be expressed as:
where the
Q1 is the heat provided by induction heating, which is related to the heating power (
P), denoted as
f (
P).
Q2 is the heat provided by the plasma arc, which is related to the cladding current (
I) and the rotation speed of the copper pipe (
v, corresponding to the residence time of the plasma arc on the copper pipe), denoted as
f (
I,
v).
Q3 is the heat dissipation of the copper pipe, which is related to the volume (
V) and the thermal conductivity (λ) of the copper pipe, denoted as
f (
V,
λ). During the cladding process, the plasma arc continuously heats the copper pipe, causing the heat input to be greater than the heat output, resulting in a continuous increase in the temperature of the cladding zone (T). To ensure a basically constant temperature to make the coating as uniform as possible, the heat input of the copper pipe must be gradually reduced during the cladding process, thus achieving a balance with heat dissipation. As a process before cladding, the heat input of the preheating system (
Q1) cannot be adjusted during the cladding process. Therefore, the only way to reduce the total heat input is to reduce the heat input of the plasma arc (
Q2). However, adjusting the rotation speed of the copper pipe (
v) during the cladding process is inconvenient, because the rotation speed needs to be matched with the swing speed of the welding gun. As a result, gradually reducing the cladding current (
I) during the cladding process become the only way to make the coating as uniform as possible and further improve the quality of the coating, which can be easily achieved in the current numerical control plasma cladding system. As shown in
Figure 15, by adjusting the cladding current in stages to control the surface temperature of the cladding zone in the range of 600–850 °C, the dilution rate and mass loss of the cladding layer have little fluctuation as a whole.
For the actual tuyere small sleeve with large-complex-curved shape, according to the above discussion, reducing the current in stages to keep the surface temperature of the tuyere small sleeve in the range of 600–850 °C is the key to obtain a high-quality coating. Furthermore, based on the cladding on copper pipe, it is first necessary to increase the size of the induction heating coil (
Figure 16) and the power of the induction power supply to ensure that the small sleeve can be heated to the required preheating temperature (600 °C).