Effect of Ni Addition on the Corrosion Resistance of NiTi Alloy Coatings on AISI 316L Substrate Prepared by Laser Cladding
Abstract
1. Introduction
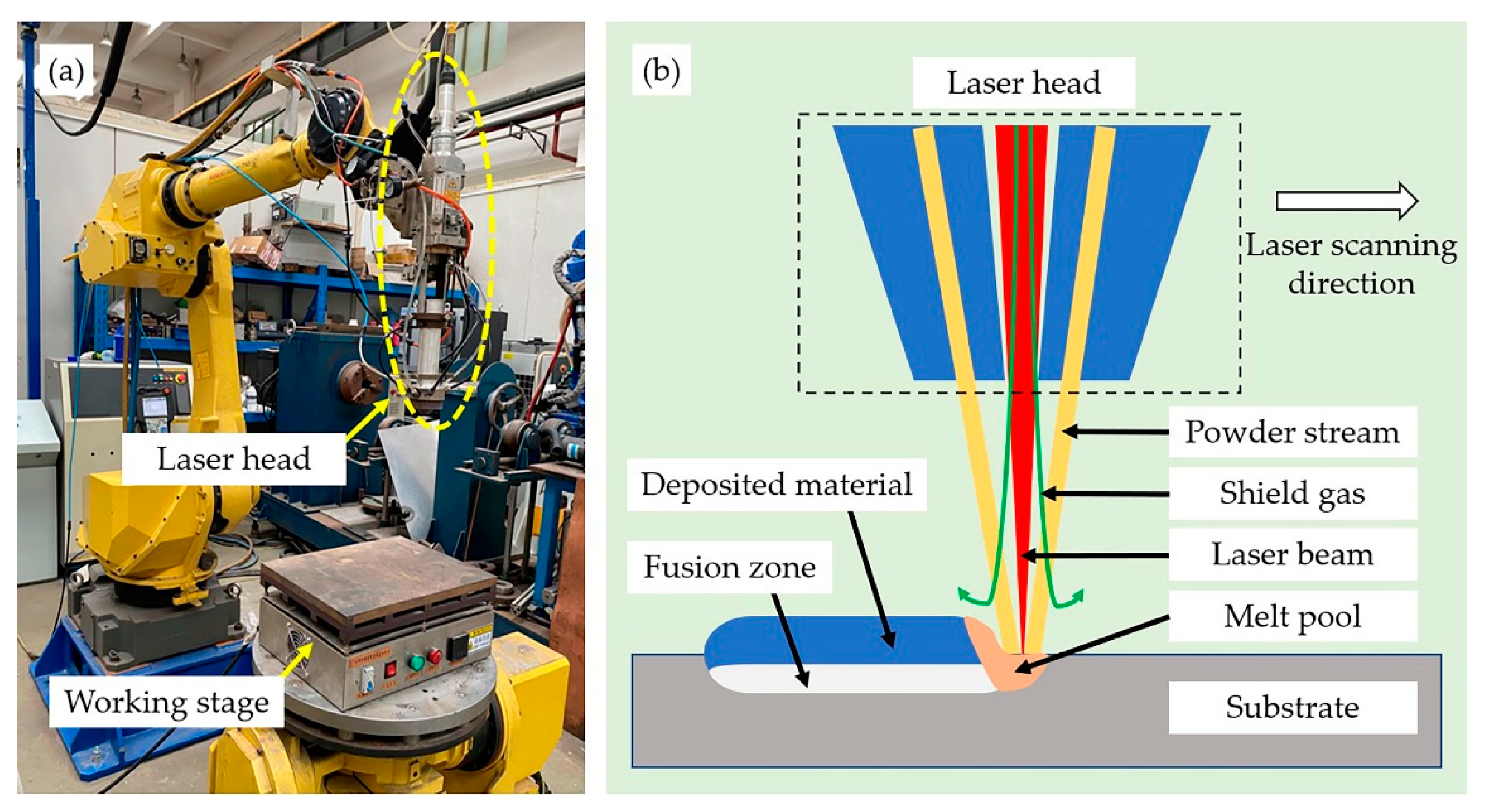
2. Experimental Procedure
2.1. Material and Specimen Preparation
2.2. Electrochemical Measurements
3. Results
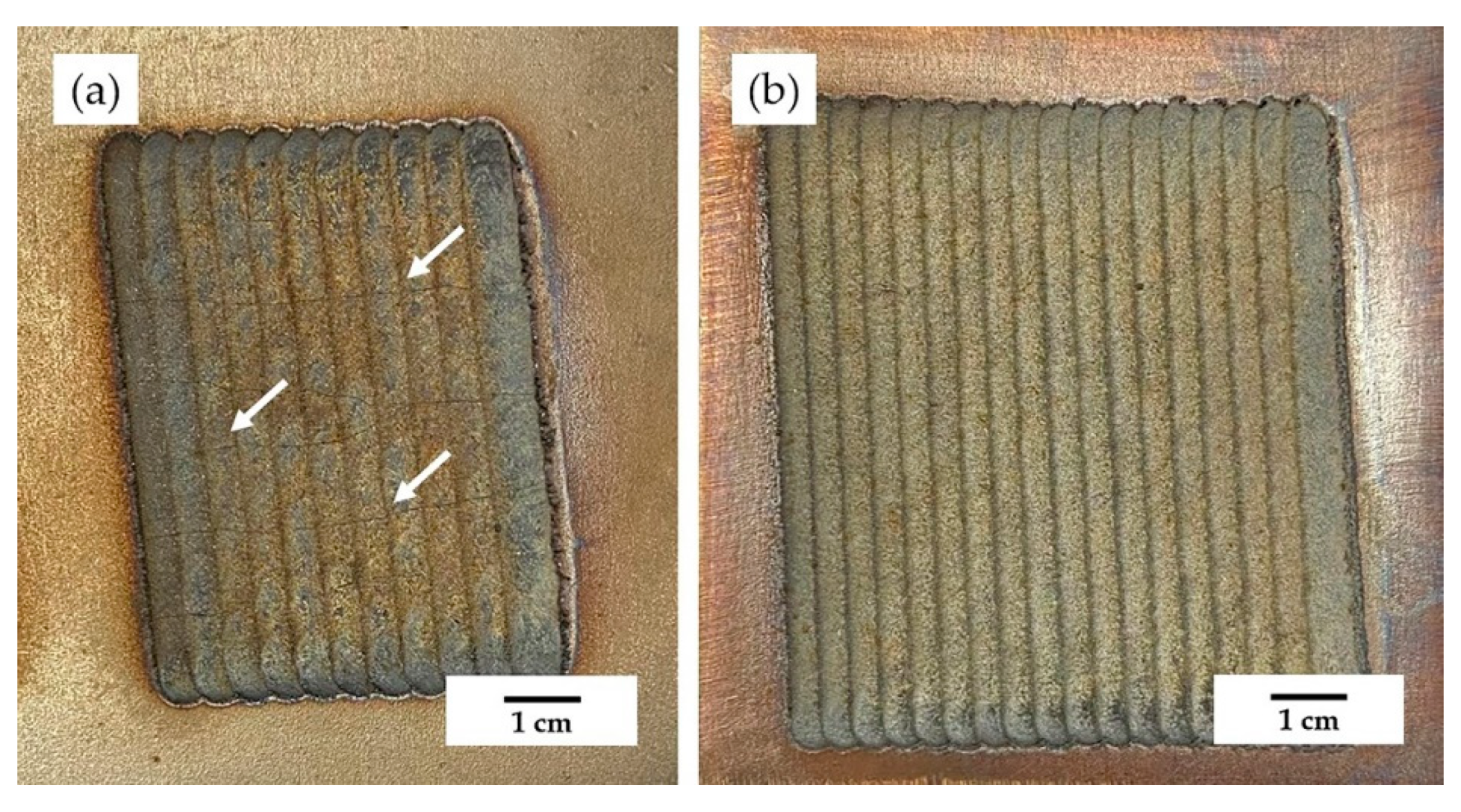
3.1. Macroscopic Morphology of the Coating
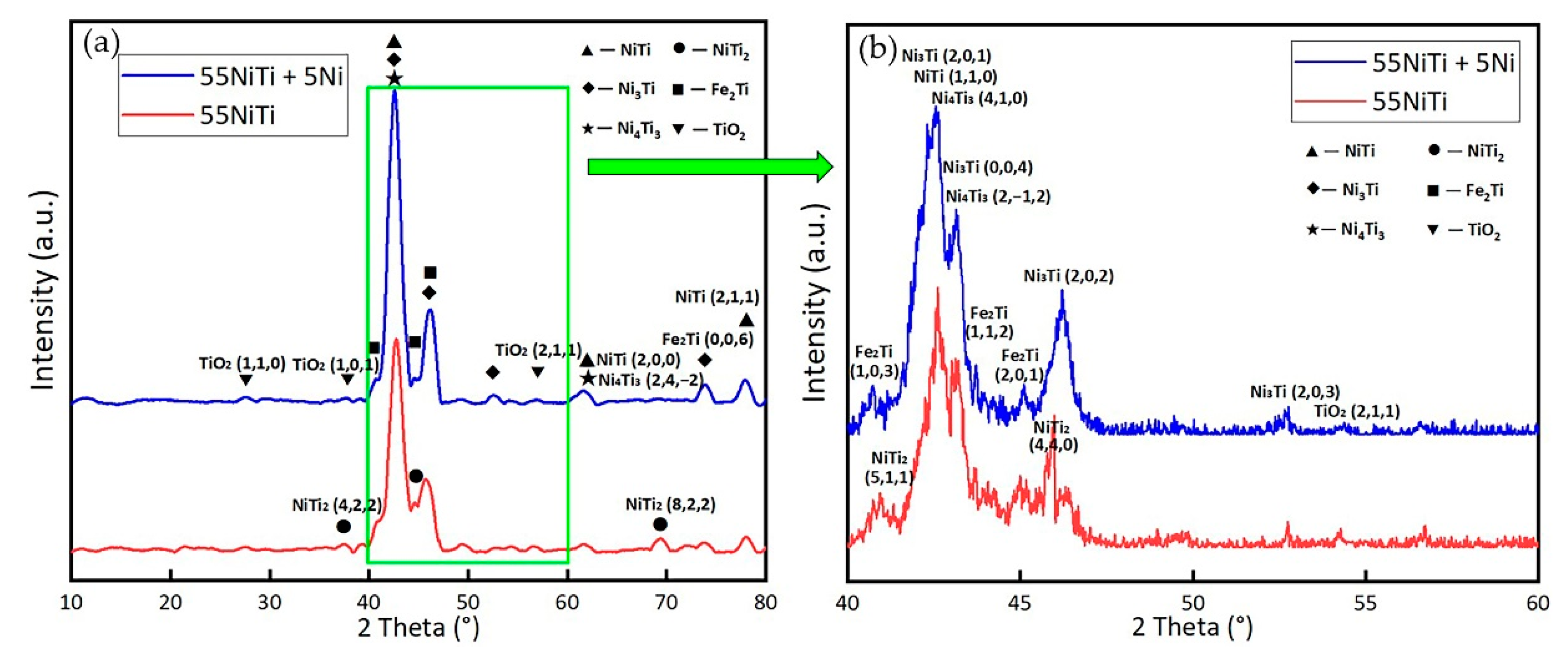
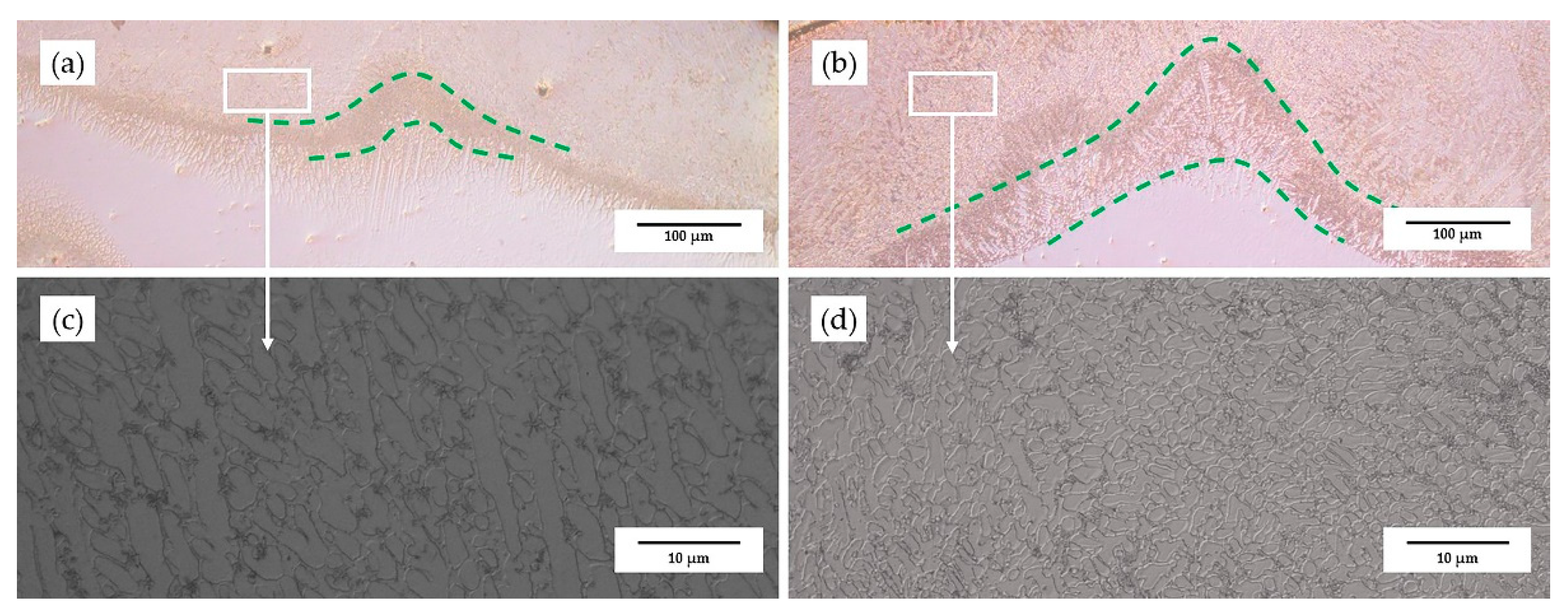
