Novel Structures of Functionalized Graphene Oxide with Hydrazide: Characterization and Bioevaluation of Antimicrobial and Cytocompatibility Features
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide
2.2. Functionalization of Graphene Oxide with Hydrazides
2.3. Characterization of Functional Materials
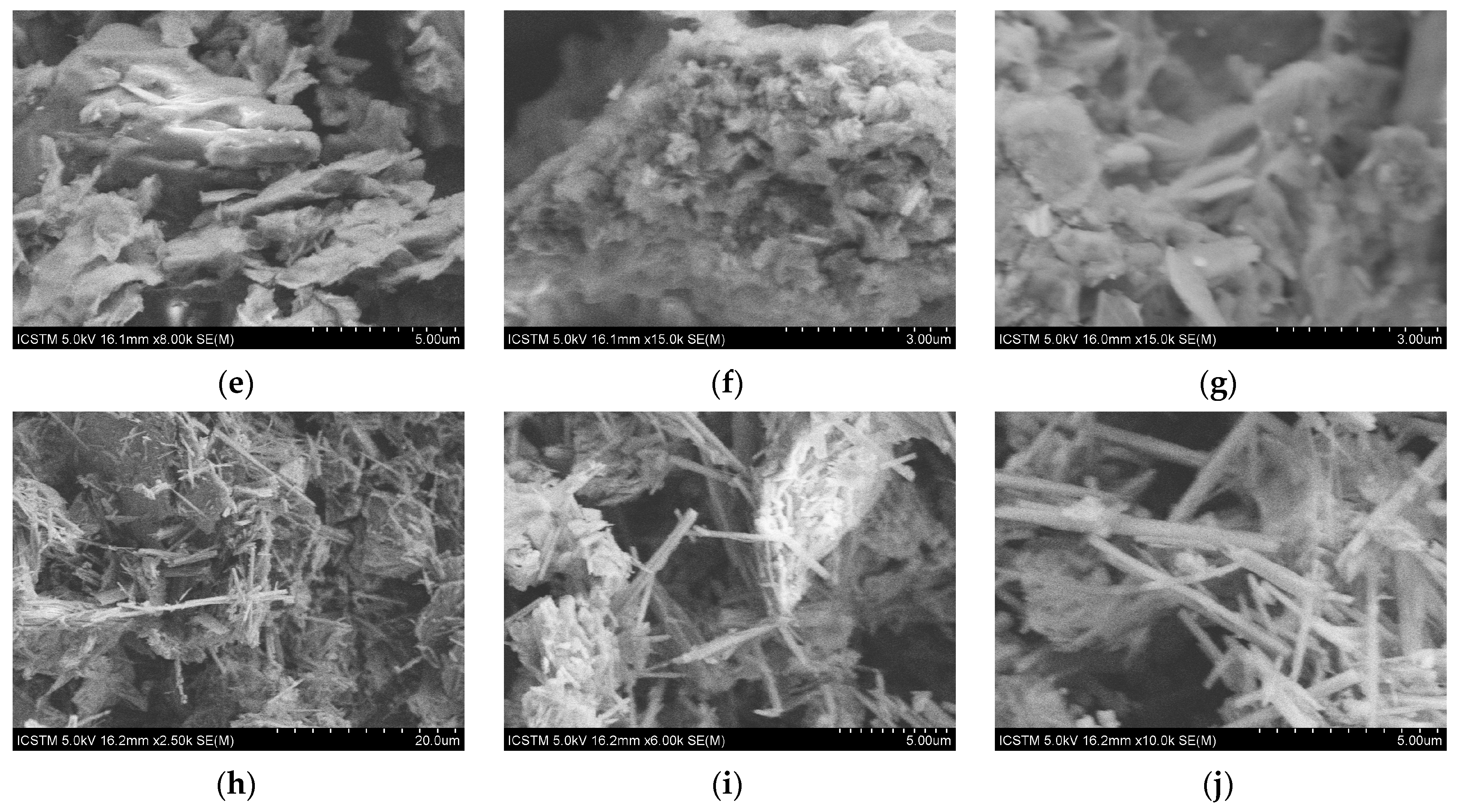
2.3.1. FE-SEM-EDS
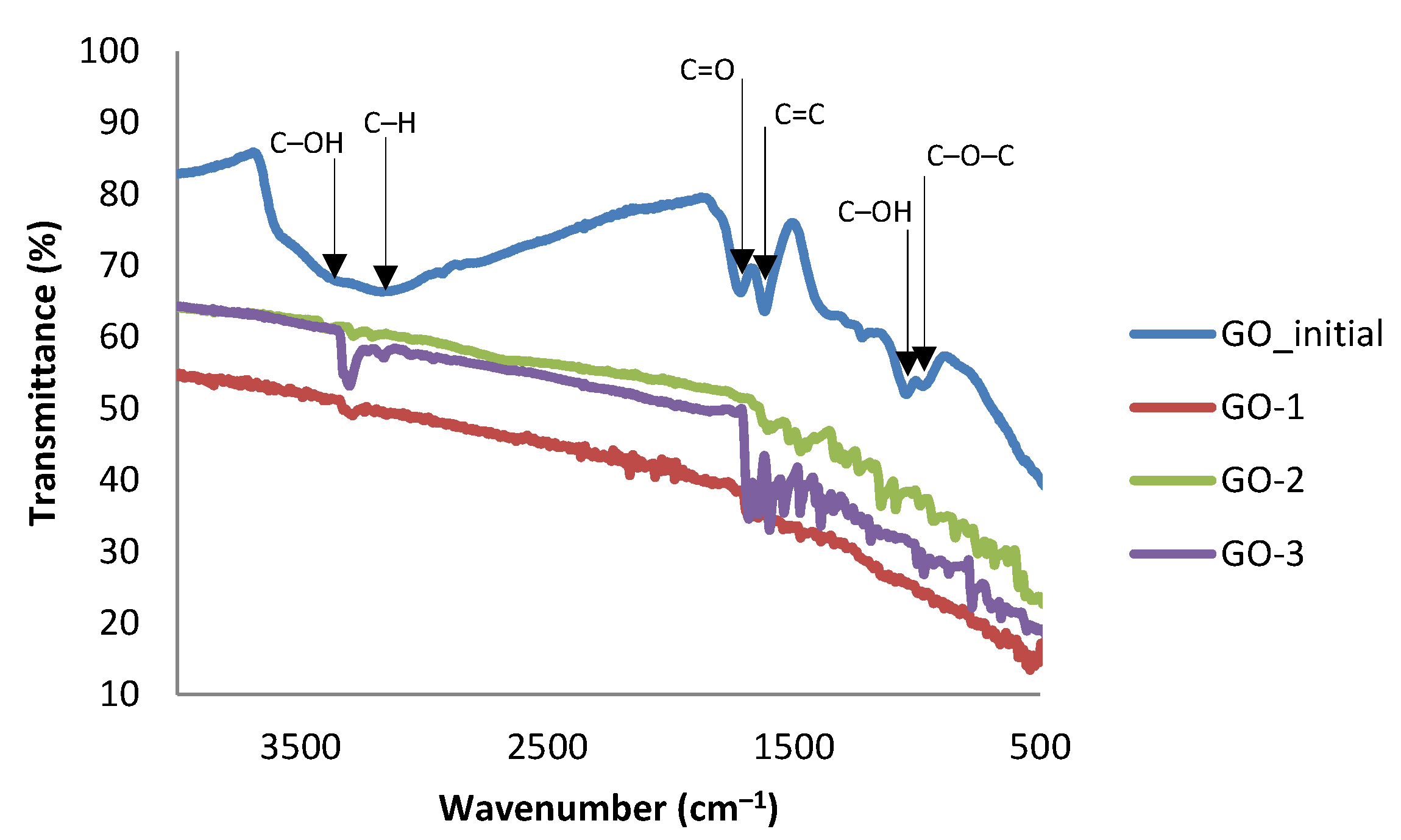
2.3.2. ATR-FTIR Spectroscopy
2.3.3. Raman Spectroscopy
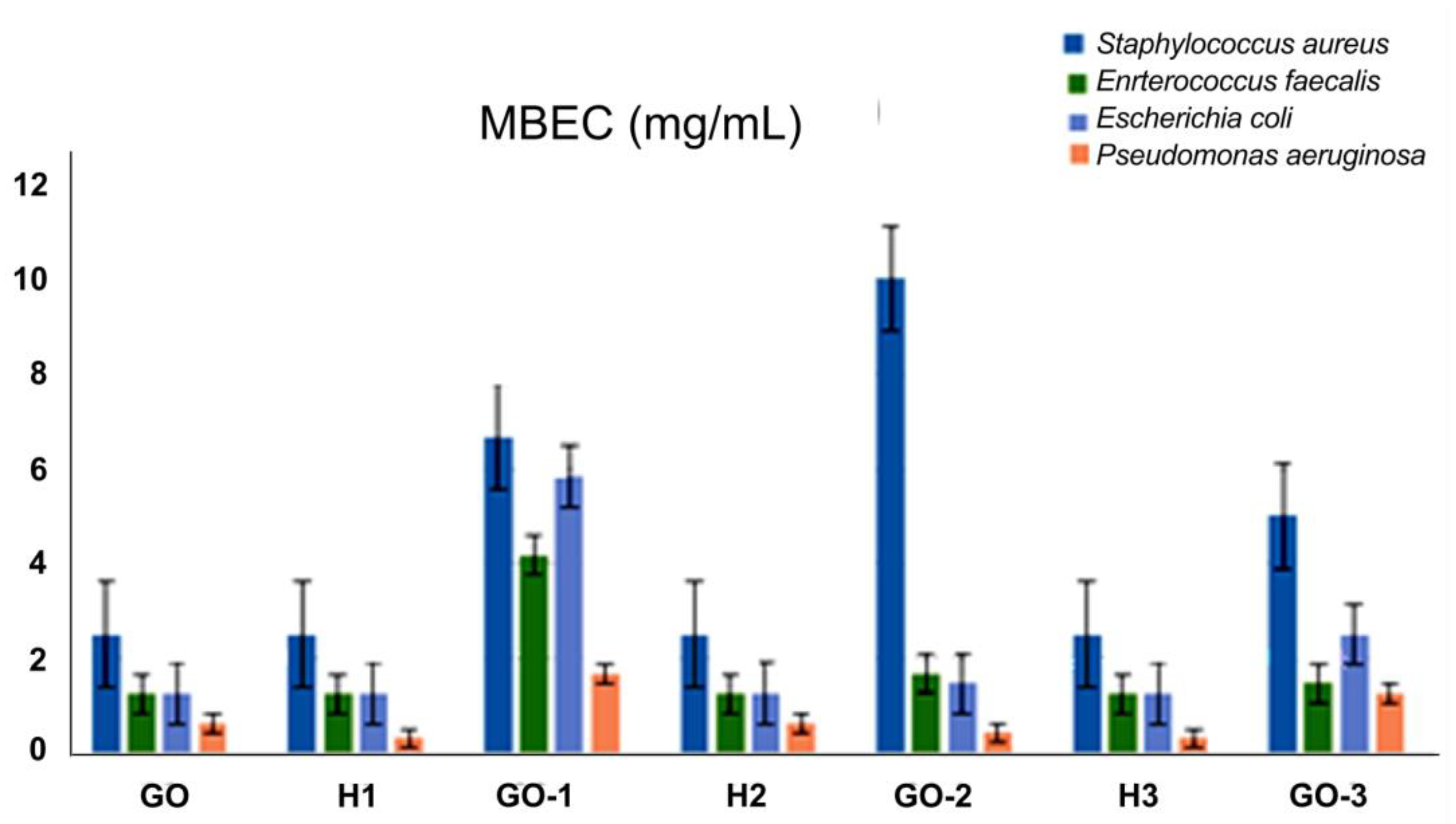
2.4. Antimicrobial Activity Assay
2.5. Biocompatiblity Assays
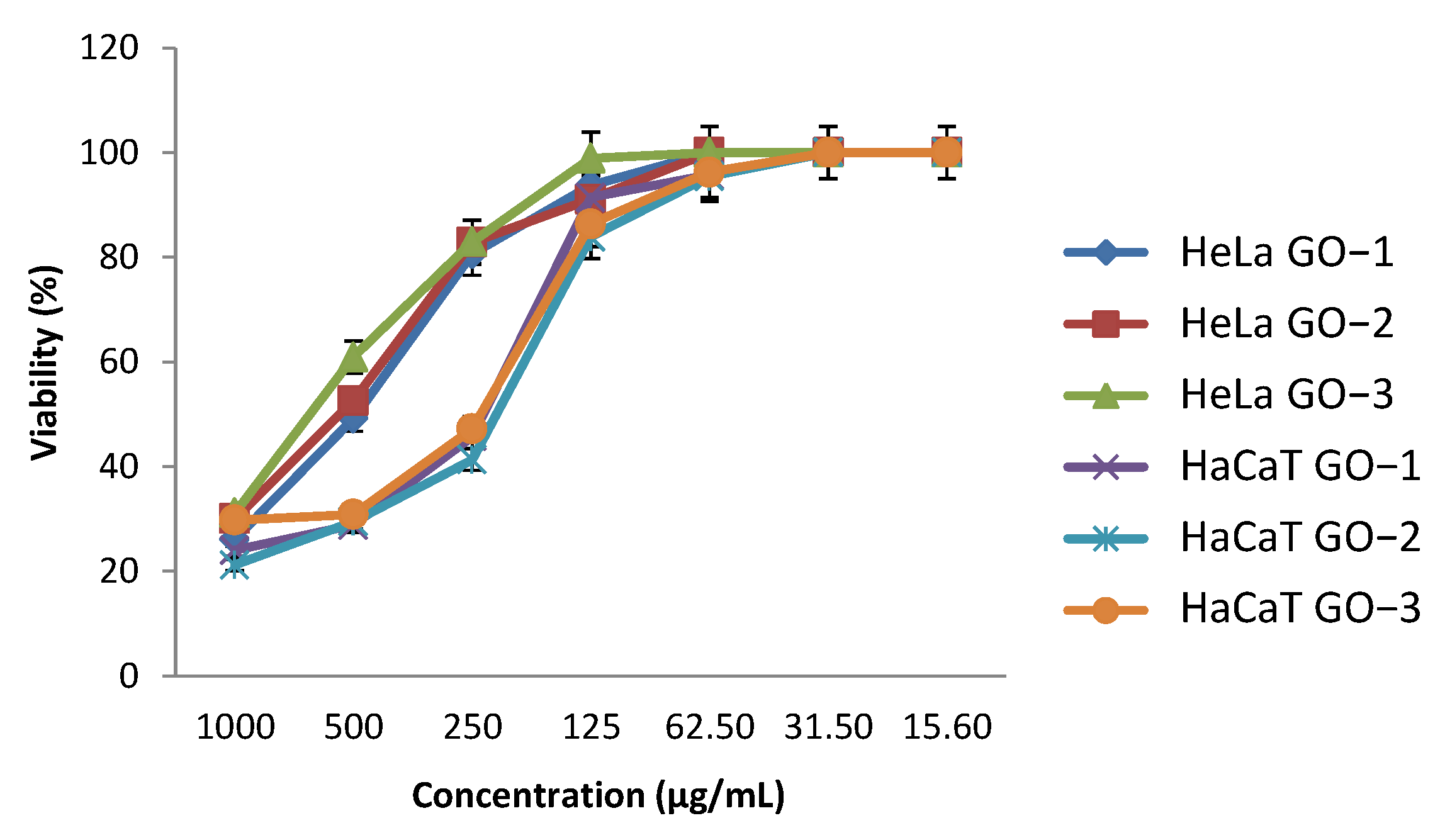
2.5.1. Human Cellular Viability Assay
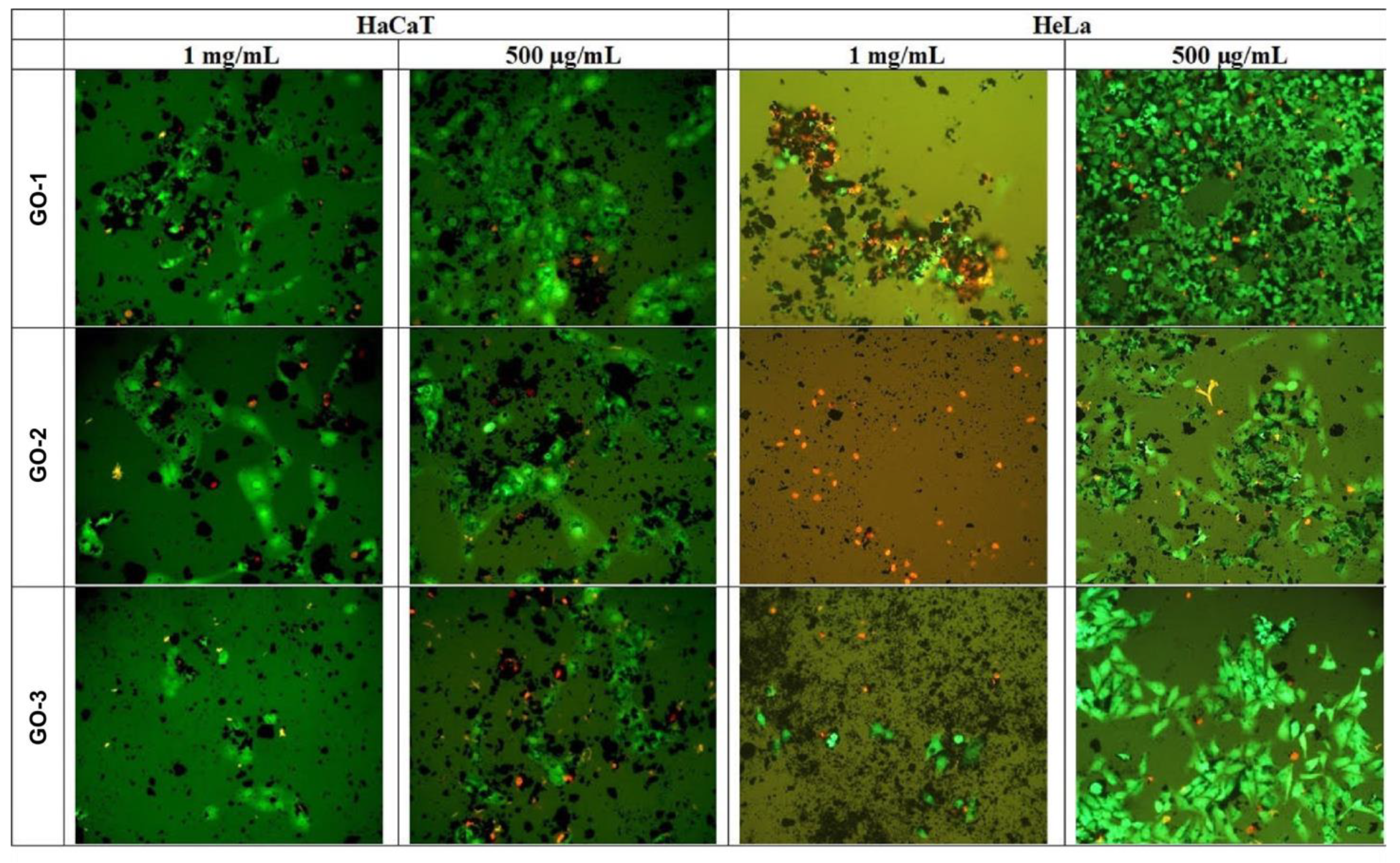
2.5.2. Fluorescein Diacetate (FDA)-Propidium Iodide (PI) Cell Staining for Microscopic Examination of Treated Cells
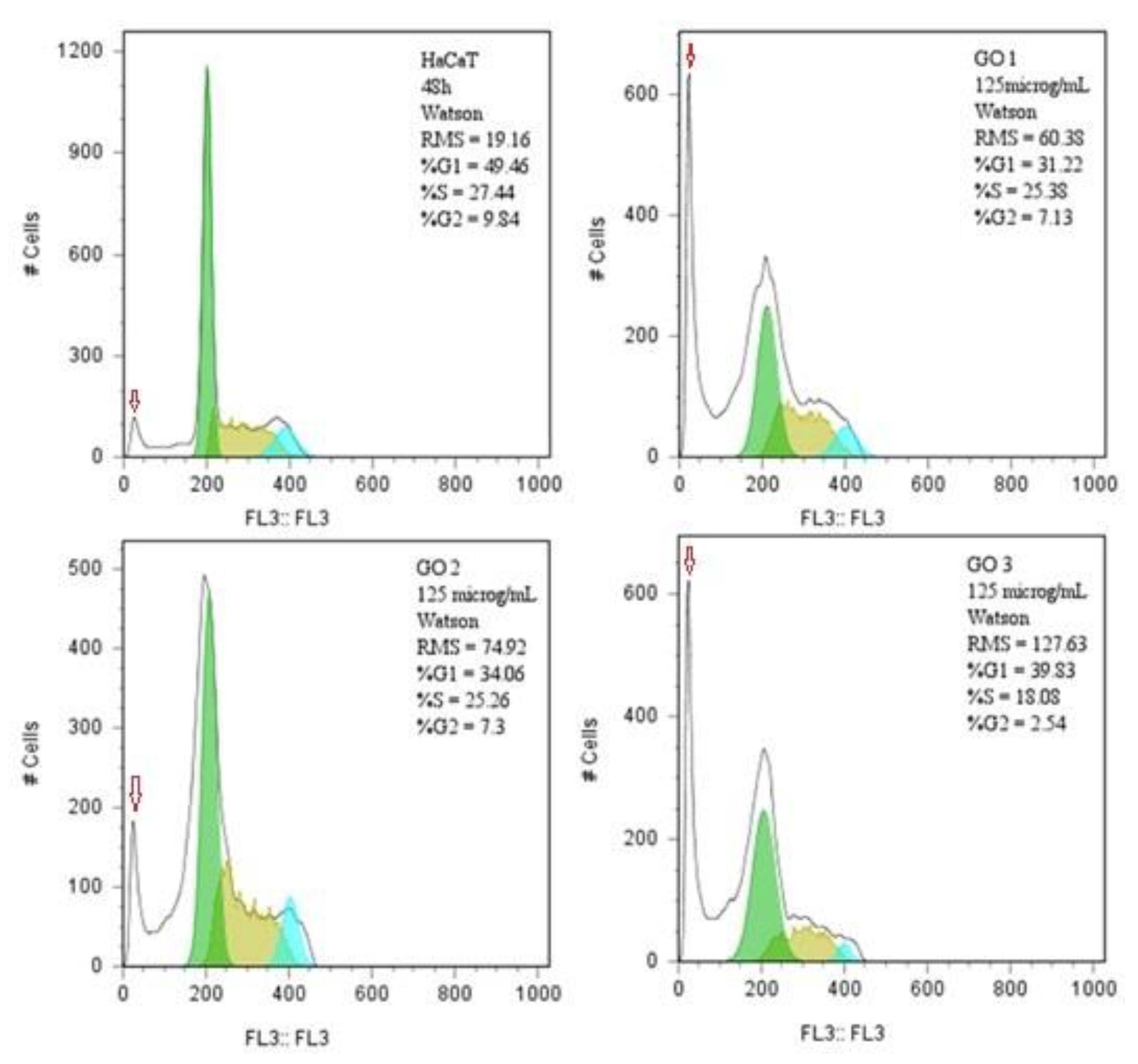
2.5.3. Assessment of the Compounds’ Influence on the Cell Cycle Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Videira-Quintela, D.; Guillén, F.; Montalvo, G.; Martin, O. Silver, copper, and copper hydroxy salt decorated fumed silica hybrid composites as antibacterial agents. Colloids Surf. B Biointerfaces 2020, 195, 111216. [Google Scholar] [CrossRef]
- Tavakolian, M.; Jafari, S.M.; van de Ven, T.G.M. A review on surface-functionalized cellulosic nanostructures as biocompatible antibacterial materials. Nano-Micro Lett. 2020, 12, 1–23. [Google Scholar] [CrossRef]
- Menazea, A.; Ahmed, M. Synthesis and antibacterial activity of graphene oxide decorated by silver and copper oxide nanoparticles. J. Mol. Struct. 2020, 1218, 128536. [Google Scholar] [CrossRef]
- Turcu, I.; Zarafu, I.; Popa, M.; Chifiriuc, M.C.; Bleotu, C.; Culita, D.; Ghica, C.; Ionita, P. Lipoic acid gold nanoparticles functionalized with organic compounds as bioactive materials. Nanomaterials 2017, 7, 43. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Song, Q.; Jiang, Z.; Li, N.; Liu, P.; Liu, L.; Tang, M.; Cheng, G. Anti-inflammatory effects of three-dimensional graphene foams cultured with microglial cells. Biomaterials 2014, 35, 6930–6940. [Google Scholar] [CrossRef]
- Luo, J.; Lai, J.; Zhang, N.; Liu, Y.; Liu, R.; Liu, X. Tannic acid induced self-assembly of three-dimensional graphene with good adsorption and antibacterial properties. ACS Sustain. Chem. Eng. 2016, 4, 1404–1413. [Google Scholar] [CrossRef]
- Jakus, A.E.; Secor, E.B.; Rutz, A.L.; Jordan, S.W.; Hersam, M.C.; Shah, R.N. Three-dimensional printing of high-content graphene scaffolds for electronic and biomedical applications. ACS Nano 2015, 9, 4636–4648. [Google Scholar] [CrossRef]
