Evolution of the Surface Structure and Functional Properties of the Electroconducting Polymer Coatings onto Porous Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
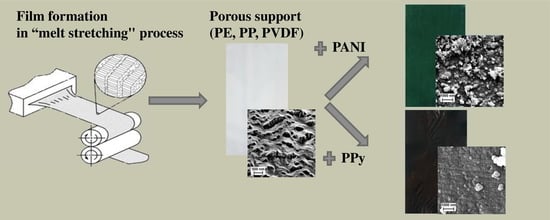
3.1. Characterization of Porous Supports
3.2. Electroconducting Polymer Coatings onto Porous Supports
3.3. Properties and Characteristics of the Electroactive Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MacDiarmid, A.G. Synthetic metals: A novel role for organic polymers. Synth. Met. 2002, 125, 11–22. [Google Scholar] [CrossRef]
- Nalwa, H.S. (Ed.) Handbook of Organic Conductive Molecules and Polymers; John Wiley & Sons: Chichester, UK; New York, NY, USA; Weinheim, Germany; Brisbane, QLD, Australia; Singapore; Toronto, ON, Canada, 1997; Volume 2, 888p. [Google Scholar]
- Joo, J.; Lee, J.K.; Baeck, J.S.; Kim, K.H.; Oh, E.J.; Epstein, J. Electrical, magnetic and structural properties of chemically and electrochemically synthesized polypyrrols. Synth. Met. 2001, 117, 45–51. [Google Scholar] [CrossRef]
- Wang, L.X.; Li, X.J.; Yang, Y.L. Preparation, properties and applications of polypyrroles. React. Funct. Polym. 2001, 47, 125–139. [Google Scholar] [CrossRef]
- Nicolas-Debarnot, D.; Poncin-Epaillard, F. Polyaniline as a new sensitive layer for gas sensor. Anal. Chim. Acta 2003, 475, 1–15. [Google Scholar] [CrossRef]
- Bar-Cohen, Y.; Zhang, Q. Electroactive polymer actuators and sensors. MRS Bull. 2008, 33, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Janmanee, R.; Chuekachang, S.; Sriwichai, S.; Baba, A.; Phanichphant, S. Functional conducting polymers in the application of SPR biosensors. J. Nanotechnol. 2012, 2012, 620309. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Adhikari, R.; Cass, P.; Bown, M.; Gunatillake, P. Electrically conductive polymers and composites for biomedical applications. RSC Adv. 2015, 5, 37553–37567. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Goroncharovskaya, I.V.; Evseev, A.K.; Sosenkin, V.E.; Goldin, M.M. The effect of electrochemical modification of activated carbons by polypyrrole on their structure characteristics, composition of surface compounds, and adsorption properties. Russ. J. Electrochem. 2017, 53, 1334–1344. [Google Scholar] [CrossRef]
- Sun, B.; Wu, T.; Wang, J.; Li, D.; Wang, J.; Gao, Q.; Bhutto, M.A.; El-Hamshary, H.; Al-Deyab, S.S.; Mo, X. Polypyrrole-coated poly(l-lacticacid-co-ε-caprolactone)/silk fibroin nanofibrous membranes promoting neural cell proliferation and differentiation with electrical stimulation. J. Mater. Chem. B 2016, 4, 6670–6679. [Google Scholar] [CrossRef]
- Liu, H.; Wang, R.; Chu, H.K.; Sun, D. Design and characterization of a conductive nanostructured polypyrrole-polycaprolactone coated magnesium/PLGA composite for tissue engineering scaffolds. J. Biomed. Mater. Res. A 2015, 103, 2966–2973. [Google Scholar] [CrossRef]
- Yuan, L.; Yao, B.; Hu, B.; Huo, K.; Chen, W.; Zhou, J. Polypyrrole-coated paper for flexible solid-state energy storage. Energy Environ. Sci. 2013, 6, 470–476. [Google Scholar] [CrossRef]
- Chung, T.C.M. Functional polyolefins for energy applications. Macromolecules 2013, 46, 6671–6698. [Google Scholar] [CrossRef]
- Gokhale, A.A.; Lee, I. Recent advances in the fabrication of nanostructured barrier films. J. Nanosci. Nanotechnol. 2014, 14, 2157–2177. [Google Scholar] [CrossRef]
- Kim, J.F.; Jung, J.T.; Wang, H.H.; Lee, S.Y.; Moore, T.; Sanguineti, A.; Drioli, E.; Lee, Y.M. Microporous PVDF membranes via thermally induced phase separation (TIPS) and stretching methods. J. Membr. Sci. 2016, 509, 94–104. [Google Scholar] [CrossRef]
