Development of Chitosan/Squid Skin Gelatin Hydrolysate Films: Structural, Physical, Antioxidant, and Antifungal Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Material and Reagents
2.2. Gelatin Extraction and Preparation of Hydrolysates (SGH)
2.3. Preparation of Chitosan/SGH Films
2.4. Characterization of the Properties of the Films
2.4.1. Optical Properties, SEM, and AFM
2.4.2. FT-IR and 1H NMR
2.4.3. Thickness, Mechanical Properties and Enzymatic Degradation
2.5. Antioxidant Activity
2.6. Antifungal Activity
2.7. Data Analysis
3. Results
3.1. Characterisation of Gelatine Hydrolysate
3.2. Properties of Films
3.2.1. Color, SEM, and AFM
3.2.2. FT-IR and 1H NMR
3.2.3. Thickness, Mechanical Properties and Enzymatic Degradation
3.3. Antioxidant and Antifungal Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SGH | squid gelatin hydrolysates |
| CH | films from chitosan |
| CH/SGH | films from chitosan and squid gelatin hydrolysates |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate acid |
| DPPH | 1,1-diphenyl-2-picrylhydrazy |
| AAPH | (2,2′-azobis-(2-amidinopropane) dihydrochloride)) |
| Trolox | (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) |
| ORAC | oxygen radical antioxidant capacity |
References
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compound: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Tharanathan, R.N. Chitin/chitosan-safe, ecofriendly packaging materials with multiple potential uses. Food Rev. Int. 2007, 23, 53–72. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudos, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 4, 840–844. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Gómez-Guillen, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Tkaczewska, J. Peptides and protein hydrolysates as food preservatives and bioactive components of edible films and coatings-A review. Trends Food Sci. Technol. 2020, 106, 298–311. [Google Scholar] [CrossRef]