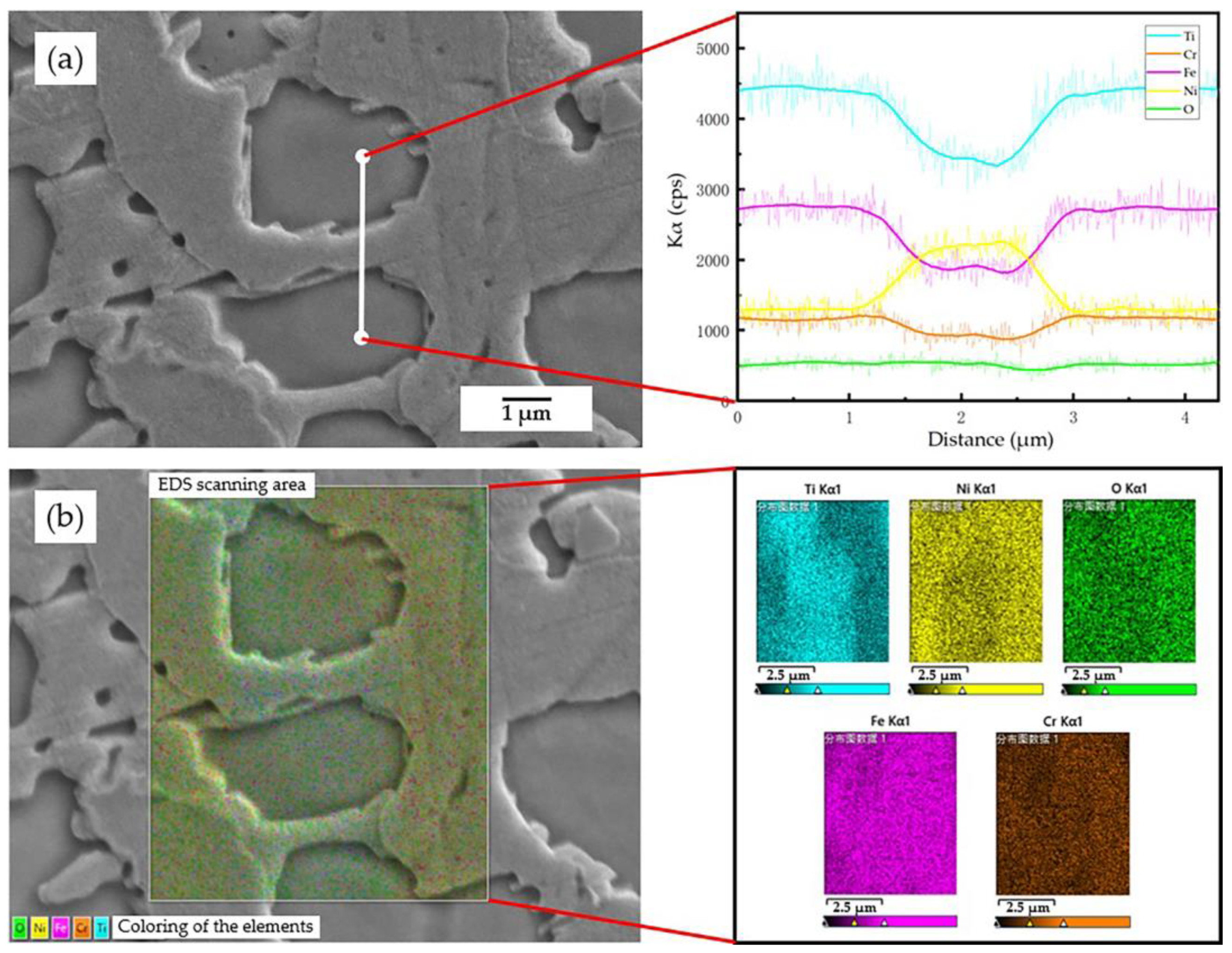
3.2. Phase Composition and Microstructure of the Coating
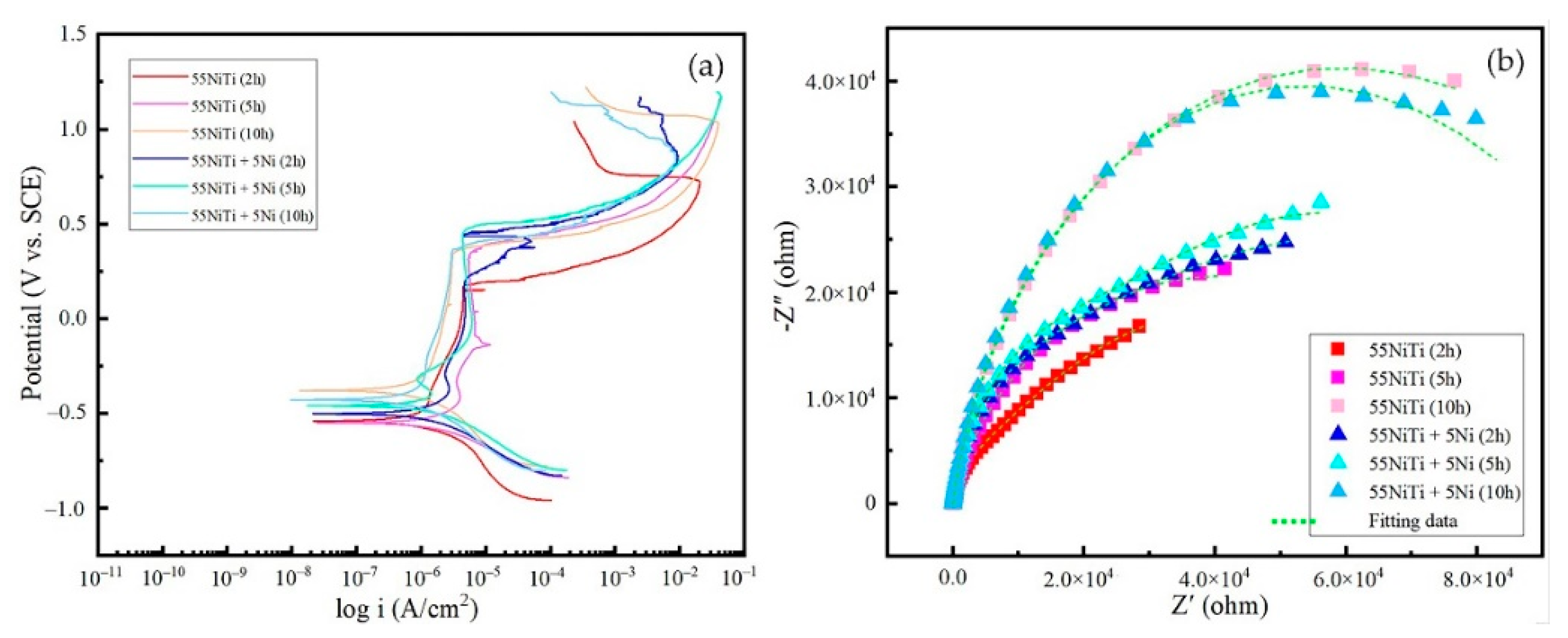
3.3. Electrochemical Behavior of the Coating
3.3.1. Open-Circuit Potential (OCP) Analysis
3.3.2. Potentiodynamic Polarization Measurement
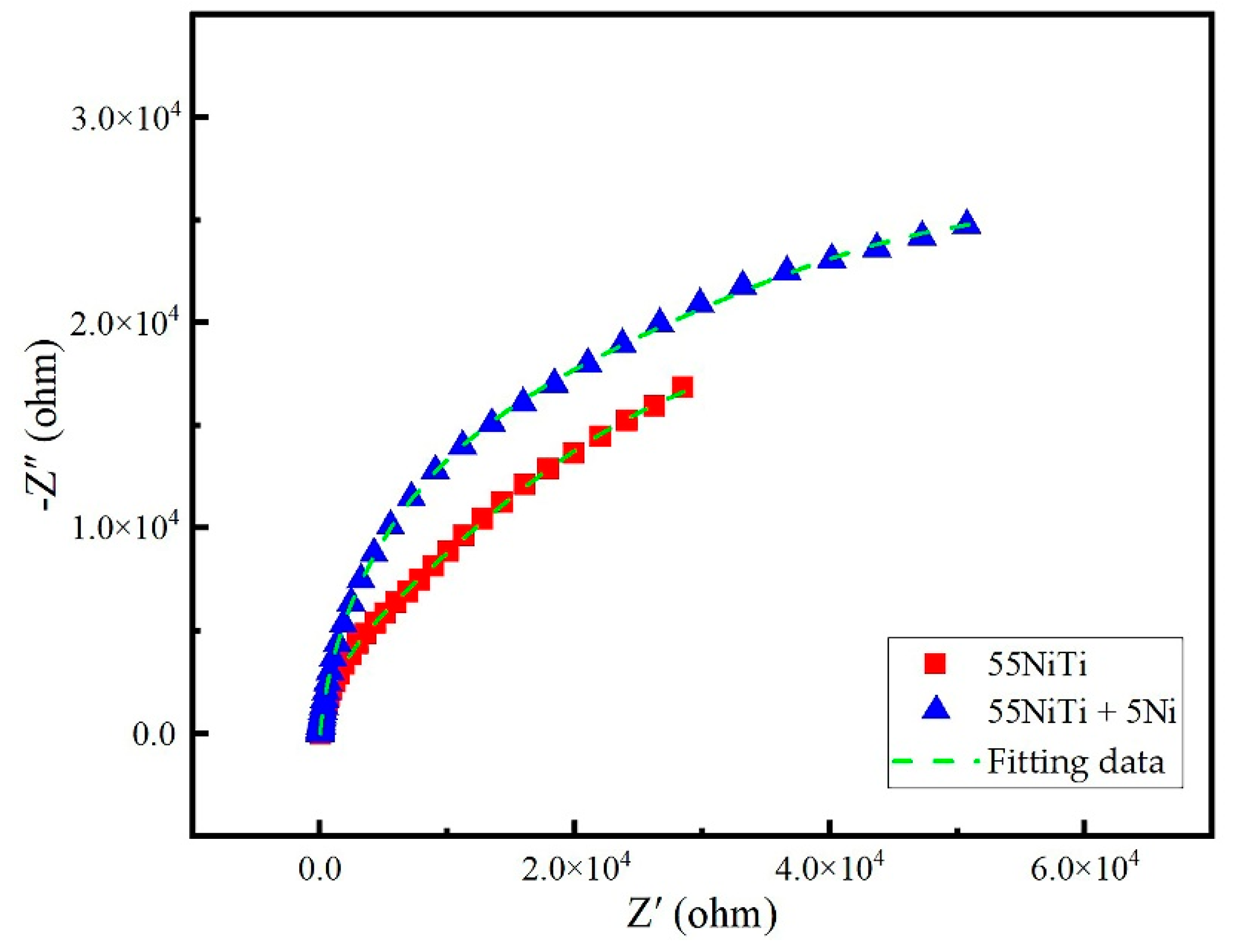
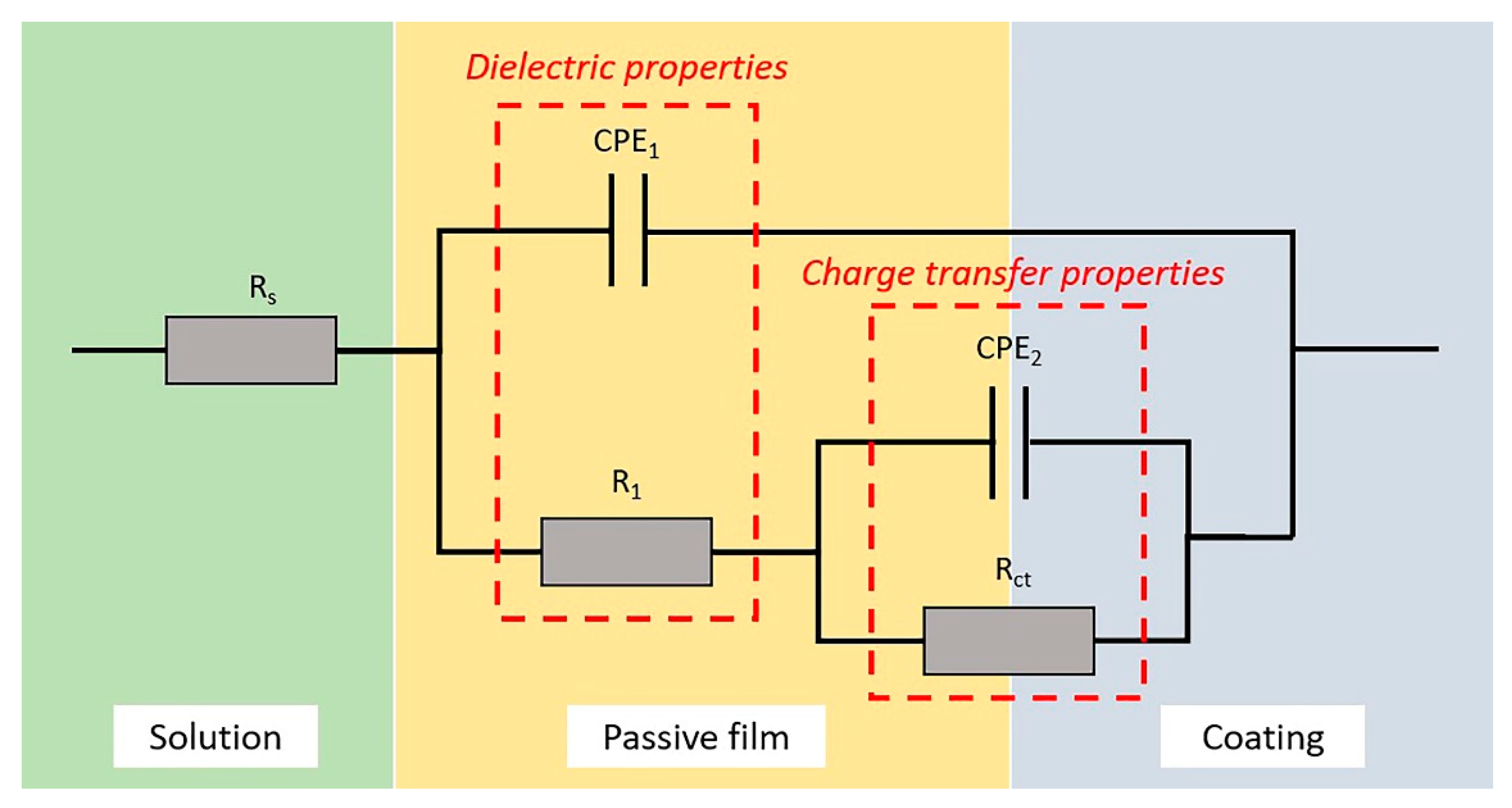
3.3.3. EIS Analysis
4. Discussion
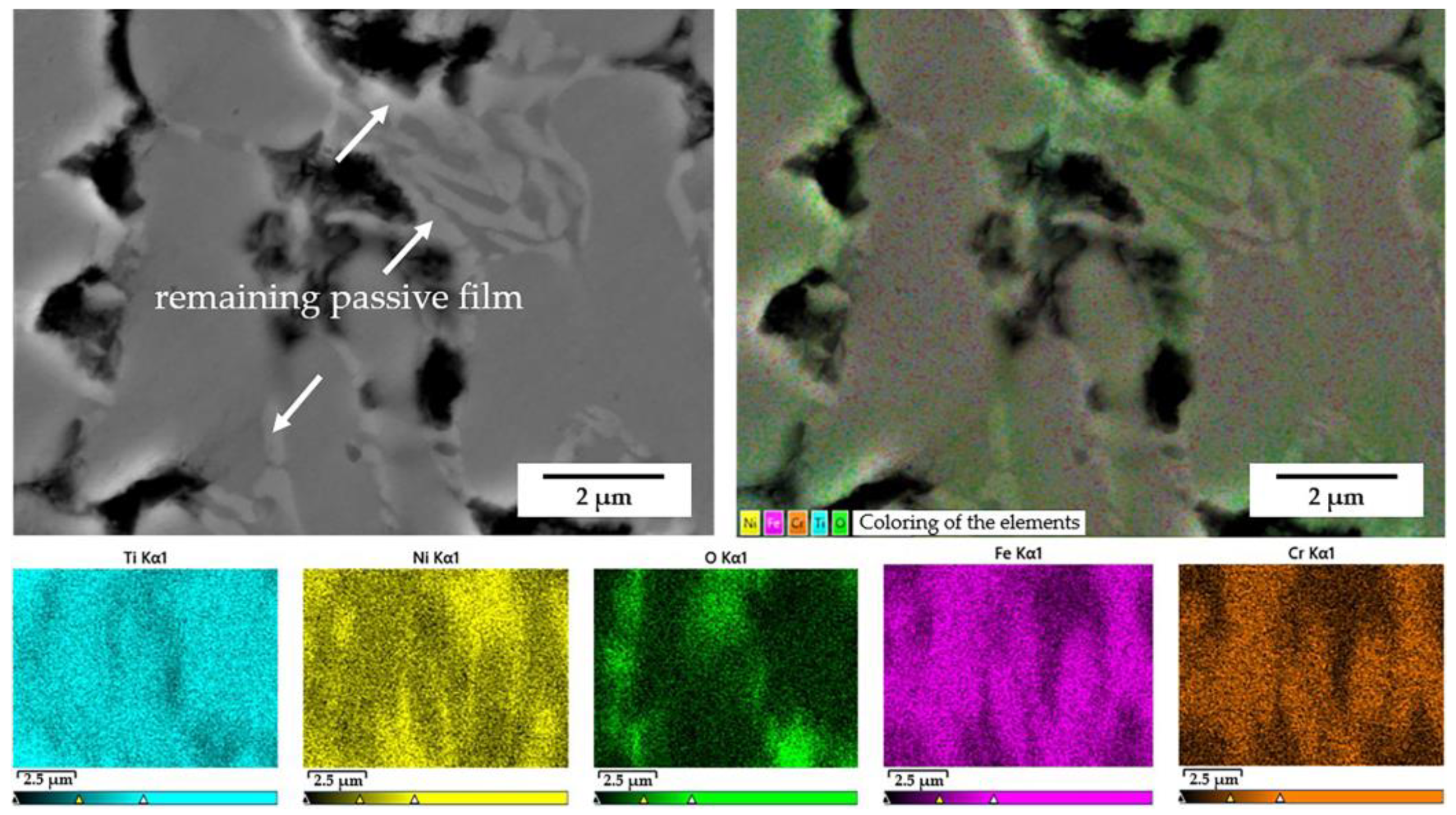