- Yarysheva, A.I.; Bagrov, D.V.; Rukhlya, E.G.; Yarysheva, L.M.; Volynskii, A.L.; Bakeev, N.F. Features of the delocalized crazing of high-density polyethylene in poly(ethylene oxide) solutions. Polym. Sci. A 2012, 54, 779–786. [Google Scholar] [CrossRef]
- Sadeghi, F.; Tabatabaei, S.H.; Ajji, A.; Carreau, P.J. Effect of PVDF characteristics on extruded film morphology and porous membranes feasibility by stretching. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1219–1229. [Google Scholar] [CrossRef]
- Elyashevich, G.K.; Kuryndin, I.S.; Lavrentyev, V.K.; Bobrovsky, A.Y.; Bukošek, V. Porous structure, permeability, and mechanical properties of polyolefin microporous films. Phys. Solid State 2012, 54, 1907–1916. [Google Scholar] [CrossRef]
- Elyashevich, G.K.; Kuryndin, I.S.; Lavrentyev, V.K.; Dmitriev, I.Y. Through permeability of polyvinylidene fluoride piezoactive porous films. Polym. Sci. A 2018, 60, 734–741. [Google Scholar] [CrossRef]
- Elyashevich, G.K.; Olifirenko, A.S.; Pimenov, A.V. Micro- and nanofiltration membranes on the base of porous polyethylene films. Desalination 2005, 184, 273–279. [Google Scholar] [CrossRef]
- Bobrovsky, A.; Shibaev, V.; Elyashevitch, G. Photopatternable fluorescent polymer composites based on stretched porous polyethylene and photopolymerizable liquid crystal mixture. J. Mater. Chem. 2008, 18, 691–695. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Guo, Z. Polyaniline coated membranes for effective separation of oil in water emulsions. J. Colloid Interface Sci. 2016, 467, 261–270. [Google Scholar] [CrossRef]
- Lee, H.S.; Hong, J. Chemical synthesis and characterization of polypyrrole coated on porous membranes and its electrochemical stability. Synth. Met. 2000, 113, 115–119. [Google Scholar] [CrossRef]
- Barra, G.M.O.; Matins, R.R.; Kafer, K.A.; Paniago, R.; Vasques, C.T.; Pires, A.T.N. Thermoplastic elastomer/polyaniline blends: Evaluation of mechanical and electromechanical properties. Polym. Test. 2008, 27, 886–892. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Kar, S.B. Role of non-linear processes in conducting polymer blends for piezo-sensors Part 2: Studies on polyaniline/SBS blends. Sens. Actuators A Phys. 2005, 120, 474–481. [Google Scholar] [CrossRef]
- Sau, K.P.; Chaki, T.K.; Khastgir, D. The effect of compressive strain and stress on electrical conductivity of conductive rubber composites. Rubber Chem. Technol. 2000, 73, 310–324. [Google Scholar] [CrossRef]
- Merlini, C.; Rosa, B.S.; Muller, D.; Ecco, L.G.; Ramoa, S.D.A.S.; Barra, G.M.O. Polypyrrole nanoparticles coated amorphous short silica fibers: Synthesis and characterization. Polym. Test. 2012, 31, 971–977. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.-M.; Zhang, Y.Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Chronakis, I.S.; Grapenson, S.; Jakob, A. Conductive polypyrrole nanofibers via electrospinning: Electrical and morphological properties. Polymer 2006, 47, 1597–1603. [Google Scholar] [CrossRef]
- Böhnstedt, W. A review of future directions in automotive battery separators. J. Power Sources 2004, 133, 59–66. [Google Scholar] [CrossRef]
- Besenhard, J.O. (Ed.) Handbook of Battery Materials; Wiley-VCH: Weinheim, Germany, 1999; 634p. [Google Scholar]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Majid, M.A.; Ooi, B.S. Functionalized PSf/SiO2 nanocomposite membrane for oil-in-water emulsion separation. Desalination 2011, 268, 266–269. [Google Scholar] [CrossRef]
- Elyashevich, G.K.; Rosova, E.Y.; Karpov, E.A. Microporous polyethylene film and method of its production. Russian. Federation Patent 140,936, 15 April 1997. [Google Scholar]