- Si, C.; Sun, Z.; Liu, F. Strain engineering of graphene: A review. Nanoscale 2016, 8, 3207–3217. [Google Scholar] [CrossRef]
- Ullah, S.; Hasan, M.; Ta, H.Q.; Zhao, L.; Shi, Q.; Fu, L.; Choi, J.-H.; Yang, R.; Liu, Z.; Rümmeli, M.H. synthesis of doped porous 3d graphene structures by chemical vapor deposition and its applications. Adv. Funct. Mater. 2019, 29, 1904457. [Google Scholar] [CrossRef]
- Maab, R.; Zhouab, Y.; Bia, H.; Yangbc, M.; Wangab, J.; Liua, Q.; Huanga, F. Multidimensional graphene structures and beyond: Unique properties, syntheses and applications. Prog. Mater. Sci. 2020, 113, 100665. [Google Scholar] [CrossRef]
- Pavan, F.R.; Maia, P.I.D.S.; Leite, S.R.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazones: Anti-Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar] [CrossRef]
- Vicini, P.; Zani, F.; Cozzini, P.; Doytchinova, I. Hydrazones of 1,2-benzisothiazole hydrazides: Synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2002, 37, 553–564. [Google Scholar] [CrossRef]
- Visbal, G.; Marchán, E.; Maldonado, A.; Simoni, Z.; Navarro, M. Synthesis and characterization of platinum–sterol hydrazone complexes with biological activity against Leishmania (L.) mexicana. J. Inorg. Biochem. 2008, 102, 547–554. [Google Scholar] [CrossRef]
- Gemma, S.; Kukreja, G.; Fattorusso, C.; Persico, M.; Romano, M.P.; Altarelli, M.; Savini, L.; Campiani, G.; Fattorusso, E.; Basilico, N.; et al. Synthesis of N1-arylidene-N2-quinolyl- and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg. Med. Chem. Lett. 2006, 16, 5384–5388. [Google Scholar] [CrossRef]
- Vicini, P.; Incerti, M.; Doytchinova, I.; La Colla, P.; Busonera, B.; Loddo, R. Synthesis and antiproliferative activity of benzo[d]isothiazole hydrazones. Eur. J. Med. Chem. 2006, 41, 624–632. [Google Scholar] [CrossRef]
- Silva, G.A.; Costa, L.M.; Brito, F.C.; Miranda, A.L.; Barreiro, E.J.; Fraga, C.A. New class of potent antinociceptive and antiplatelet 10H-phenothiazine-1-acylhydrazone derivatives. Bioorg. Med. Chem. 2004, 12, 3149–3158. [Google Scholar] [CrossRef]
- Kulandasamy, R.; Adhikari, A.V.; Stables, J.P. A new class of anticonvulsants possessing 6Hz activity: 3,4-Dialkyloxy thiophene bishydrazones. Eur. J. Med. Chem. 2009, 44, 4376–4384. [Google Scholar] [CrossRef]
- Sztanke, M.; Sztanke, K. Biologically important hydrazide-containing fused azaisocytosines as antioxidant agents. Redox Rep. 2017, 22, 572–581. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Damu, G.L.; Zhang, L.; Geng, R.-X.; Zhou, C.-H. Synthesis and biological evaluation of novel benzimidazole derivatives and their binding behavior with bovine serum albumin. Eur. J. Med. Chem. 2012, 55, 164–175. [Google Scholar] [CrossRef]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist? J. Enzym. Inhib. Med. Chem. 2015, 31, 345–360. [Google Scholar] [CrossRef]