- Ezquerra-Brauer, J.M.; Aubourg, S.P. Recent trends for the employment of jumbo squid (Dosidicus gigas) by-products as a source of bioactive compounds with nutritional, functional and preservative applications: A review. Int. J. Food Sci. Technol. 2019, 54, 987–998. [Google Scholar] [CrossRef] [Green Version]
- Giménez, B.; Estaca, J.G.; Alemán, A.; Guillén, M.C.G.; Montero, M. Improvement of the antioxidant properties of squid skin gelatin films by the addition of hydrolysates from squid gelatin. Food Hydrocoll. 2009, 23, 1322–1327. [Google Scholar] [CrossRef] [Green Version]
- Nuanmanto, S.; Prodpran, T.; Benjakul, S. Potential use of gelatin hydrolysate as plasticizer in fish myofibrillar protein film. Food Hydrocoll. 2015, 47, 61–68. [Google Scholar] [CrossRef]
- de Morais Lima, M.; Bianchini, D.; Guerrra Dias, A.; da Rosa Zavareze, E.; Prentice, C.; da Silveira Moreira, A. Biodegrdable films based on chitosan, xanthan gum, and fish protein hydrolysate. J. Appl. Polym. Sci. 2017, 134, 44899. [Google Scholar] [CrossRef]
- da Roche, M.; Alemán, A.; Baccan, G.C.; López-Caballero, M.E.; Gómez-Guillen, C.; Montero, P. Anti-inflammatory, antioxidant, and antimicrobial effects of underutilized fish protein hydrolysate. J. Aquat. Food Prod. Technol. 2018, 27, 592–608. [Google Scholar] [CrossRef] [Green Version]
- Marroquín-Cardona, A.G.; Johnson, N.M.; Phillips, T.D.; Hayes, A.W. Mycotoxins in a changing global environment-A review. Food Chem. Toxicol. 2014, 69, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Cota-Arriola, O.; Cortez-Rocha, M.O.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M.; Plascencia-Jatomea, M. Controlled release matrices and micro/nanoparticles of chitosan with antimicrobial potential: Development of new strategies for microbial control in agriculture. J. Sci. Food Agric. 