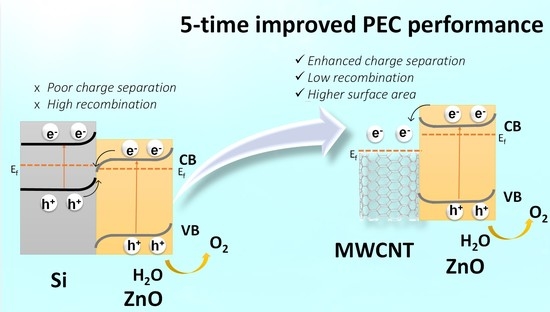
CNT-ZnO Core-Shell Photoanodes for Photoelectrochemical Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
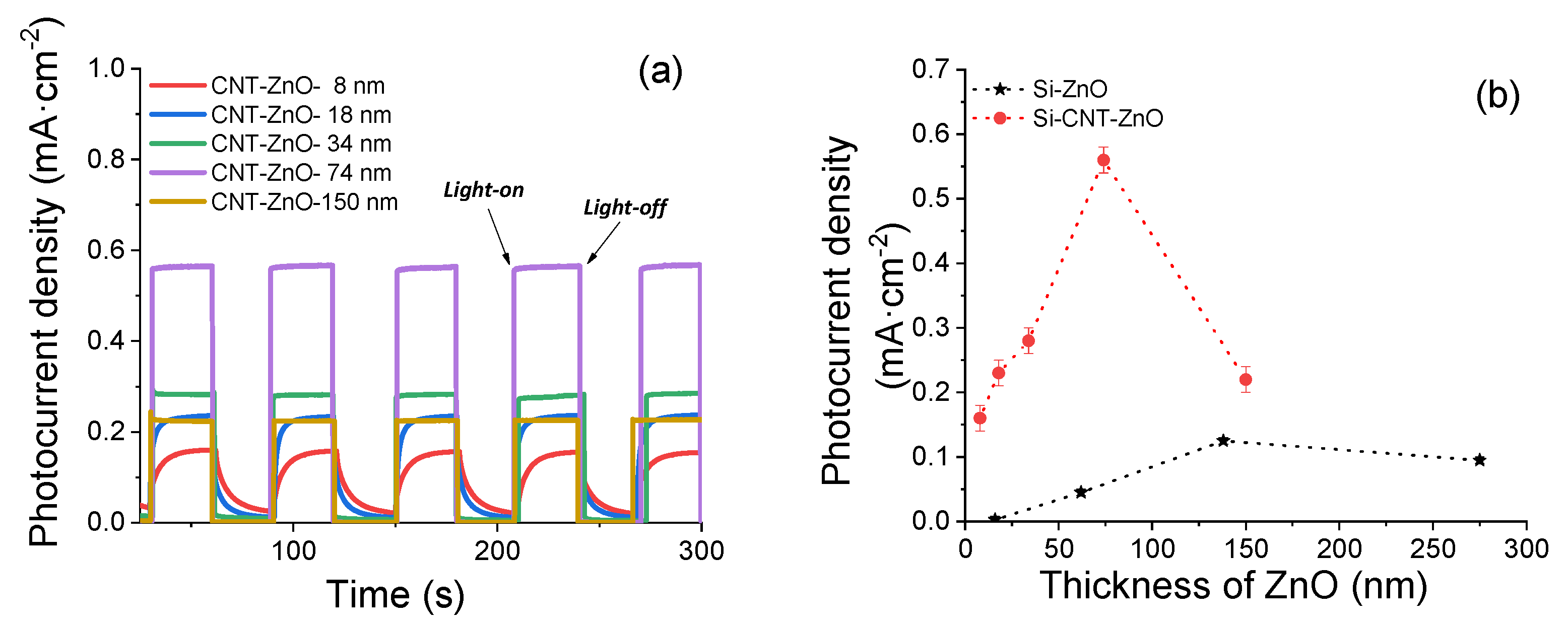
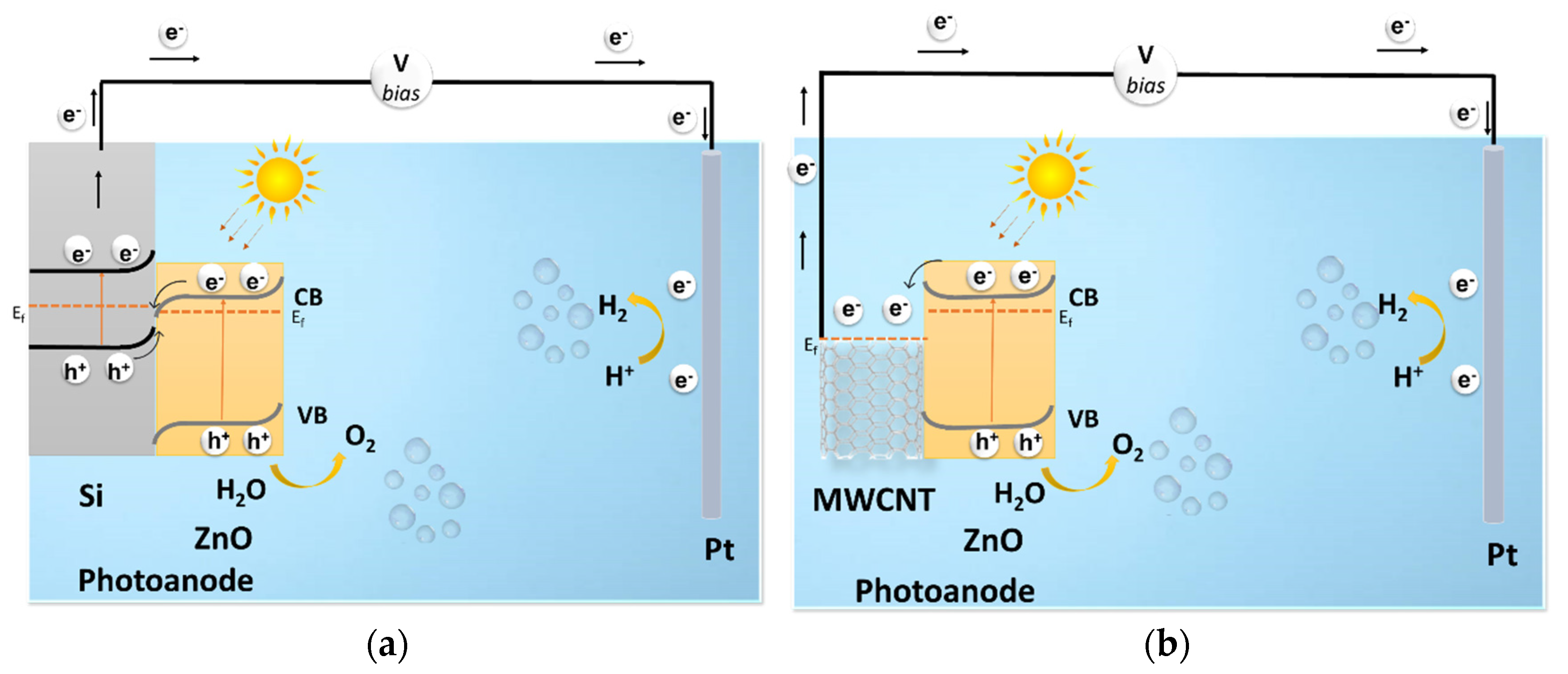
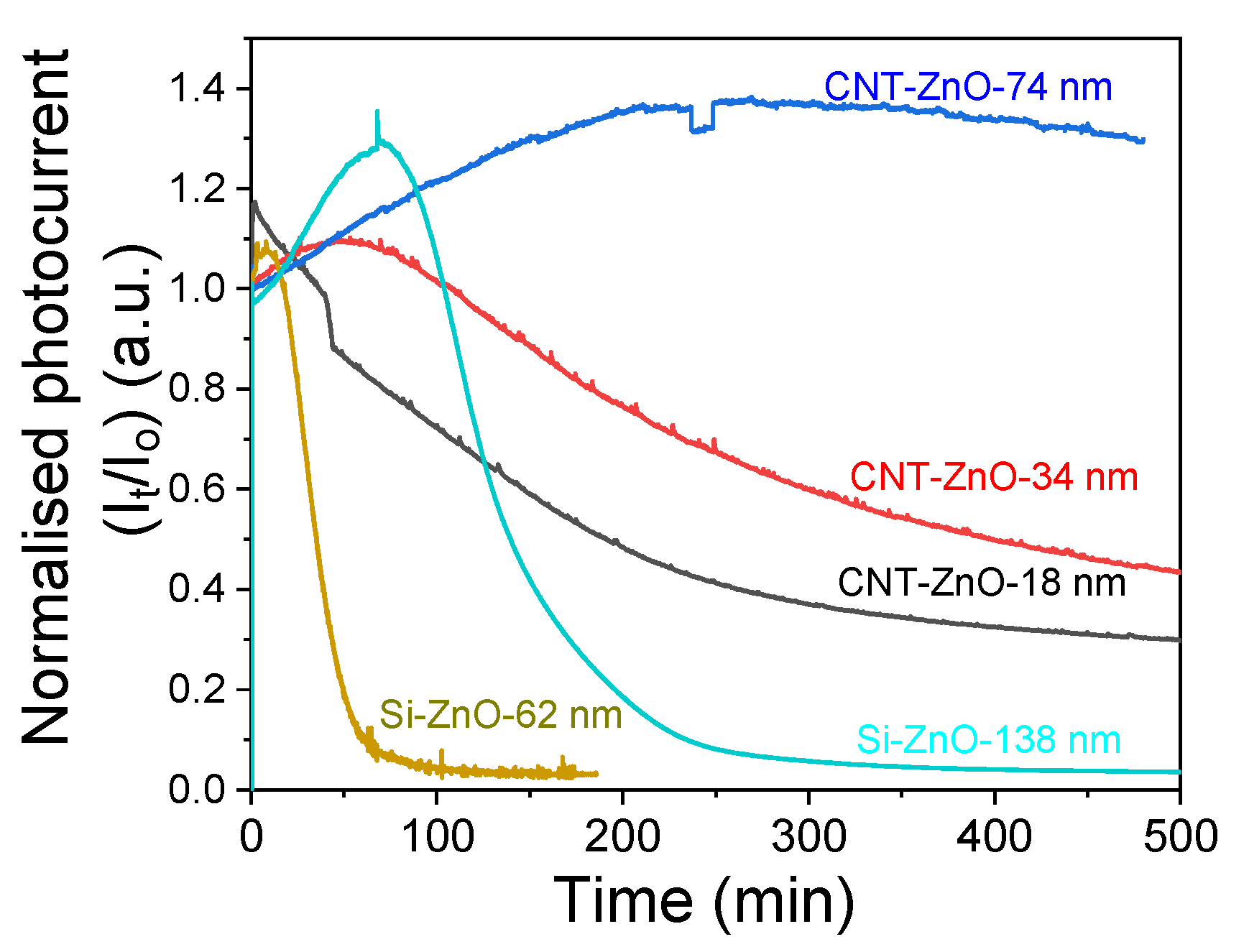
3. Results
- Transfer across the interface layer for the oxygen evolution reaction (OER) (faradaic process);
- Recombination with electrons at the surface (non-faradaic);
- Promotion of Zn2+ dissolution due to a reaction with the unsaturated O2− of ZnO [17]. (Photo corrosion).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Varghese, O.K.; Grimes, C.A. Appropriate strategies for determining the photoconversion efficiency of water photoelectrolysis cells: A review with examples using titania nanotube array photoanodes. Sol. Energy Mater. Sol. Cells 2008, 92, 374–384. [Google Scholar] [CrossRef]
- Panwar, N.L.; Kaushik, S.C.; Kothari, S. Role of renewable energy sources in environmental protection: A review. Renew. Sustain. Energy Rev. 2011, 15, 1513–1524. [Google Scholar] [CrossRef]
- PennState, 9.4. Hydrogen Storage. Available online: https://www.e-education.psu.edu/eme812/node/726 (accessed on 20 January 2021).