- E Boyd, A. Sulfonylurea receptors, ion channels, and fruit flies. Diabetes 1988, 37, 847–850. [Google Scholar] [CrossRef]
- Al-Otaibi, F. An overview of structurally diversified anticonvulsant agents. Acta Pharm. 2019, 69, 321–344. [Google Scholar] [CrossRef]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med. Chem. 2014, 6, 1149–1165. [Google Scholar] [CrossRef]
- Rotaru, I.D.; Nuta, D.C.; Chirita, I.C.; Caproiu, M.T.; Limban, C.; Missir, A.V.; Chirita, C. New synthesis in the N-[4[(phenylcarbamoyl)amino]-phenyl]benzenesulfonamide derivatives series. Farmacia 2016, 64, 828–833. [Google Scholar]
- De Souza, G.D.; Rodrigues, M.A.; Silva-Caldeira, P.; Maia, E.C.P.; Botelho, F.; De Campos, T.A.; Franca, E.; De Almeida, K.J.; Guerra, W. A new complex of palladium(II) with 2-furoic hydrazide: Synthesis, characterization, theoretical calculations and biological studies. Croat. Chem. Acta 2013, 86, 201–206. [Google Scholar] [CrossRef]
- Haseena Banu, B.; Prasad, K.V.S.R.G.; Bharathi, K. Biological importance of quinazolin-4-one scaffold and its derivatives—A brief update. Int. J. Pharm. Pharm. Sci. 2015, 7, 1–7. [Google Scholar]
- Bratu, M.; Olaru, O.T.; Chirita, I.C.; Dinu, M.; Anghel, A.I. Phytobiological testing of some compounds with 4(3H)-quinazolone structure. Farmacia 2014, 62, 929–941. [Google Scholar]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Tudose, M.; Anghel, E.M.; Culita, D.; Somacescu, S.; Calderon-Moreno, J.; Tecuceanu, V.; Dumitrascu, F.D.; Dracea, O.; Popa, M.; Marutescu, L.; et al. Covalent coupling of tuberculostatic agents and graphene oxide: A promising approach for enhancing and extending their antimicrobial applications. Appl. Surf. Sci. 2019, 471, 553–565. [Google Scholar] [CrossRef]
- Zarafu, I.; Turcu, I.; Culiță, D.C.; Petrescu, S.; Popa, M.; Chifiriuc, M.C.; Limban, C.; Telehoiu, A.; Ioniță, P. Antimicrobial features of organic functionalized graphene-oxide with selected amines. Materials 2018, 11, 1704. [Google Scholar] [CrossRef]
- Avramescu, S.; Petrescu, S.; Culita, D.C.; Tudose, M.; Hanganu, A.; Zarafu, I.; Ionita, P. A mixed organic functionalized silica-graphene oxide as advanced material for pollutant removal. J. Nanopart. Res. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Capasso, C.; Supuran, C.T. Sulfa and trimethoprim-like drugs—Antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J. Enzym. Inhib. Med. Chem. 2013, 29, 379–387. [Google Scholar] [CrossRef]