2013, 93, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Pei, J.; Zhu, S.; Song, Y.; Xiong, X.; Xue, F. Development of chitosan/peptide films: Physical, antibacterial and antioxidant properties. Coatings 2020, 10, 1193. [Google Scholar] [CrossRef]
- Cuevas-Acuña, D.A.; Robles-Sánchez, R.M.; Torres-Arreola, W.; Márquez-Ríos, E.; Ezquerra-Brauer, J.M. Collagen from jumbo squid fin: Extracting conditions and influence of the protease system on collagen hydrolysate antioxidant activity. CyTA-J. Food 2016, 14, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Adler-Nissen, J. Enzymatic Hydrolysis of Food Proteins, 1st ed.; Adler-Nisse, J., Ed.; Elsevier: New York, NY, USA, 1986; pp. 9–131. [Google Scholar]
- Vazquez-Ortiz, F.A.; Higuera-Ciapara, I.; Caire, G.; Hernández-Watanabe, G. High performance liquid chromatographic determination of free amino acids in shrimp. J. Liq. Chromatogr. 1995, 10, 2050–2068. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice- Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Prior, R.L.; Hoang, H.; Gu, L.; Wu, X.; Bacchiocca, M.; Howard, L.; Hampsch-Woodill, M.; Huang, D.; Ou, B.; Jacob, R. Assays for hydrophilic and lipophilic antioxidant capacity (oxygen radical absorbance capacity (ORACFL) of plasma and other biological and food samples. J. Agric. Food Chem. 2003, 5, 3273–3279. [Google Scholar] [CrossRef]
- Arias-Moscoso, J.L.; Soto-Valdez, H.; Plascencia-Jatomea, M.; Vidal-Quintanar, R.L.; Rouzaud-Sández, O.; Ezquerra-Brauer, J.M. Composites of chitosan with acid-soluble collagen from jumbo squid (Dosidicus gigas) by-products. Polym. Int. 2011, 60, 924–931. [Google Scholar] [CrossRef]
- Moreno-Osorio, L.; Garcia, M.; Villalobos-Carbajal, R. Effect of polygodial on mechanical, optical and barrier properties of chitosan films. J. Food Process. Preserv. 2010, 34, 219–234. [Google Scholar] [CrossRef]