- Ogden, J.M. Prospects for building a hydrogen energy infrastructure. Annu. Rev. Energy Environ. 1999, 24, 227–279. [Google Scholar] [CrossRef]
- Kim, J.H.; Hansora, D.; Sharma, P.; Jang, J.W.; Lee, J.S. Toward practical solar hydrogen production-an artificial photosynthetic leaf-to-farm challenge. Chem. Soc. Rev. 2019, 48, 1908–1971. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; An, X.; Han, H.; Chen, J.Q.; Li, C. Photoelectrocatalytic materials for solar water splitting. Adv. Energy Mater. 2018, 8, 1–36. [Google Scholar] [CrossRef]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef]
- Ros, C.; Andreu, T.; Morante, J.R. Photoelectrochemical water splitting: A road from stable metal oxides to protected thin film solar cells. J. Mater. Chem. A 2020, 8, 10625–10669. [Google Scholar] [CrossRef]
- Li, J.; Wu, N. Semiconductor-based photocatalysts and photoelectrochemical cells for solar fuel generation: A review. Catal. Sci. Technol. 2015, 5, 1360–1384. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.W. Thermodynamic oxidation and reduction potentials of photocatalytic semiconductors in aqueous solution. Chem. Mater. 2012, 24, 3659–3666. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting-materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef] [Green Version]
- Osterloh, F.E. Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting. Chem. Soc. Rev. 2013, 42, 2294–2320. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Zinc oxide based photocatalysis: Tailoring surface-bulk structure and related interfacial charge carrier dynamics for better environmental applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Shaikh, A.F.; Arbuj, S.S.; Tamboli, M.S.; Naik, S.D.; Rane, S.B.; Kale, B.B. ZnSe/ZnO nano-heterostructures for enhanced solar light hydrogen generation. ChemistrySelect 2017, 2, 9174–9180. [Google Scholar] [CrossRef]
- Kegel, J.; Povey, I.M.; Pemble, M.E. Zinc oxide for solar water splitting: A brief review of the material’s challenges and associated opportunities. Nano Energy 2018, 54, 409–428. [Google Scholar] [CrossRef]
- McCafferty, E. Thermodynamics of corrosion: Pourbaix diagrams. In Introduction to Corrosion Science; Springer: New York, NY, USA, 2010; p. 328. [Google Scholar]
- Becker, J.P.; Pust, S.E.; Hüpkes, J. Effects of the electrolyte species on the electrochemical dissolution of polycrystalline ZnO:Al thin films. Electrochim. Acta 2013, 112, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Weng, B.; Qi, M.Y.; Han, C.; Tang, Z.R.; Xu, Y.J. Photocorrosion inhibition of semiconductor-based photocatalysts: Basic principle, current development, and future perspective. ACS Catal. 2019, 9, 4642–4687. [Google Scholar] [CrossRef]
- Fekete, M.; Riedel, W.; Patti, A.F.; Spiccia, L. Photoelectrochemical water oxidation by screen printed ZnO nanoparticle films: Effect of pH on catalytic activity and stability. Nanoscale 2014, 6, 7585–7593. [Google Scholar] [CrossRef]