- Stecoza, C.E.; Caproiu, M.T.; Draghici, C.; Chifiriuc, M.C.; Dracea, N.O. Synthesis, characterization and antimicrobial activity evaluation of some new derivatives of 6,11-dihydrodibenzo[b,e]thiepin 5,5-dioxide. Rev. Chim. 2009, 60, 137–141. [Google Scholar]
- Patrinoiu, G.; Calderón-Moreno, J.M.; Chifiriuc, M.C.; Saviuc, C.; Birjega, R.; Carp, O. Tunable ZnO spheres with high anti-biofilm and antibacterial activity via a simple green hydrothermal route. J. Colloid Interface Sci. 2016, 462, 64–74. [Google Scholar] [CrossRef]
- AAT Bioquest Inc. Quest Graph™ IC50 Calculator. 2020. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 20 August 2020).
- Nitulescu, G.M.; Matei, L.; Aldea, I.M.; Draghici, C.; Olaru, O.T.; Bleotu, C. Ultrasound-assisted synthesis and anticancer evaluation of new pyrazole derivatives as cell cycle inhibitors. Arab. J. Chem. 2019, 12, 816–824. [Google Scholar] [CrossRef]
- Zanni, E.; Chandraiahgari, C.R.; De Bellis, G.; Montereali, M.R.; Armiento, G.; Ballirano, P.; Polimeni, A.; Sarto, M.S.; Uccelletti, D. Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans. Nanomaterials 2016, 6, 179. [Google Scholar] [CrossRef]
- Jiao, T.; Hu, J.; Zhang, Q.; Xiao, Y. Preparation and Self-assembly of Functionalized Nanocomposites and Nanomaterials—Relationship Between Structures and Properties. In Nanomaterials—Toxicity and Risk Assessment; IntechOpen: London, UK, 2015; pp. 177–210. [Google Scholar]
- Dobrota, A.S.; Pašti, I.A.; Mentus, S.V.; Skorodumova, N.V. A general view on the reactivity of the oxygen-functionalized graphene basal plane. Phys. Chem. Chem. Phys. 2016, 18, 6580–6586. [Google Scholar] [CrossRef]
- Loryuenyong, V.; Totepvimarn, K.; Eimburanapravat, P.; Boonchompoo, W.; Buasri, A. preparation and characterization of reduced graphene oxide sheets via water-based exfoliation and reduction methods. Adv. Mater. Sci. Eng. 2013, 2013, 1–5. [Google Scholar] [CrossRef]
- Andrei, V.A.; Radulescu, C.; Malinovschi, V.; Marin, A.; Coaca, E.; Mihalache, M.; Mihailescu, C.N.; Dulama, I.D.; Teodorescu, S.; Bucurica, I.A. Aluminum oxide ceramic coatings on 316l austenitic steel obtained by plasma electrolysis oxidation using a pulsed unipolar power supply. Coatings 2020, 10, 318. [Google Scholar] [CrossRef]
- Malinovschi, V.; Marin, A.H.; Andrei, V.; Coaca, E.; Mihailescu, C.; Lungu, C.P.; Radulescu, C.; Dulama, I.D. Obtaining and characterization of PEO layers prepared on CP-Ti in sodium dihydrogen phosphate dihydrate acidic electrolyte solution. Surf. Coat. Technol. 2019, 375, 621–636. [Google Scholar] [CrossRef]