4.1. The Impact of the Coating Microstructure and Composition on Corrosion Resistance
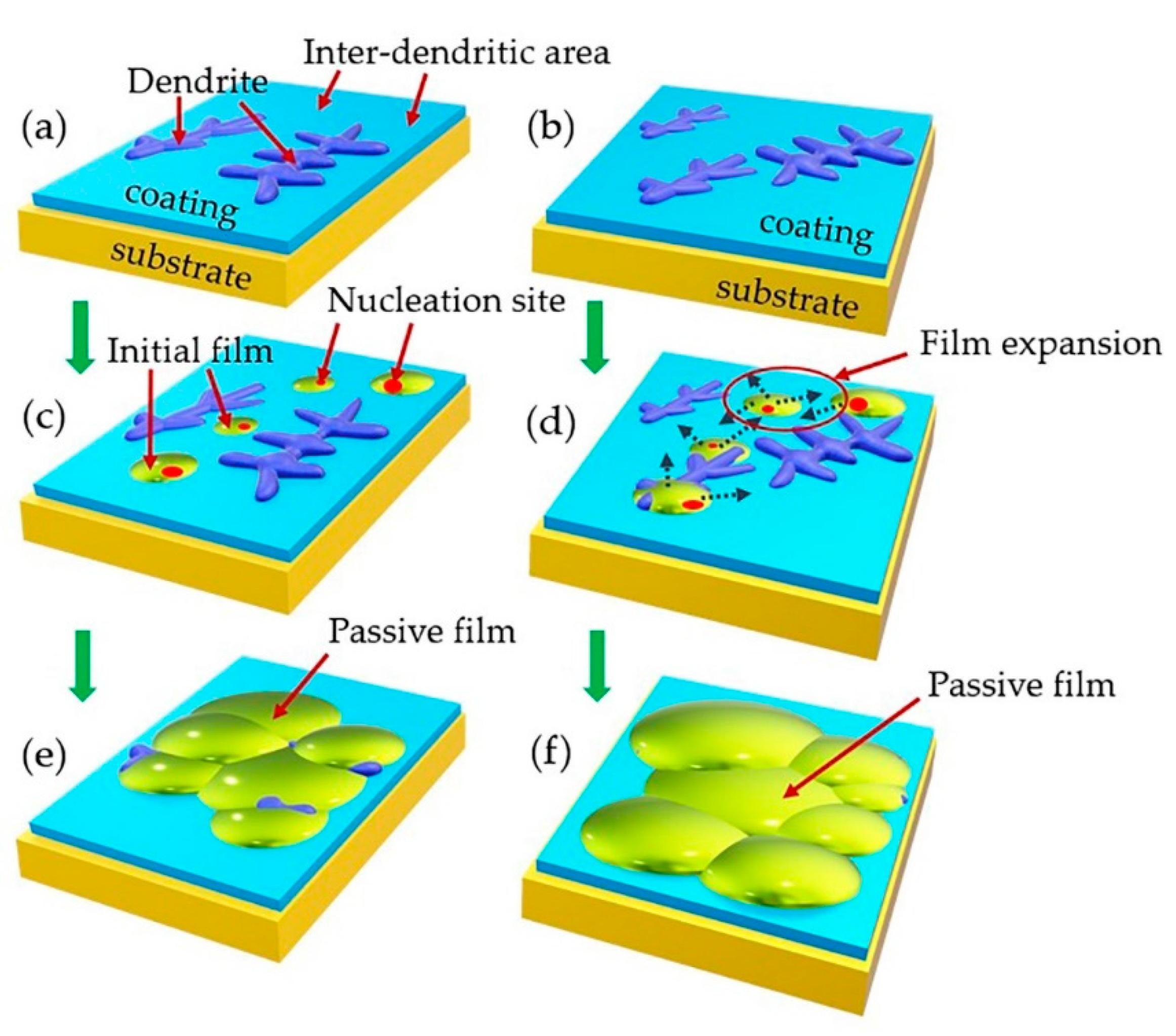
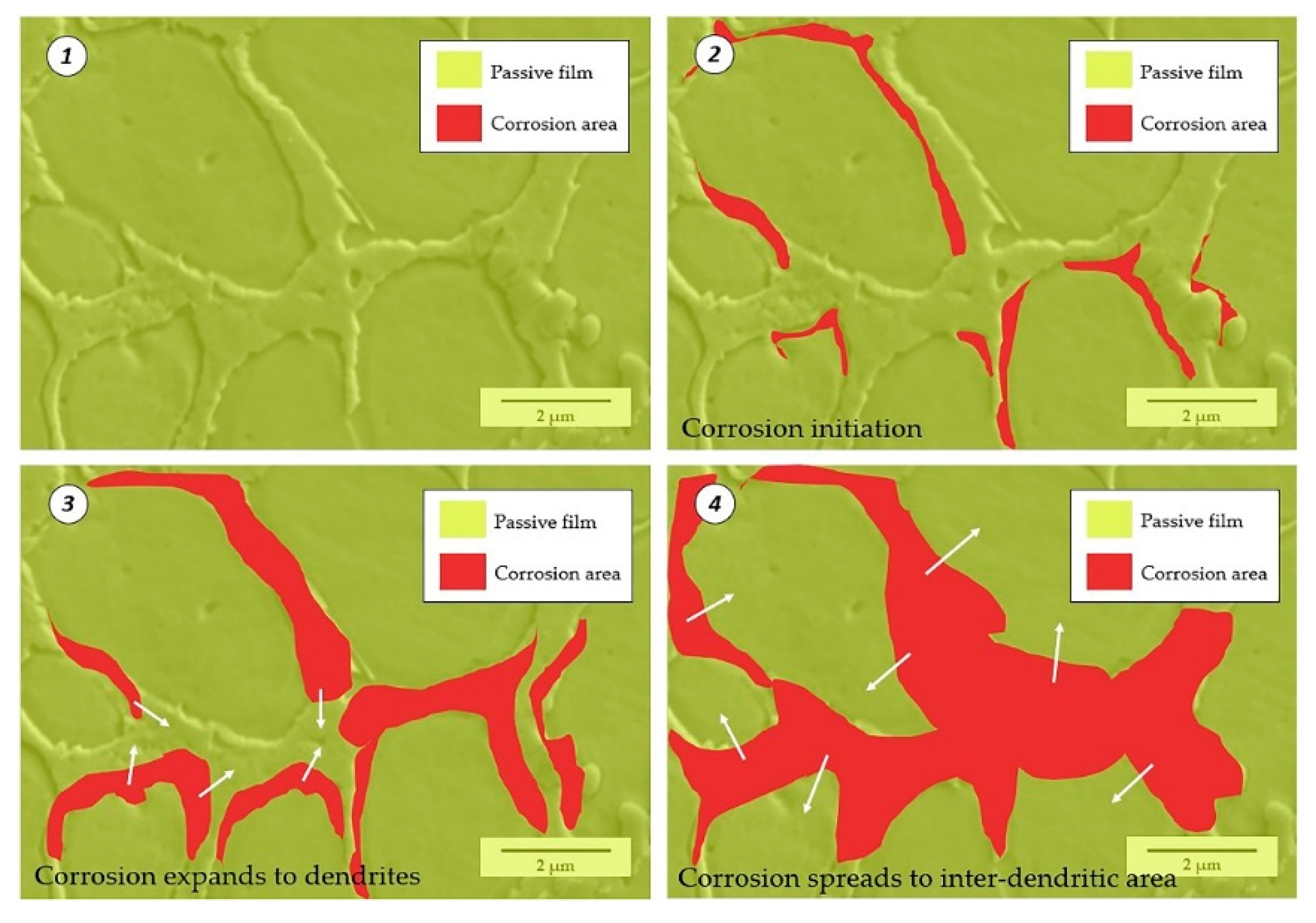
4.2. Corrosion Initiation and Expansion Process
4.3. The Impact of Immersion Duration on Corrosion Resistance of the Coating
5. Conclusions
- (1)
- With the addition of Ni, the microstructure of the coatings became finer and denser; in the bottom area, the average grain size of