- Liu, C.F.; Lu, Y.J.; Hu, C.C. Effects of anions and ph on the stability of ZnO nanorods for photoelectrochemical water splitting. ACS Omega 2018, 3, 3429–3439. [Google Scholar] [CrossRef]
- Liu, M.; Nam, C.Y.; Black, C.T.; Kamcev, J.; Zhang, L. Enhancing water splitting activity and chemical stability of zinc oxide nanowire photoanodes with ultrathin titania shells. J. Phys. Chem. C 2013, 117, 13396–13402. [Google Scholar] [CrossRef]
- Reddy, N.K.; Winkler, S.; Koch, N.; Pinna, N. Electrochemical water oxidation of ultrathin cobalt oxide-based catalyst supported onto aligned ZnO nanorods. ACS Appl. Mater. Interfaces 2016, 8, 3226–3232. [Google Scholar] [CrossRef]
- Shao, M.; Ning, F.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical nanowire arrays based on ZnO core-layered double hydroxide shell for largely enhanced photoelectrochemical water splitting. Adv. Funct. Mater. 2014, 24, 580–586. [Google Scholar] [CrossRef]
- Mao, Y.; Cheng, Y.; Wang, J.; Yang, H.; Li, M.; Chen, J.; Chao, M.; Tong, Y.; Liang, E. Amorphous NiO electrocatalyst overcoated ZnO nanorod photoanodes for enhanced photoelectrochemical performance. New J. Chem. 2016, 40, 107–112. [Google Scholar] [CrossRef]
- Li, C.; Wang, T.; Luo, Z.; Zhang, D.; Gong, J. Transparent ALD-grown Ta2O5 protective layer for highly stable ZnO photoelectrode in solar water splitting. Chem. Commun. 2015, 51, 7290–7293. [Google Scholar] [CrossRef]
- El-Shazly, A.N.; Hegazy, A.H.; Rashad, M.M.; El-Shahat, M.F.; Allam, N.K. Ultrathin ALD TiO2 shells for enhanced photoelectrochemical solar fuel generation. J. Alloy. Compd. 2018, 739, 178–183. [Google Scholar] [CrossRef]
- Scheuermann, A.G.; McIntyre, P.C. Atomic Layer Deposited Corrosion Protection: A Path to Stable and Efficient Photoelectrochemical Cells. J. Phys. Chem. Lett. 2016, 7, 2867–2878. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Feng, S.; Liu, S.; Li, X.; Wang, L.; Lu, W. A three-dimensional interconnected hierarchical FeOOH/TiO2/ZnO nanostructural photoanode for enhancing the performance of photoelectrochemical water oxidation. Nanoscale 2015, 7, 19178–19183. [Google Scholar] [CrossRef] [PubMed]
- Hernández, S.; Cauda, V.; Chiodoni, A.; Dallorto, S.; Sacco, A.; Hidalgo, D.; Celasco, E.; Pirri, C.F. Optimization of 1D ZnO@TiO2 core-shell nanostructures for enhanced photoelectrochemical water splitting under solar light illumination. ACS Appl. Mater. Interfaces 2014, 6, 12153–12167. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Q.; Jiang, J.; Mao, Y. Morphology-tunable synthesis of ZnO nanoforest and its photoelectrochemical performance. Nanoscale 2014, 6, 8769–8780. [Google Scholar] [CrossRef]
- Jeong, K.; Deshmukh, P.R.; Park, J.; Sohn, Y.; Shin, W.G. ZnO-TiO2 core-shell nanowires: A sustainable photoanode for enhanced photoelectrochemical water splitting. ACS Sustain. Chem. Eng. 2018, 6, 6518–6526. [Google Scholar] [CrossRef]