- David, M.; Serban, A.; Radulescu, C.; Danet, A.F.; Florescu, M. Bioelectro chemical evaluation of plant extracts and gold nanozyme-based sensors for total antioxidant capacity determination. Bioelectrochemistry 2019, 129, 124–134. [Google Scholar] [CrossRef]
- Radulescu, C.; Olteanu, R.L.; Stihi, C.; Florescu, M.; Lazurca, D.; Dulama, I.D.; Stirbescu, R.M.; Teodorescu, S. Chemometric Assessment of Spectroscopic Techniques and Antioxidant Activity for Hippophae rhamnoides L. Extracts Obtained by Different Isolation Methods. Anal. Lett. 2019, 52, 2393–2415. [Google Scholar] [CrossRef]
- Zarafu, I.; Matei, L.; Bleotu, C.; Ionita, P.; Tatibouët, A.; Păun, A.; Nicolau, I.; Hanganu, A.; Limban, C.; Nuta, D.; et al. Synthesis, characterization, and biologic activity of new acyl hydrazides and 1,3,4-oxadiazole derivatives. Molecules 2020, 25, 3308. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Srivastava, V.C. Simple synthesis of large graphene oxide sheets via electrochemical method coupled with oxidation process. ACS Omega 2018, 3, 10233–10242. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew Energy Env. Eng. 2014, 2, 58–63. [Google Scholar]
- Guerrero-Contreras, J.; Caballero-Briones, F. Graphene oxide powders with different oxidation degree, prepared by synthesis variations of the Hummers method. Mater. Chem. Phys. 2015, 153, 209–220. [Google Scholar] [CrossRef]
- Rădulescu, C.; Ioniţă, I.; Hossu, A. Synthesis of linear bis-thiazolo[2,3-d][8,9-d]trans-quinacridone. Dye. Pigment. 2005, 65, 175–177. [Google Scholar] [CrossRef]
- Innocenti, F.; Milani, A.; Castiglioni, C. Can Raman spectroscopy detect cumulenic structures of linear carbon chains? J. Raman Spectrosc. 2009, 41, 226–236. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Negrea, A.; Bacinschi, Z.; Bucurica, I.A.; Teodorescu, S.; Stirbescu, R. A new material for bipolar plates used in fuel cells. Rom. J. Phys. 2016, 61, 527–535. [Google Scholar]
- King, A.; Davies, B.R.; Noorbehesht, N.; Newman, P.; Church, T.L.; Harris, A.T.; Razal, J.; Minett, A.I. A new raman metric for the characterisation of graphene oxide and its derivatives. Sci. Rep. 2016, 6, 19491. [Google Scholar] [CrossRef]
- Verma, S.; Dutta, R.K. Development of cysteine amide reduced graphene oxide (CARGO) nano-adsorbents for enhanced uranyl ions removal from aqueous medium. J. Environ. Chem. Eng. 2017, 5, 4547–4558. [Google Scholar] [CrossRef]