the 55NiTi + 5Ni coating was less than half the grain size of the 55NiTi coating.
- (2)
- The results of electrochemical experiments show that the 55NiTi + 5Ni coating had a nobler corrosion potential and smaller corrosion current density, which was due to the homogeneous and thick passive film formed on the surface of the 55NiTi + 5Ni coating.
- (3)
- The initiation of the corrosion was selective. It initiated preferentially at the junctions of dendrites and inter-dendritic areas, then spread to the dendrites and finally covered the inter-dendritic areas.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otsuka, K.; Ren, X. Physical metallurgy of Ti–Ni-based shape memory alloys. Prog. Mater. Sci. 2005, 50, 511–678. [Google Scholar] [CrossRef]
- Buehler, W.J.; Gilfrich, J.V.; Wiley, R.C. Effect of Low–Temperature Phase Changes on the Mechanical Properties of Alloys near Composition TiNi. J. Appl. Phys. 1963, 34, 1475–1477. [Google Scholar] [CrossRef]
- Tobushi, H.; Ikai, A.; Yamada, S.; Tanaka, K.; Lexcellent, C. Thermomechanical properties of TiNi shape memory alloy. J. Phys. IV. 1996, 6, 385–393. [Google Scholar] [CrossRef][Green Version]
- Jackson, C.; Wagner, H.; Wasilewski, R. 55-Nitinol-The Alloy with a Memory: It’s Physical Metallurgy Properties, and Applications; NASA: Washington, DC, USA, January 1972; p. 5110.