- Hassan, M.A.; Waseem, A.; Johar, M.A.; Bagal, I.V.; Ha, J.S.; Ryu, S.W. Single-step fabrication of 3D hierarchical ZnO/ZnS heterojunction branched nanowires by MOCVD for enhanced photoelectrochemical water splitting. J. Mater. Chem. A 2020, 8, 8300–8312. [Google Scholar] [CrossRef]
- Hu, W.-C.; Chen, Y.-A.; Hsieh, P.-Y.; Tsao, C.-W.; Chiu, Y.-H.; Chang, T.-F.M.; Chen, C.-Y.; Sone, M.; Hsu, Y.-J. Reduced graphene oxides-wrapped ZnO with notable photocatalytic property. J. Taiwan Inst. Chem. Eng. 2020, 112, 337–344. [Google Scholar] [CrossRef]
- Zhang, Z.; Choi, M.; Baek, M.; Hwang, I.; Cho, C.; Deng, Z.; Lee, J.; Yong, K. Design and roles of RGO-wrapping in charge transfer and surface passivation in photoelectrochemical enhancement of cascade-band photoanode. Nano Res. 2017, 10, 2415–2430. [Google Scholar] [CrossRef]
- Liu, C.; Qiu, Y.; Wang, F.; Wang, K.; Liang, Q.; Chen, Z. Design of core–shell-structured ZnO/ZnS hybridized with graphite-like c3n4 for highly efficient photoelectrochemical water splitting. Adv. Mater. Interfaces 2017, 4, 1–11. [Google Scholar] [CrossRef]
- Fu, H.; Xu, T.; Zhu, S.; Zhu, Y. Photocorrosion inhibition and enhancement of photocatalytic activity for ZnO via hybridization with C60. Environ. Sci. Technol. 2008, 42, 8064–8069. [Google Scholar] [CrossRef]
- Choi, H.; Lee, N.; Park, H.; Choi, Y.; Yuk, H.; Lee, J.; Lee, S.J.; Lee, E.J.; Jeon, H. Tuning the electronic band structure and optoelectrical characteristics of ALD-grown Zn(O,S) buffer layers for SnS solar cells. Optik 2021, 228, 165921. [Google Scholar] [CrossRef]
- Shiraishi, M.; Ata, M. Work function of carbon nanotubes. Carbon N. Y. 2001, 39, 1913–1917. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sharma, R. Fabrication and characterization of zinc oxide/multi-walled carbon nanotube Schottky barrier diodes. J. Electron. Mater. 2018, 47, 3037–3044. [Google Scholar] [CrossRef]
- Marinho, B.; Ghislandi, M.; Tkalya, E.; Koning, C.E.; de With, G. Electrical conductivity of compacts of graphene, multi-wall carbon nanotubes, carbon black, and graphite powder. Powder Technol. 2012, 221, 351–358. [Google Scholar] [CrossRef]
- Chaudhary, D.; Singh, S.; Vankar, V.D.; Khare, N. ZnO nanoparticles decorated multi-walled carbon nanotubes for enhanced photocatalytic and photoelectrochemical water splitting. J. Photochem. Photobiol. A Chem. 2018, 351, 154–161. [Google Scholar] [CrossRef]
- Vietmeyer, F.; Seger, B.; Kamat, P.V. Anchoring ZnO particles on functionalized single wall carbon nanotubes. Excited state interactions and charge collection. Adv. Mater. 2007, 19, 2935–2940. [Google Scholar] [CrossRef]