- Smith, S.; Waters, V.; Jahnke, N.; Ratjen, F. Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis. Cochrane Database Syst. Rev. 2020, 2021, CD009528. [Google Scholar] [CrossRef]
- Rashdan, H.; Nasr, S.; El-Refai, H.A.; Abelaziz, M. A novel approach of potent antioxidant and antimicrobial agents containing coumarin moiety accompanied with cytotoxicity studies on the newly synthesized derivatives. J. Appl. Pharm. Sci. 2017, 7, 186–196. [Google Scholar] [CrossRef][Green Version]






| Sample | GO | GO-1 | GO-2 | GO-3 | |
|---|---|---|---|---|---|
| Element | |||||
| C | 62.84 ± 0.29 | 76.69 ± 0.33 | 76.05 ± 0.29 | 76.36 ± 0.38 | |
| N | nd * | 7.87 ± 0.08 | 5.99 ± 0.08 | 8.00 ± 0.18 | |
| O | 33.42 ± 0.22 | 11.82 ± 0.18 | 13.08 ± 0.22 | 14.97 ± 0.35 | |
| Na | nd * | nd * | 0.06 ± 0.01 | nd * | |
| Mg | nd * | nd * | 0.04 ± 0.01 | nd * | |
| Al | 0.14 ± 0.01 | 0.05 ± 0.01 | 0.13 ± 0.01 | 0.04 ± 0.01 | |
| Si | nd * | 0.05 ± 0.01 | 0.21 ± 0.01 | 0.15 ± 0.01 | |
| S | 2.15 ± 0.04 | 2.25 ± 0.01 | 1.59 ± 0.01 | nd * | |
| Cl | 0.82 ± 0.05 | 1.10 ± 0.01 | 0.72 ± 0.01 | 0.28 ± 0.01 | |
| K | 0.63 ± 0.03 | 0.17 ± 0.01 | 1.82 ± 0.02 | 0.20 ± 0.02 | |
| Ca | nd * | nd * | 0.31 ± 0.02 | nd * | |
| Wavenumber (cm−1) & Relative Intensity * | Vibrational Assignment | |||
|---|---|---|---|---|
| GO | GO-1 | GO-2 | GO-3 | |
| 3680 w | 3687 w | 3690 w | 3689 w | O–H stretching |
| 3641 w | 3646 w | 3639 w | 3641 w | O–H stretching |
| 3620 w | 3627 w | 3622 w | 3629 w | O–H stretching |
| 3408 m | 3412 m | 3413 m | 3410 m | –OH stretch in carboxylic; C–OH group (GO) |
| – | 3316 s | 3318 s | 3327 s | N–H proton stretching vibration from amide |
| – | 3293 s | 3289 m | 3302 s | N–H proton stretching vibration |
| – | – | 3229 m | – | N–H stretching vibration of sulfonamide |
| 3169 m | 3161 m | 3170 m | 3162 m | C–H stretching of hydrocarbon |
| – | 3107 m | – | 3051 w | C–H stretching from furan cycle; C–H stretching of hydrocarbon |
| 2976 m | 2978 w | 2980 m | 2971 w | C–H stretching vibration |
| 2350 w | 2355 m | 2354 w | 2354 w | C=O stretching vibration |
| – | 2206 w | – | – | C–N, N-H stretching hydrazide |
| – | 2108 w | – | – | C=C stretching vibration from furan cycle |
| – | 1989 w | – | – | C=C stretching vibration from furan cycle |
| – | 1962 w | – | – | C=C stretching vibration from furan cycle |
| 1720 m | 1724 m | 1727 m | 1731 m | C=O stretching vibrations in carboxylic (GO) |
| – | – | 1682 m | 1682 m | C=C aromatic ring; N-C=O stretching on quinazolin-4-one |
| 1630 s | 1633 s | 1632 s | 1635 s | C=C aromatic stretch (GO); C=O with molecular bond δ from amide |