- Yoneyama, T.; Miyazaki, S. Shape Memory Alloys for Biomedical Applications; Woodhead publishing limited: Cambridge, UK, 2009; p. 101. [Google Scholar]
- Khanlari, K.; Ramezani, M.; Kelly, P. 60NiTi: A Review of Recent Research Findings, Potential for Structural and Mechanical Applications, and Areas of Continued Investigations. Trans. Indian Inst. Met. 2017, 71, 781–799. [Google Scholar] [CrossRef]
- Saburi, T.; Yoshida, M.; Nenno, S. Deformation behavior of shape memory Ti–Ni alloy crystals. Scr. Metall. 1984, 18, 363–366. [Google Scholar] [CrossRef]
- Miyazaki, S.; Kimura, S.; Otsuka, K.; Suzuki, Y. The habit plane and transformation strains associated with the martensitic transformation in Ti-Ni single crystals. Scr. Metall. 1984, 18, 883–888. [Google Scholar] [CrossRef]
- Abd El-Fattah, H.A.; El-Mahallawi, I.; Shazly, M.H.; Khalifa, W.A. Microstructure Evolution of NiTi Magnetron Sputtered Thin Film on Different Substrates. Key Eng. Mater. 2020, 835, 68–74. [Google Scholar] [CrossRef]
- Samal, S.; Tyc, O.; Cizek, J.; Klecka, J.; Lukáč, F.; Molnárová, O.; de Prado, E.; Weiss, Z.; Kopeček, J.; Heller, L.; et al. Fabrication of Thermal Plasma Sprayed NiTi Coatings Possessing Functional Properties. Coatings 2021, 11, 610. [Google Scholar] [CrossRef]
- Pasang, T.; Tavlovich, B.; Yannay, O.; Jackson, B.; Fry, M.; Tao, Y.; Turangi, C.; Wang, J.C.; Jiang, C.P.; Sato, Y.; et al. Directionally-Dependent Mechanical Properties of Ti6Al4V Manufactured by Electron Beam Melting (EBM) and Selective Laser Melting (SLM). Materials 2021, 14, 603. [Google Scholar] [CrossRef]
- Vokoun, D.; Klimša, L.; Vetushka, A.; Duchoň, J.; Racek, J.; Drahokoupil, J.; Kopeček, J.; Yu, Y.-S.; Koothan, N.; Kei, C.-C. Al2O3 and Pt Atomic Layer Deposition for Surface Modification of NiTi Shape Memory Films. Coatings 2020, 10, 746. [Google Scholar] [CrossRef]
- Dai, J.; Liu, Z.; Yu, B.; Ruan, Q.; Chu, P.K. Effects of Ti, Ni, and Dual Ti/Ni Plasma Immersion Ion Implantation on the Corrosion and Wear Properties of Magnesium Alloy. Coatings 2020, 10, 313. [Google Scholar] [CrossRef]
- Verdian, M.; Raeissi, K.; Salehi, M. Corrosion performance of HVOF and APS thermally sprayed NiTi intermetallic coatings in 3.5% NaCl solution. Corros. Sci. 2010, 52, 1052–1059. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, W. Laser surface cladding: The state of the art and challenges. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2010, 224, 1041–1060. [Google Scholar] [CrossRef]
- Vilar, R. Laser cladding. J. Laser Appl. 1999, 11, 64–79. [Google Scholar] [CrossRef]
- Norhafzan, B.; Aqida, S.N.; Chikarakara, E.; Brabazon, D. Surface modification of AISI H13 tool steel by laser cladding with NiTi powder. Appl. Phys. A 2016, 122, 348. [Google Scholar] [CrossRef]
- Chiu, K.Y.; Cheng, F.T.; Man, H.C. Cavitation erosion resistance of AISI 316L stainless steel laser surface-modified with NiTi. Mater. Sci. Eng. A 2005, 392, 348–358. [Google Scholar] [CrossRef]
- Fu, B.; Feng, K.; Li, Z. Study on the effect of Cu addition on the microstructure and properties of NiTi alloy fabricated by laser cladding. Mater. Lett. 2018, 220, 148–151. [Google Scholar] [CrossRef]
- Lepule, M.L.; Obadele, B.A.; Andrews, A.; Olubambi, P.A. Corrosion and wear behaviour of ZrO2 modified NiTi coatings on AISI 316 stainless steel. Surf. Coat. Technol. 2015, 261, 21–27. [Google Scholar] [CrossRef]
- Okamoto, H.; Okamoto, H. Phase Diagrams for Binary Alloys; ASM International: Materials Park, OH, USA, 2000; Volume 44. [Google Scholar]
- Hornbuckle, B.C.; Xiao, X.Y.; Noebe, R.D.; Martens, R.; Weaver, M.L.; Thompson, G.B. Hardening behavior and phase decomposition in very Ni-rich Nitinol alloys. Mater. Sci. Eng. A 2015, 639, 336–344. [Google Scholar] [CrossRef]
- Khanlari, K.; Ramezani, M.; Kelly, P.; Cao, P.; Neitzert, T. Mechanical and microstructural characteristics of as-sintered and solutionized porous 60NiTi. Intermetallics 2018, 100, 32–43. [Google Scholar] [CrossRef]
- Feng, Y.; Du, Z.; Hu, Z. Study on the Effect of Ni Addition on the Microstructure and Properties of NiTi Alloy Coating on AISI 316 L Prepared by Laser Cladding. Materials 2021, 14, 4373. [Google Scholar] [CrossRef]
- Du, Z.; Hu, Z.; Feng, Y.; Mo, F. The Effect of Powder Composition on the Microstructure and Corrosion Resistance of Laser Cladding 60NiTi Alloy Coatings on SS 316L. Metals 2021, 11, 1104. [Google Scholar] [CrossRef]
- Zhou, S.; Xiong, Z.; Dai, X.; Liu, J.; Zhang, T.; Wang, C. Microstructure and oxidation resistance of cryomilled NiCrAlY coating by laser induction hybrid rapid cladding. Surf. Coat. Technol. 2014, 258, 943–949. [Google Scholar] [CrossRef]
- Zhuang, Q.; Zhang, P.; Li, M.; Yan, H.; Yu, Z.; Lu, Q. Microstructure, Wear Resistance and Oxidation Behavior of Ni-Ti-Si Coatings Fabricated on Ti6Al4V by Laser Cladding. Materials 2017, 10, 1248. [Google Scholar] [CrossRef]
- Carvalho, P.A.; Vilar, R. Laser alloying of zine with aluminum: Solidification structure. Surf. Coat. Technol. 1997, 91, 158–166. [Google Scholar] [CrossRef]
- Pouquet, J.; Miranda, R.M.; Quintino, L.; Williams, S. Dissimilar laser welding of NiTi to stainless steel. Int. J. Adv. Manuf. Technol. 2011, 61, 205–212. [Google Scholar] [CrossRef]