- Li, M.; Chang, K.; Wang, T.; Liu, L.; Zhang, H.; Li, P.; Ye, J. Hierarchical nanowire arrays based on carbon nanotubes and Co3O4 decorated ZnO for enhanced photoelectrochemical water oxidation. J. Mater. Chem. A 2015, 3, 13731–13737. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Du, H.; Kong, J.; Lu, X.; Ke, L.; Sun, X.W. Multi-walled carbon nanotubes modified ZnO nanorods: A photoanode for photoelectrochemical cell. Electrochim. Acta 2014, 143, 188–195. [Google Scholar] [CrossRef]
- Park, J.G.; Cheng, Q.; Lu, J.; Bao, J.; Li, S.; Tian, Y.; Liang, Z.; Zhang, C.; Wang, B. Thermal conductivity of MWCNT/epoxy composites: The effects of length, alignment and functionalization. Carbon N. Y. 2012, 50, 2083–2090. [Google Scholar] [CrossRef]
- Prasadam, V.P.; Ramirez, F.V.; Papakonstantinou, I.; Parkin, I.P.; Bahlawane, N. Thermoresponsive black VO2 –carbon nanotube composite coatings for solar energy harvesting. ACS Appl. Nano Mater. 2020, 3, 8848–8857. [Google Scholar] [CrossRef]
- Coy, E.; Siuzdak, K.; Pavlenko, M.; Załęski, K.; Graniel, O.; Ziółek, M.; Balme, S.; Miele, P.; Weber, M.; Bechelany, M.; et al. Enhancing photocatalytic performance and solar absorption by schottky nanodiodes heterojunctions in mechanically resilient palladium coated TiO2/Si nanopillars by atomic layer deposition. Chem. Eng. J. 2020, 392, 123702. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.W.; Zou, Y.; Fan, Z.; Ji, X.; Niu, C.; Wang, L. Rational design of CdS@ZnO core-shell structure via atomic layer deposition for drastically enhanced photocatalytic H2 evolution with excellent photostability. Nano Energy 2017, 39, 183–191. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.C.; Chou, C.Y.; Yi, S.R.; Chen, H.D. Deposition of heterojunction of ZnO on hydrogenated TiO2 nanotube arrays by atomic layer deposition for enhanced photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 28685–28697. [Google Scholar] [CrossRef]
- Kulmas, M.; Paterson, L.; Höflich, K.; Bashouti, M.Y.; Wu, Y.; Göbelt, M.; Ristein, J.; Bachmann, J.; Meyer, B.; Christiansen, S. Composite Nanostructures of TiO2 and ZnO for water splitting application: Atomic layer deposition growth and density Functional Theory Investigation. Adv. Funct. Mater. 2016, 26, 4882–4889. [Google Scholar] [CrossRef]
- Pavlenko, M.; Siuzdak, K.; Coy, E.; Załęski, K.; Jancelewicz, M.; Iatsunskyi, I. Enhanced solar-driven water splitting of 1D core-shell Si/TiO2/ZnO nanopillars. Int. J. Hydrogen Energy 2020, 45, 26426–26433. [Google Scholar] [CrossRef]
- Basheer, H.J.; Pachot, C.; Lafont, U.; Devaux, X.; Bahlawane, N. Low-temperature thermal CVD of superblack carbon nanotube coatings. Adv. Mater. Interfaces 2017, 4, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Basheer, H.J.; Baba, K.; Bahlawane, N. Thermal conversion of ethanol into carbon nanotube coatings with adjusted packing density. ACS Omega 2019, 4, 10405–10410. [Google Scholar] [CrossRef] [PubMed]
- Prasadam, V.P.; Gautier, N.; Bahlawane, N. CNT nanoengineering for thermally stable selective solar absorption. Mater. Today Commun. 2021, 28, 102552. [Google Scholar] [CrossRef]