| 1616 m | 1614 m | 1616 m | 1615 m | C=C stretching vibration from aromatic ring (GO) |
| 1562 w | 1545 w | 1554 w | 1570 w | C=C stretching vibration (GO) |
| – | 1538 w | 1533 w | 1539 w | C–N, N–H stretching vibration from hydrazide group |
| – | 1516 w | 1517 m | 1523 w | C–N, N–H stretching vibration from hydrazide group |
| – | 1470 w | 1471 m | 1473 m | C–N of stretching vibration from hydrazide group |
| – | 1455 w | 1446 m | 1444 m | C–N stretching vibration from hydrazide group |
| 1404 m | 1405 m | 1408 m | 1409 m | C–O–H stretching vibration (GO) |
| 1388 m | 1390 m | 1389 m | 1389 m | C-OH stretching vibration (GO) |
| 1347 w | 1334 w | 1331 w | 1347 w | C–OH stretching vibration from GO; S=O stretching of –SO2–NH– group (sulfonamide) |
| – | 1319 m | 1322 m | 1326 m | C–N stretching vibration from hydrazide group |
| 1238 m | 1240 m | 1239 m | 1236 m | C–O–C stretching vibration (GO) |
| 1221 m | 1227 m | 1224 m | 1229 m | C–OH stretching vibration (GO) |
| – | – | 1180 s | – | S=O stretch from –SO2–NH– group (sulfonamide) |
| – | 1187 m | – | 1145 m | C=O–C stretch of furan cycle; N–C=O on cycle |
| 1085 m | 1082 m | 1085 m | 1086 m | C–O–C stretching vibration (GO) |
| – | - | 903 w | – | S–N stretching vibration from –SO2–N |
| – | 802 w | – | – | C–H stretching vibration from furan cycle |
| – | 787 w | – | – | C–H stretching vibration from furan cycle |
| – | 752 w | – | – | C–H stretching vibration from furan cycle |
| 698 w | 706 w | 716 w | 697 w | C–H stretching vibration (GO) |
| Wavenumber (cm−1) & Relative Intensity * | Vibrational Assignment | |||
|---|---|---|---|---|
| GO | GO-1 | GO-2 | GO-3 | |
| – | – | 1680 w | 1680 w | C=C aromatic stretch; N-C=O stretching vibration from quinazolin-4-one |
| 1622 w | 1623 w | 1628 w | 1631 w | C=C aromatic stretch (GO) |
| 1573 s | 1579 s | 1558 s | 1571 s | C=C stretching vibration mode of sp2 hybridized carbon atoms (GO) |
| – | 1533 m | 1538 m | 1542 m | C–N, N–H stretching vibration from hydrazide group |
| – | 1516 m | 1517 m | 1523 m | C–N, N–H stretching vibration from hydrazide group |
| – | 1471 m | 1473 m | 1479 m | C–N of stretching vibration from hydrazide group |
| – | 1452 w | 1457 m | 1459 m | C–N stretching vibration from hydrazide group |
| 1424 w | 1417 w | 1428 w | 1419 w | C–O–H stretching vibration (GO) |
| 1348 s | 1355 s | 1346 s | 1351 s | C–OH stretching vibration from GO |
| 1230 w | 1223 w | 1227 w | 1216 w | C–O–C stretching vibration (GO) |
| 1220 w | 1217 w | 1223 w | 1229 m | C–OH stretching vibration (GO) |
| – | – | 1165 m | – | S=O stretch from –SO2-NH– group (sulfonamide) |