- ASTM D882-10. Standard test methods for tensile properties of thin plastic sheeting. In Annual Book of American Society for Testing and Materials Standards; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar] [CrossRef]
- Han, T.; Nwe, N.; Furuike, T.; Tokura, S.; Tamura, H. Methods of N-acetylated chitosan scaffolds and its in vitro biodegradation by lysozyme. J. Biomed. Sci. Eng. 2012, 5, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Byun, Y.; Kim, Y.T.; Whiteside, S. Characterization of an antioxidant polylactic acid (PLA) film prepared with a-tocopherol, BHT and polyethylene glycol using film cast extruder. J. Food Eng. 2010, 100, 239–244. [Google Scholar] [CrossRef]
- Martínez-Camacho, A.P.; Cortez-Rocha, M.O.; Ezquerra-Brauer, J.M.; Rodríguez-Feliz, F.; Castillo-Ortega, M.M.; Yepiz-Gómez, M.; Plascencia-Jatomea, M. Chitosan chomposite films: Thermal, structural, mechanical and antifungal properties. Cabohydr. Polym. 2010, 82, 305–315. [Google Scholar] [CrossRef]
- Uriarte-Montoya, M.H.; Santacruz-Ortega, H.C.; Cinco-Moroyoqui, F.J.; Rouzaud-Sández, O.; Plascencia-Jatomea, M.; Ezquerra-Brauer, J.M. Giant squid gelatin: Chemical composition and biophysical characterization. Food Res. Int. 2011, 44, 3243–3249. [Google Scholar] [CrossRef]
- Suárez-Jiménez, G.M.; Robles-Sanchez, R.M.; Yepiz-Plascencia, G.; Burgos-Hernandez, A.; Ezquerra-Brauer, J.M. In vitro antioxidant, antimutagenic and antiproliferative activities of collagen hydrolysates of jumbo squid (Dosidicus gigas) byproducts. Food Sci. Technol. 2015, 35, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Suárez-Jiménez, G.M.; Burgos-Hernández, A.; Torres-Arreola, W.; López-Saiz, C.M.; Velázquez-Contreras, C.A.; Ezquerra-Brauer, J.M. Bioactive peptides from collagen hydrolysates from squid (Dosidicus gigas) by-products fractionated by ultrafiltration. Int. J. Food Sci. Technol. 2019, 54, 1054–1061. [Google Scholar] [CrossRef]