- Bozzolo, G.; Noebe, R.D.; Mosca, H.O. Site preference of ternary alloying additions to NiTi: Fe, Pt, Pd, Au, Al, Cu, Zr and Hf. J. Alloys Compd. 2005, 389, 80–94. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Z.; Ai, J.; Bu, S.; Liu, H. Preparation of Coating on the Titanium Surface by Micro-Arc Oxidation to Improve Corrosion Resistance. Coatings 2021, 11, 230. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.; Zhang, B.; Li, Y.; Wang, F. An investigation on the continuous and uniform thin membrane passive film formed on sputtered nanocrystalline stainless steel. Corros. Sci. 2016, 104, 71–83. [Google Scholar] [CrossRef]
- Robin, A.; Meirelis, J.P. Influence of fluoride concentration and pH on corrosion behavior of titanium in artificial saliva. J. Appl. Electrochem. 2007, 37, 511–517. [Google Scholar] [CrossRef]
- Figueira, N.; Silva, T.M.; Carmezim, M.J.; Fernandes, J.C.S. Corrosion behaviour of NiTi alloy. Electrochim. Acta 2009, 54, 921–926. [Google Scholar] [CrossRef]
- Wever, D.J.; Veldhuizen, A.G.; Vries, J.d.; Busscher, H.J.; Uges, D.R.A.; Horn, J.R.v. Electrochemical and surface characterization of a nickel–titanium alloy. Biomaterials 1998, 19, 761–769. [Google Scholar] [CrossRef]
- Cheng, F.; Shi, P.; Man, H.C. A preliminary study of TiO2 deposition on NiTi by a hydrothermal method. Surf. Coat. Technol. 2004, 187, 26–32. [Google Scholar] [CrossRef]
- Cheng, Y.; Cai, W.; Li, H.; Zheng, Y. Surface modification of NiTi alloy with tantalum to improve its biocompatibility and radiopacity. J. Mater. Sci. 2006, 41, 4961–4964. [Google Scholar] [CrossRef]
- Shen, F.; Tao, W.; Li, L.; Zhou, Y.; Wang, W.; Wang, S. Effect of microstructure on the corrosion resistance of coatings by extreme high speed laser cladding. Appl. Surf. Sci. 2020, 517, 146085. [Google Scholar] [CrossRef]
- Shokouhfar, M.; Allahkaram, S.R. Effect of incorporation of nanoparticles with different composition on wear and corrosion behavior of ceramic coatings developed on pure titanium by micro arc oxidation. Surf. Coat. Technol. 2017, 309, 767–778. [Google Scholar] [CrossRef]
- Wang, Q.-Y.; Pei, R.; Liu, S.; Wang, S.-L.; Dong, L.-J.; Zhou, L.-J.; Xi, Y.-C.; Bai, S.-L. Microstructure and corrosion behavior of different clad zones in multi-track Ni-based laser-clad coating. Surf. Coat. Technol. 2020, 402, 126310. [Google Scholar] [CrossRef]
- Olaseinde, O.A. Comparative Study of the Effect of Temperature on the Corrosion Behaviour of 2205 Duplex Stainless Steel and 316 Austenitic Stainless Steel in Acidic Chloride Environment. Adv. Mater. Phys. Chem. 2015, 5, 185. [Google Scholar] [CrossRef]
- Stoynov, Z.; Grafov, B.; Savova-Stoynova, B.; Elkin, V. Electrochemical Impedance; Publishing House Science: Moscow, Russia, 1991. [Google Scholar]
- Gaberšček, M.; Pejovnik, S. Impedance spectroscopy as a technique for studying the spontaneous passivation of metals in electrolytes. Electrochim. Acta 1996, 41, 1137–1142. [Google Scholar] [CrossRef]
- Shi, Z.-P.; Wang, Z.-B.; Wang, J.-Q.; Qiao, Y.-X.; Chen, H.-N.; Xiong, T.-Y.; Zheng, Y.-G. Effect of Ni Interlayer on Cavitation Erosion Resistance of NiTi Cladding by Tungsten Inert Gas (TIG) Surfacing Process. Acta Metall. Sin. (Engl. Lett.) 2019, 33, 415–424. [Google Scholar] [CrossRef]









| Powders | Ni | Ti | Fe | Nb | Co | C | Si | O |
|---|---|---|---|---|---|---|---|---|
| Pure Ni | Bal. | – | 0.003 | – | 0.020 | 0.020 | 0.003 | 0.006 |
| 55NiTi | 56.46 | Bal. | 0.005 | 0.010 | 0.005 | 0.005 | – | 0.037 |
| Fe | Cr | Ni | Mo | Mn | Si | P | C | S |
|---|---|---|---|---|---|---|---|---|
| Bal. | 16.32 | 10.12 | 2.04 | 0.92 | 0.34 | 0.026 | 0.016 | 0.015 |
| Coating | Position | Ni (at.%) | Ti (at.%) | Fe (at.%) | Cr (at.%) | O (at.%) | Major Phase |
|---|---|---|---|---|---|---|---|
| 55NiTi | 1 | 31.40 | 32.83 | 17.21 | 10.73 | 7.83 | NiTi |
| 2 | 58.12 | 27.45 | 9.40 | 4.12 | 0.91 | Ni3Ti | |
| 3 | 14.72 | 24.54 | 15.21 | 5.41 | 40.12 | TiO2 | |
| 55NiTi + 5Ni | 4 | 29.84 | 30.15 | 19.86 | 12.40 | 7.75 | NiTi |
| 5 | 61.82 | 21.28 | 7.21 | 5.41 | 4.28 | Ni3Ti | |
| 6 | 16.97 | 20.31 | 16.46 | 7.48 | 38.78 | TiO2 |
| Sample | 55NiTi + 5Ni | 55NiTi | 316L |
|---|---|---|---|
| OCP (V) | −0.495 | −0.367 | 0.201 |
| Sample | Ecorr (V) | Icorr (μA/cm2) | Corrosion Rate (mpy) |
|---|---|---|---|
| 55NiTi + 5Ni | −0.50 | 2.12 | 1.32 |
| 55NiTi | −0.61 | 5.47 | 3.50 |
| 316L | 0.17 | 60.80 | 31.14 |
| Coatings | Rs (Ω·cm2) | R1 (Ω·cm2) | Rct (Ω·cm2) | CPE1 (Ω−1·cm2·sn) | n1 | CPE2 (Ω−1·cm2·sn) | n2 |
|---|---|---|---|---|---|---|---|
| 55NiTi | 8.40 | 4.12 × 104 | 2.14 × 105 | 3.05 × 10−5 | 0.81 | 6.21 × 10−5 | 0.88 |
| 55NiTi + 5Ni | 10.82 | 7.53 × 104 | 2.35 × 105 | 5.70 × 10−5 | 0.97 | 4.86 × 10−5 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Du, Z.; Hu, Z. Effect of Ni Addition on the Corrosion Resistance of NiTi Alloy Coatings on AISI 316L Substrate Prepared by Laser Cladding. Coatings 2021, 11, 1139. https://doi.org/10.3390/coatings11091139
Feng Y, Du Z, Hu Z. Effect of Ni Addition on the Corrosion Resistance of NiTi Alloy Coatings on AISI 316L Substrate Prepared by Laser Cladding. Coatings. 2021; 11(9):1139. https://doi.org/10.3390/coatings11091139
Chicago/Turabian StyleFeng, Yuqiang, Zexu Du, and Zhengfei Hu. 2021. "Effect of Ni Addition on the Corrosion Resistance of NiTi Alloy Coatings on AISI 316L Substrate Prepared by Laser Cladding" Coatings 11, no. 9: 1139. https://doi.org/10.3390/coatings11091139
APA StyleFeng, Y., Du, Z., & Hu, Z. (2021). Effect of Ni Addition on the Corrosion Resistance of NiTi Alloy Coatings on AISI 316L Substrate Prepared by Laser Cladding. Coatings, 11(9), 1139. https://doi.org/10.3390/coatings11091139