- Yamada, A.; Sang, B.; Konagai, M. Atomic layer deposition of ZnO transparent conducting oxides. Appl. Surf. Sci. 1997, 112, 216–222. [Google Scholar] [CrossRef]
- Wang, R.P.; Xu, G.; Jin, P. Size dependence of electron-phonon coupling in ZnO nanowires. Phys. Rev. B-Condens. Matter Mater. Phys. 2004, 69, 5–8. [Google Scholar] [CrossRef]
- Basheer, H.J.; Baba, K.; Bahlawane, N. Thermal chemical vapor deposition of superblack randomly oriented carbon nanotube coatings. Phys. Status Solidi (A) 2020, 217, 1900704. [Google Scholar] [CrossRef]
- Blanksby, S.J.; Ellison, B.G. Bond dissociation energies of organic molecules. Acc. Chem. Res. 2003, 36, 255–263. [Google Scholar] [CrossRef]
- Haschke, S.; Mader, M.; Schlicht, S.; Roberts, A.M.; Angeles-Boza, A.M.; Barth, J.A.; Bachmann, J. Direct oxygen isotope effect identifies the rate-determining step of electrocatalytic OER at an oxidic surface. Nat. Commun. 2018, 9, 4565. [Google Scholar] [CrossRef] [PubMed]
- Doepke, A.; Han, C.; Back, T.; Cho, W.; Dionysiou, D.D.; Shanov, V.; Halsall, H.B.; Heineman, W.R. Analysis of the electrochemical oxidation of multiwalled carbon nanotube tower electrodes in sodium hydroxide. Electroanalysis 2012, 24, 1501–1508. [Google Scholar] [CrossRef]
- Govatsi, K.; Seferlis, A.; Neophytides, S.G.; Yannopoulos, S.N. Influence of the morphology of ZnO nanowires on the photoelectrochemical water splitting efficiency. Int. J. Hydrogen Energy 2018, 43, 4866–4879. [Google Scholar] [CrossRef]
- Yang, H.; Kim, E.; Kim, S.H.; Jeong, M.S.; Shin, H. Hole trap, charge transfer and photoelectrochemical water oxidation in thickness-controlled TiO2 anatase thin films. Appl. Surf. Sci. 2020, 529, 147020. [Google Scholar] [CrossRef]
- Paulauskas, I.E.; Jellison, G.E.; Boatner, L.A.; Brown, G.M. Photoelectrochemical stability and alteration products of n-type single-crystal zno photoanodes. Int. J. Electrochem. 2011, 2011, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Souza, F.L.; Lopes, K.P.; Longo, E.; Leite, E.R. The influence of the film thickness of nanostructured α-Fe2O3 on water photooxidation. Phys. Chem. Chem. Phys. 2009, 11, 1215–1219. [Google Scholar] [CrossRef]
- Septiani, N.L.W.; Saputro, A.G.; Kaneti, Y.V.; Maulana, A.L.; Fathurrahman, F.; Lim, H.; Yuliarto, B.; Nugraha; Dipojono, H.K.; Golberg, D.; et al. Hollow zinc oxide microsphere-multiwalled carbon nanotube composites for selective detection of sulfur dioxide. ACS Appl. Nano Mater. 2020, 3, 8982–8996. [Google Scholar] [CrossRef]
- Ida, S.; Kearney, K.; Futagami, T.; Hagiwara, H.; Sakai, T.; Watanabe, M.; Rockett, A.; Ishihara, T. Photoelectrochemical H2 evolution using TiO2-coated CaFe2O4 without an external applied bias under visible light irradiation at 470 nm based on device modeling. Sustain. Energy Fuels 2017, 1, 280–287. [Google Scholar] [CrossRef]