- Gómez-Guillen, M.C.; López-Caballero, M.E.; Alemán, A.; López de Lacey, A.; Giménez, B.; Montero, P. Antioxidant and antimicrobial peptide fractions from squid and tuna skin gelatin. In Sea By-Products as Real Material: New Ways of Application; Le Bihan, A., Ed.; Transworld Research Network: Kerala, India, 2010; pp. 89–115. [Google Scholar]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assay comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Regentein, J.M.; Ren, J. Changes in the antioxidant activity of loach (Misgurnusan guillicaudatus) protein hydrolysates during a simulated gastrointestinal digestion. Food Chem. 2010, 120, 810–816. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardize method for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Chan-Higuera, J.E.; Carbonell-Barrachina, A.A.; Cárdenas-López, J.L.; Kačániová, M.; Burgos-Hernández, A.; Ezquerra-Brauer, J.M. Jumbo squid (Dosidicus gigas) skin pigments: Chemical analysis and evaluation of antimicrobial and antimutagenic potential. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 349–353. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Huang, G.Q.; Xu, T.C.; Liu, L.N.; Xiao, J.X. Comparative study of the Maillard reaction of chitosan oligosaccharide and glucose with soybean protein isolate. Int. J. Biol. Macromol. 2019, 131, 601–607. [Google Scholar] [CrossRef] [PubMed]
- Ocak, B. Film-forming ability of collagen hydrolysate extracted from leather solid wastes with chitosan. Environ. Sci Pollut. Res. Int. 2018, 25, 4643–4655. [Google Scholar] [CrossRef] [PubMed]
- Kassai, M.R. Determination of the degree of N-acetylation for chitin and chitosan by various NMR spectroscopy techniques: A review. Carbohyd. Polym. 2010, 79, 801–810. [Google Scholar] [CrossRef]