| – | 1181 m | – | 1128 w | C=O–C stretching from furan cycle; N–C=O on cycle |
| 1080 m | 1089 m | 1091 m | 1090 m | C–O–C stretching vibration (GO) |
| – | – | 910 m | – | S–N stretching vibrations from –SO2–N |
| – | 801 m | – | – | C–H stretching vibration from furan cycle |
| – | 780 m | – | – | C–H stretching vibration from furan cycle |
| – | 751 m | – | – | C–H stretching vibration from furan cycle |
| 690 m | 704 m | 721 m | 695 m | C–H stretching vibration (GO) |
| – | – | 691 m | – | S=O stretching from –SO2 group |
| – | 659 m | – | – | C–H stretching vibration from furan cycle |
| – | – | 635 m | – | S=O stretching vibration from –SO2 group |
| – | 584 m | 584 m | 585 m | C=O stretching vibration from amide group |
| Cell Line | HeLa | HaCaT | ||||
|---|---|---|---|---|---|---|
| Functionalized graphene oxide | GO-1 | GO-2 | GO-3 | GO-1 | GO-2 | GO-3 |
| IC50 (µg/mL) | 417.69 | 448.47 | 569.62 | 201.43 | 181.13 | 186.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarafu, I.; Limban, C.; Radulescu, C.; Dulama, I.D.; Nuta, D.C.; Chirita, C.; Chifiriuc, M.C.; Badiceanu, C.D.; Popa, M.; Bleotu, C.; et al. Novel Structures of Functionalized Graphene Oxide with Hydrazide: Characterization and Bioevaluation of Antimicrobial and Cytocompatibility Features. Coatings 2022, 12, 45. https://doi.org/10.3390/coatings12010045
Zarafu I, Limban C, Radulescu C, Dulama ID, Nuta DC, Chirita C, Chifiriuc MC, Badiceanu CD, Popa M, Bleotu C, et al. Novel Structures of Functionalized Graphene Oxide with Hydrazide: Characterization and Bioevaluation of Antimicrobial and Cytocompatibility Features. Coatings. 2022; 12(1):45. https://doi.org/10.3390/coatings12010045
Chicago/Turabian StyleZarafu, Irina, Carmen Limban, Cristiana Radulescu, Ioana Daniela Dulama, Diana Camelia Nuta, Cornel Chirita, Mariana Carmen Chifiriuc, Carmellina Daniela Badiceanu, Marcela Popa, Coralia Bleotu, and et al. 2022. "Novel Structures of Functionalized Graphene Oxide with Hydrazide: Characterization and Bioevaluation of Antimicrobial and Cytocompatibility Features" Coatings 12, no. 1: 45. https://doi.org/10.3390/coatings12010045
APA StyleZarafu, I., Limban, C., Radulescu, C., Dulama, I. D., Nuta, D. C., Chirita, C., Chifiriuc, M. C., Badiceanu, C. D., Popa, M., Bleotu, C., Dragu, L. D., Stirbescu, R. M., Bucurica, I. A., Stanescu, S. G., & Ionita, P. (2022). Novel Structures of Functionalized Graphene Oxide with Hydrazide: Characterization and Bioevaluation of Antimicrobial and Cytocompatibility Features. Coatings, 12(1), 45. https://doi.org/10.3390/coatings12010045