- Pawar, A.U.; Kim, C.W.; Kang, M.J.; Kang, Y.S. Crystal facet engineering of ZnO photoanode for the higher water splitting efficiency with proton transferable nafion film. Nano Energy 2016, 20, 156–167. [Google Scholar] [CrossRef]
- Xie, S.; Lu, X.; Zhai, T.; Li, W.; Yu, M.; Liang, C.; Tong, Y. Enhanced photoactivity and stability of carbon and nitrogen co-treated ZnO nanorod arrays for photoelectrochemical water splitting. J. Mater. Chem. 2012, 22, 14272–14275. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Jiang, J.; Wan, Z.; Su, Y.; Tang, H. Insight into charge carrier separation and solar-light utilization: rGO decorated 3D ZnO hollow microspheres for enhanced photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2020, 564, 322–332. [Google Scholar] [CrossRef] [PubMed]
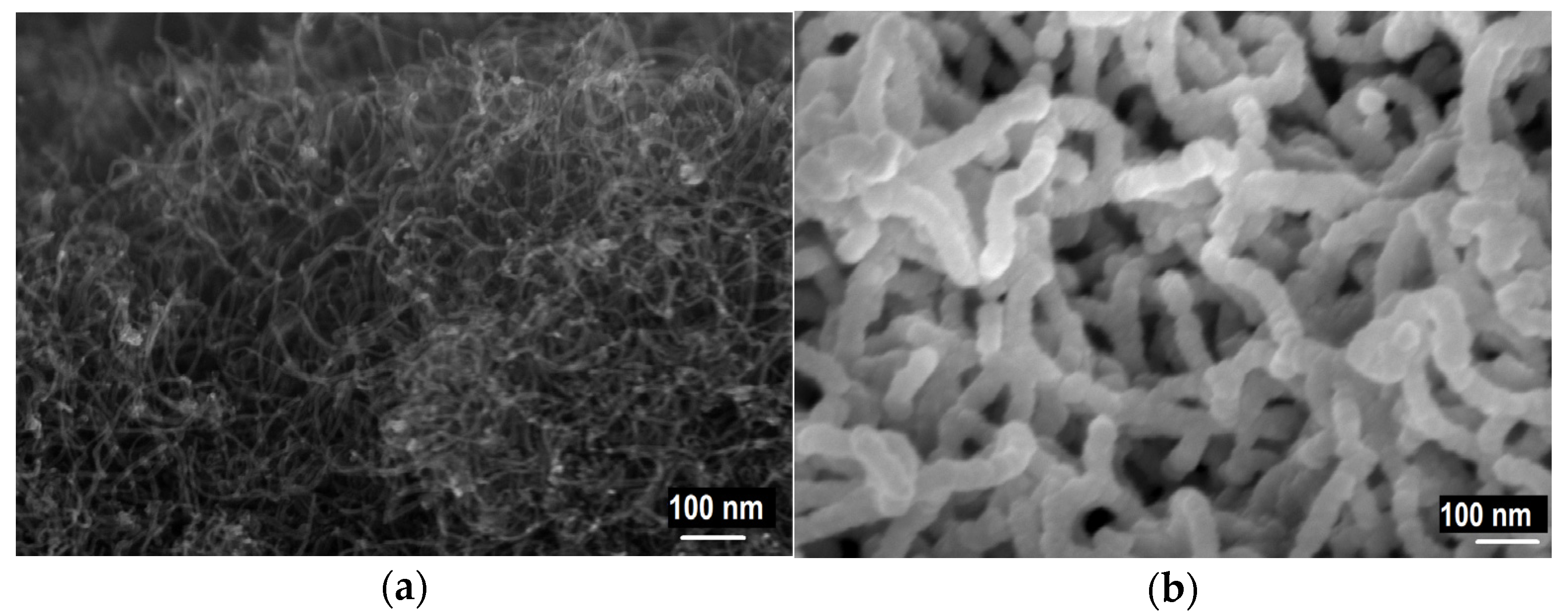
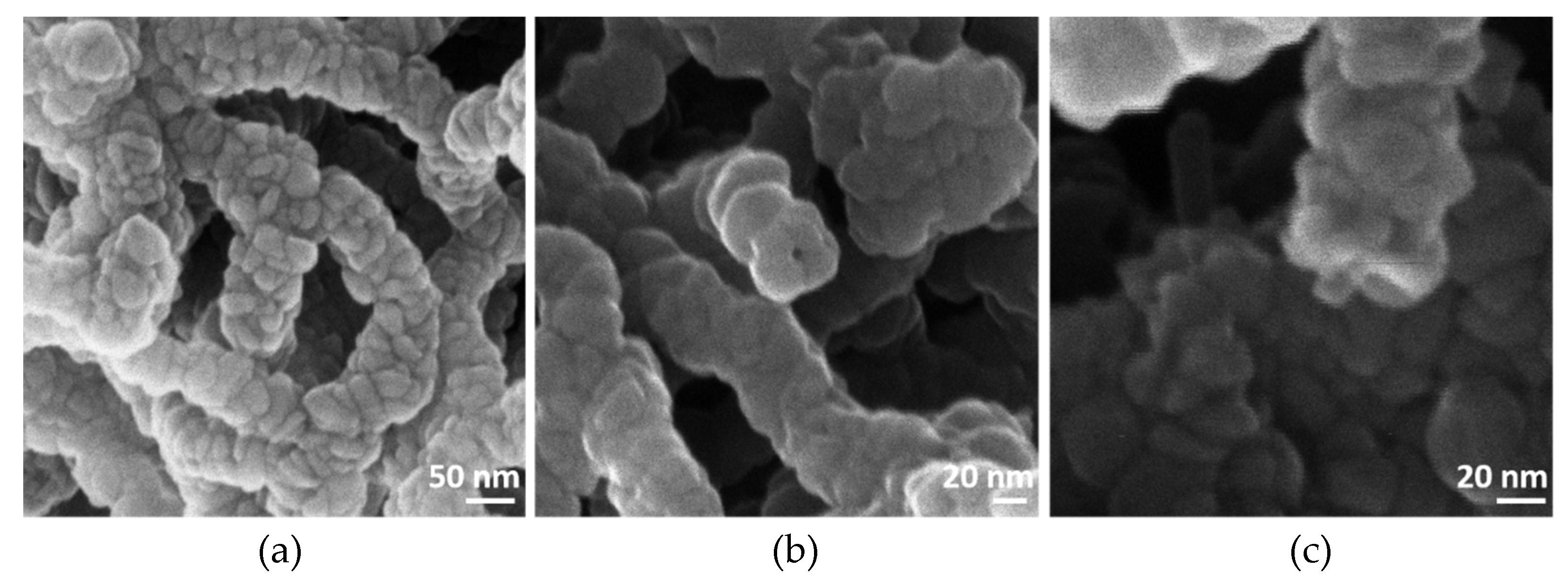

| Material | Synthesis/Remark | Dimensions | Current Density | Reference |
|---|---|---|---|---|
| ZnO thin film covered with nafion | Hydrothermal/ enhancement by (002) crystal facet orientation | ZnO thickness of 800 nm; nafion thickness of 2.5 µm | 0.4 mA·cm−2 (1.23 VRHE) photocurrent stability for 5 h | [67] |
| ZnO rods covered with C3N4 layer | Electrodeposition/ enhancement by surface passivation | ZnO rods of 2 µm length; C3N4 of 15 nm thickness | 0.45 mA·cm−2 (1.23 VRHE) photocurrent stability for 1 h | [68] |
| ZnO rods covered with C3N4 sheets in-between | Electrodeposition/ enhancement by surface passivation | 400 nm ZnO rod arrays | 0.4 mA·cm−2 (1.23 VRHE) photocurrent stability for 1 h | [35] |
| RGO dispersed between ZnO sphere | Solvothermal/ enhancement by heterostructure | 1–2 µm diameter ZnO sphere | 0.1 mA·cm−2 (1.23 VRHE) photocurrent stability for 3 h | [69] |
| ZnO particle on CNT composite | Sol-Gel/enhancement by heterostructure | 30 nm ZnO particle | 0.45 mA·cm−2 (1.23 VRHE) photocurrent stability for 20 min | [41] |
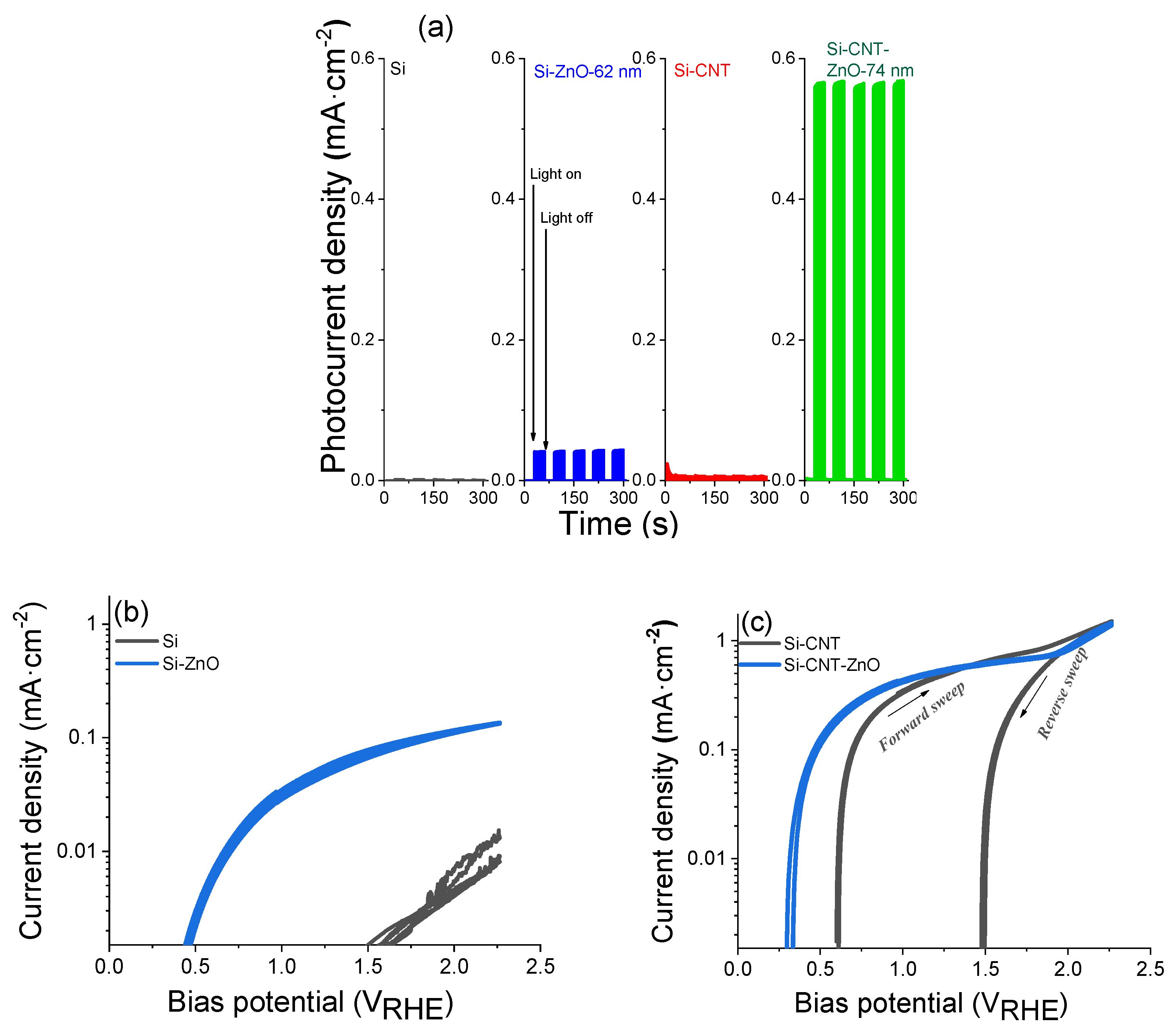
| CNT-ZnO core–shell nanocomposite | CVD-ALD/enhancement by heterostructure | 3 µm thick-CNT film; 74 nm thickness-ZnO shell layer | 0.55 mA·cm−2 (1.23 VRHE) photocurrent stability for 8 h | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prasadam, V.P.; Huerta Flores, A.M.; Audinot, J.-N.; Bahlawane, N. CNT-ZnO Core-Shell Photoanodes for Photoelectrochemical Water Splitting. Coatings 2022, 12, 47. https://doi.org/10.3390/coatings12010047
Prasadam VP, Huerta Flores AM, Audinot J-N, Bahlawane N. CNT-ZnO Core-Shell Photoanodes for Photoelectrochemical Water Splitting. Coatings. 2022; 12(1):47. https://doi.org/10.3390/coatings12010047
Chicago/Turabian StylePrasadam, Vasu Prasad, Ali Margot Huerta Flores, Jean-Nicolas Audinot, and Naoufal Bahlawane. 2022. "CNT-ZnO Core-Shell Photoanodes for Photoelectrochemical Water Splitting" Coatings 12, no. 1: 47. https://doi.org/10.3390/coatings12010047