- Sothornvit, R.; Krochta, J.M. Oxygen permeability and mechanical properties of films from hydrolyzed whey protein. J. Agric. Food Chem. 2000, 48, 3913–3916. [Google Scholar] [CrossRef]
- Nogueira Campos, M.G.; Innocentini Mei, L.H.; Rodrigues Santos, A. Sorbitol-plasticized and neutralized chitosan membranes as skin substitutes. Mater. Res. 2015, 18, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.C. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Sébastien, F.; Stéphane, G.; Copinet, A.; Coma, V. Novel biodegradable films made from chitosan and poly(lactic acid) with antifungal properties against mycotoxinogen strains. Carbohydr. Polym. 2006, 65, 185–193. [Google Scholar] [CrossRef]
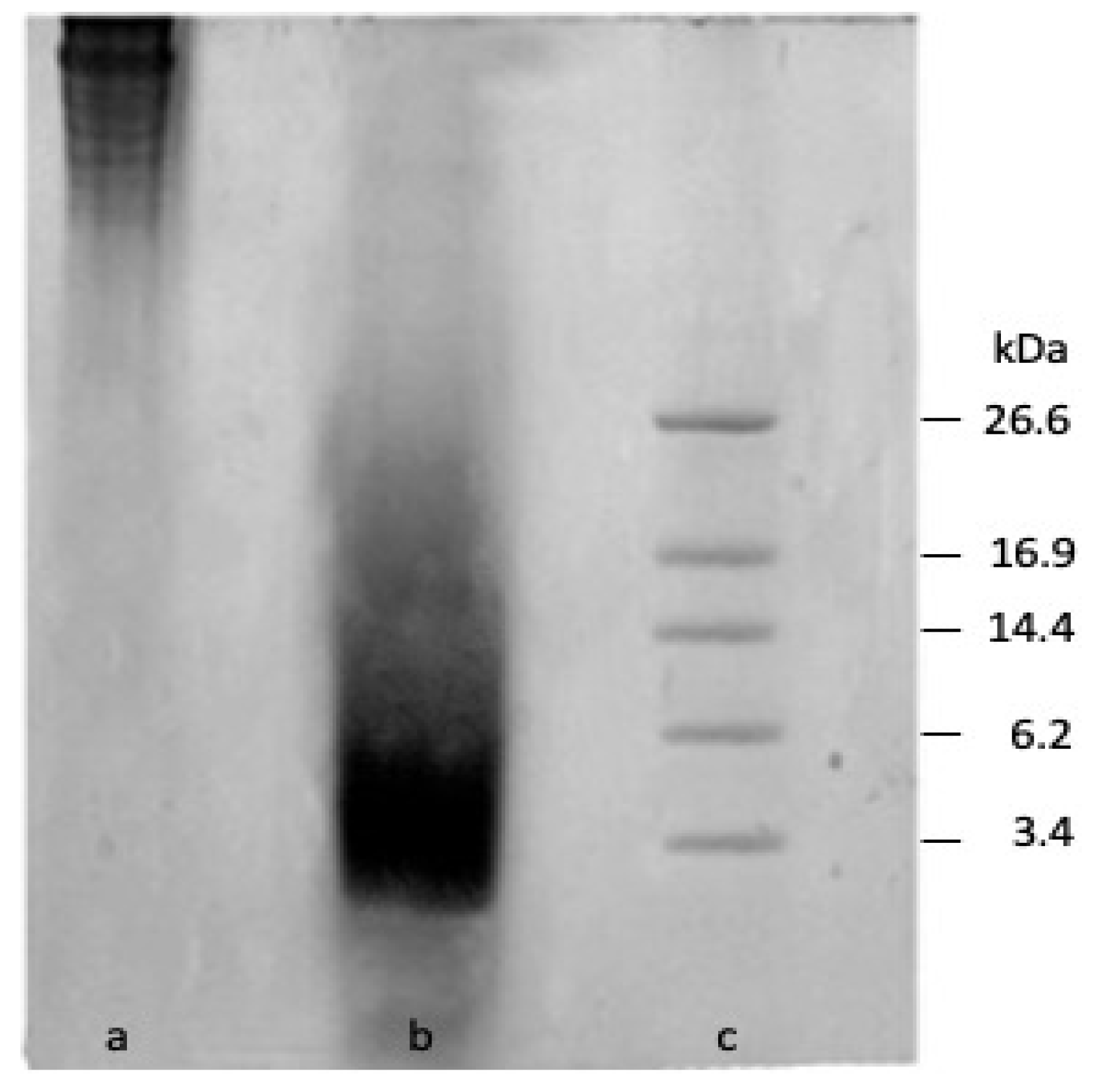

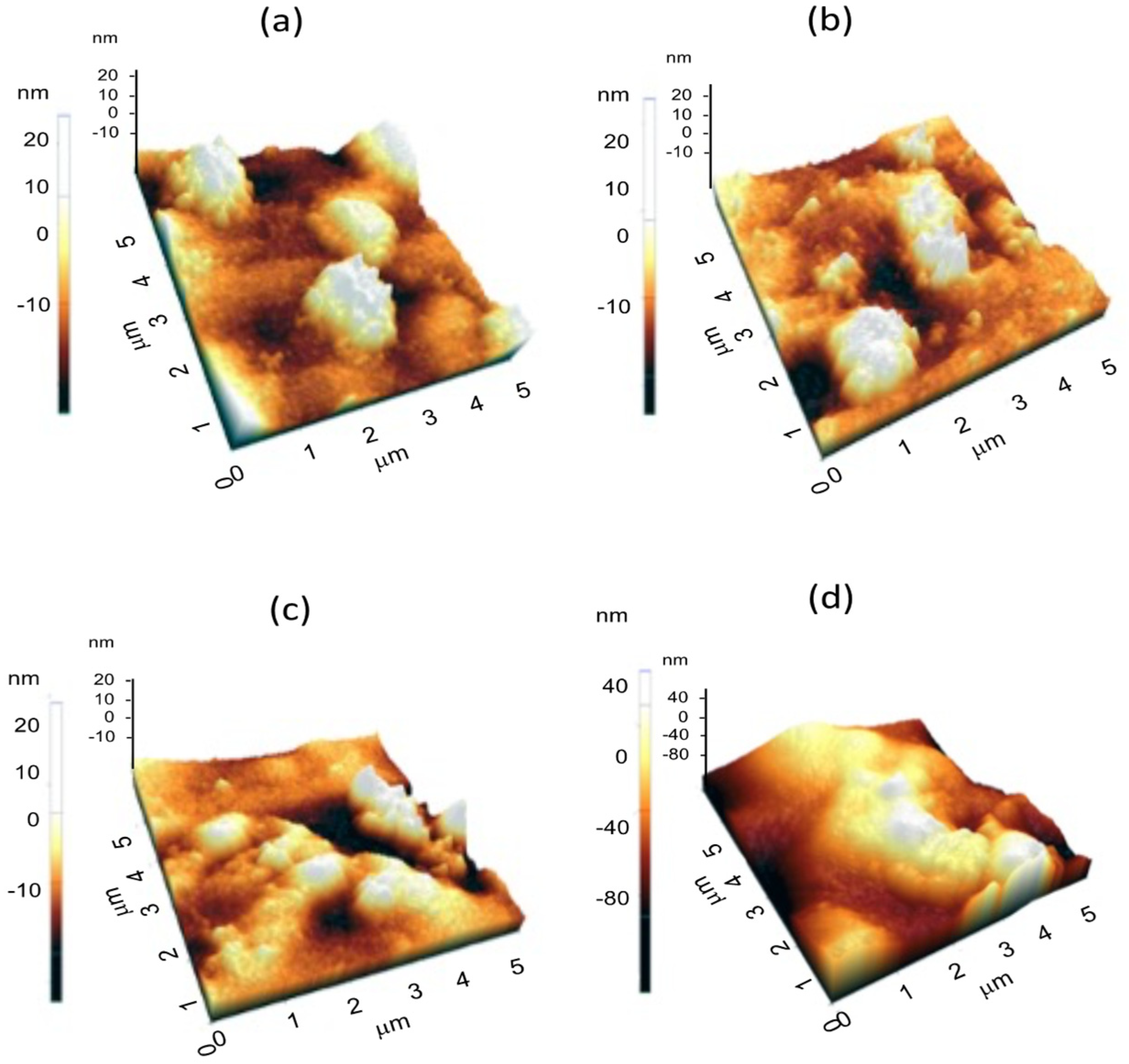

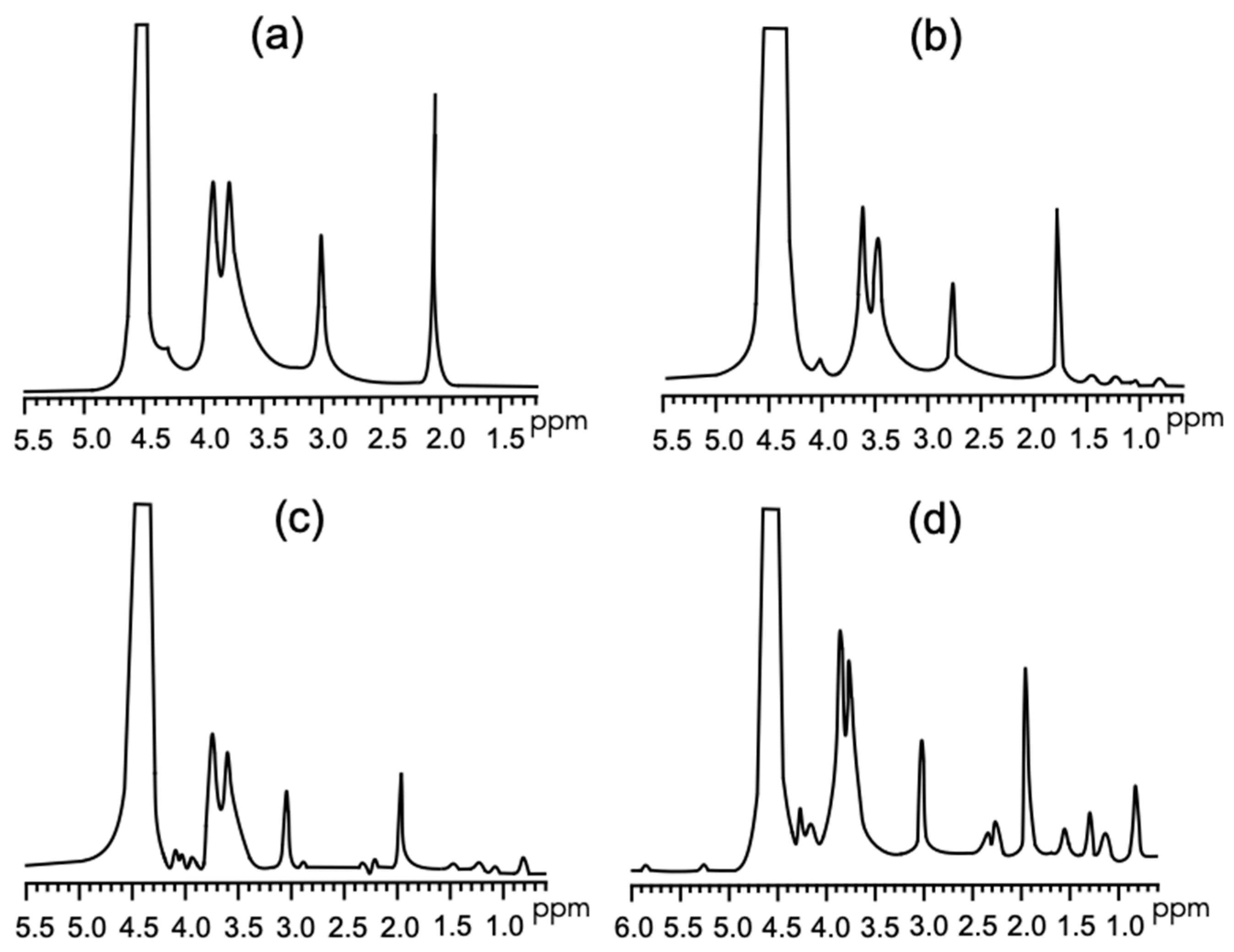

| Parameter | 100 CH/0 SGH | 90 CH/10 SGH | 80 CH/20 SGH | 60 CH/40 SGH |
|---|---|---|---|---|
| L | 79.7 ± 0.4 a | 31.6 ± 1.3 b | 16.4 ± 0.6 c | 13.5 ± 0.4 d |
| A | −6.8 ± 0.2 c | 24.7 ± 0.7 a | 25.1 ± 0.3 a | 19.9 ± 0.3 b |
| B | 29.1 ± 1.6 a | 27.9 ± 0.9 a | 18.3 ± 0.5 b | 11.1 ± 0.2 c |
| ΔE* | 31.4 ± 1.0 c | 78.3 ± 1.2 b | 90.2 ± 1.3 a | 90.7 ± 0.8 a |
| Film | Average Roughness |
|---|---|
| 100 CH/0 SGH | 3.11 ± 0.93 a |
| 90 CH/10 SGH | 4.35 ± 1.11 ab |
| 80 CH/20 SGH | 5.50 ± 0.05 c |
| 60 CH/40 SGH | 8.38 ± 0.65 d |
| Parameter | 100 CH/0 SGH | 90 CH/10 SGH | 80 CH/20 SGH | 60 CH/40 SGH |
|---|---|---|---|---|
| Thickness (μM) | 34.6 ± 1.1 a | 31.2 ± 1.3 b | 30.4 ± 1.5 b | 29.6 ± 1.6 b |
| TS (MPa) | 77.5 ± 5.7 a | 58.1 ± 5.3 b | 55.1 ± 2.3 b | 47.3 ± 5.7 c |
| EB (%) | 23.1 ± 3.0 a | 12.6 ± 1.9 b | 5.8 ± 1.0 c | 4.5 ± 1.2 c |
| EM (MPa) | 2625 ± 242 a | 2129 ± 141 b | 2392 ± 238 b | 1869 ± 145 c |
| ED (%) 2 | 87.2 ± 2.7 a | 72.8 ± 2.5 b | 56.4 ± 3.1 c | 35.8 ± 2.8 d |
| Assay | 100 CH/0 SGH | 90 CH/10 SGH | 80 CH/20 SGH | 60 CH/40 SGH |
|---|---|---|---|---|
| ABTS (μM TE/100 mg) | 3.3 ± 1.2 a | 30.1 ± 3.9 b | 57.7 ± 2.2 c | 88.9 ± 2.5 d |
| DPPH (%) | 2.8 ± 0.4 a | 6.1 ± 0.8 b | 6.6 ± 1.0 b,c | 8.7 ± 1.7 c |
| Spore size (μm) | 51 ± 2.5 b | 50 ± 2.2 b | 42 ± 2.6 c | 91 ± 9.0 a |
| Hyphae size (μm) | ND | ND | ND | 47.8 ± 5.9 a |
| Fungistatic index (%) 3 | 25 ± 3.9 b | 28 ± 1.6 b | 34 ± 3.1 a | 0 ± 0.4 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuevas-Acuña, D.A.; Plascencia-Jatomea, M.; Santacruz-Ortega, H.d.C.; Torres-Arreola, W.; Ezquerra-Brauer, J.M. Development of Chitosan/Squid Skin Gelatin Hydrolysate Films: Structural, Physical, Antioxidant, and Antifungal Properties. Coatings 2021, 11, 1088. https://doi.org/10.3390/coatings11091088
Cuevas-Acuña DA, Plascencia-Jatomea M, Santacruz-Ortega HdC, Torres-Arreola W, Ezquerra-Brauer JM. Development of Chitosan/Squid Skin Gelatin Hydrolysate Films: Structural, Physical, Antioxidant, and Antifungal Properties. Coatings. 2021; 11(9):1088. https://doi.org/10.3390/coatings11091088
Chicago/Turabian StyleCuevas-Acuña, Dulce Alondra, Maribel Plascencia-Jatomea, Hisila del Carmen Santacruz-Ortega, Wilfrido Torres-Arreola, and Josafat Marina Ezquerra-Brauer. 2021. "Development of Chitosan/Squid Skin Gelatin Hydrolysate Films: Structural, Physical, Antioxidant, and Antifungal Properties" Coatings 11, no. 9: 1088. https://doi.org/10.3390/coatings11091088
APA StyleCuevas-Acuña, D. A., Plascencia-Jatomea, M., Santacruz-Ortega, H. d. C., Torres-Arreola, W., & Ezquerra-Brauer, J. M. (2021). Development of Chitosan/Squid Skin Gelatin Hydrolysate Films: Structural, Physical, Antioxidant, and Antifungal Properties. Coatings, 11(9), 1088. https://doi.org/10.3390/coatings11091088