- Elyashevich, G.K.; Kuryndin, I.S.; Dmitriev, I.Y.; Lavrentyev, V.K.; Saprykina, N.N.; Bukošek, V. Orientation efforts as regulatory factor of structure formation in permeable porous polyvinylidene fluoride films. Chin. J. Polym. Sci. 2019, 37, 1283–1289. [Google Scholar] [CrossRef]
- Gerasimov, D.I.; Kuryndin, I.S.; Lavrentyev, V.K.; Temnov, D.E.; Elyashevich, G.K. Piezoelectric properties of the oriented porous poly(vynilidene) fluoride films. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2020; Volume 2308, pp. 030001-1–030001-6. [Google Scholar] [CrossRef]
- Skotheim, T.A.; Reynolds, J. (Eds.) Conjugated Polymers: Theory, Synthesis, Properties, and Characterization, 3rd ed.; Handbook of Conducting Polymers; CRC Press Taylor & Francis Group: Boca Raton, FL, USA, 2006; 1024p. [Google Scholar]
- Kesting, R.E. Synthetic Polymer Membranes. A Structural Perspective, 2nd ed.; John Wiley & Sons: New York, NY, USA; Chichester, UK, 1985; 348p. [Google Scholar]
- Hermans, P.H.; Weidinger, A. On the determination of the crystalline fraction of polyethylenes from X-ray diffraction. Macromol. Chem. 1961, 44, 24–36. [Google Scholar] [CrossRef]
- Stauffer, D.; Aharony, A. Introduction to Percolation Theory; Taylor and Francis: London, UK, 1994; 181p. [Google Scholar]
- Novikov, D.V.; Lavrentyev, V.K.; Elyashevich, G.K.; Bukošek, V. Disorder–Order transition in microporous oriented polyethylene films. Phys. Solid State 2012, 54, 1903–1906. [Google Scholar] [CrossRef]
- Elyashevich, G.K.; Kuryndin, I.S.; Rosova, E.Y.; Gerasimov, D.I.; Vylegzhanina, M.E. Piezoactive composite systems based on porous polyvinylidene fluoride films and conducting polymer layers as electrodes. Phys. Complex Syst. 2021, 2, 25–32. [Google Scholar] [CrossRef]
- Boinovich, L.B.; Emelyanenko, A.M. Hydrophobic materials and coatings: Principles of design, properties and applications. Russ. Chem. Rev. 2008, 77, 583–600. [Google Scholar] [CrossRef]
- Kornberg, A.B.; Thompson, M.R.; Zhu, S. Flexible conductive substrate incorporating a submicrometer co-continuous polyaniline phase within polyethylene by controlled crazing. ACS Appl. Polym. Mater. 2021, 3, 1880–1889. [Google Scholar] [CrossRef]
- Yang, J.; Hou, J.; Zhu, W.; Xu, M.; Wan, M. Substituted polyaniline-polypropylene film composites: Preparation and properties. Synth. Met. 1996, 80, 283–289. [Google Scholar] [CrossRef]
- Malmonge, L.F.; Lopes, G.d.A.; Langiano, S.d.C.; Malmonge, J.A.; Cordeiro, J.M.M.; Mattoso, L.H.C. A new route to obtain PVDF/PANI conducting blends. Eur. Polym. J. 2006, 42, 3108–3113. [Google Scholar] [CrossRef]
- Dispenza, C.; Sabatino, M.A.; Deghiedy, N.; Casaletto, M.P.; Spadaro, G.; Piazza, S.; El-Rehim, H.A.A. In-situ polymerization of polyaniline in radiation functionalized polypropylene films. Polymer 2015, 67, 128–138. [Google Scholar] [CrossRef]
- Chang, Y.Z.; Shi, W.H.; Han, G.Y.; Song, H.; Hou, W.J. Fabrication on the flexible supercapacitor based on the polypyrrole deposited on polyethylene/polypropylene non-woven film. Russ. J. Electrochem. 2020, 56, 947–958. [Google Scholar] [CrossRef]
- Huang, X.; Yu, Y.-H.; De Llergo, O.L.; Marquez, S.M.; Cheng, Z. Facile polypyrrole thin film coating on polypropylene membrane for efficient solar-driven interfacial water evaporation. RSC Adv. 2017, 7, 9495–9499. [Google Scholar] [CrossRef]
- Ray, S.; Easteal, A.J.; Cooney, R.P.; Edmonds, N.R. Structure and properties of melt-processed PVDF/PMMA/polyaniline blends. Mater. Chem. Phys. 2009, 113, 829–838. [Google Scholar] [CrossRef]
- Elyashevich, G.K.; Sidorovich, A.V.; Smirnov, M.A.; Kuryndin, I.S.; Bobrova, N.V.; Trchová, M.; Stejskal, J. Thermal and structural stability of composite systems based on polyaniline deposited on porous polyethylene films. Polym. Degrad. Stab. 2006, 91, 2786–2792. [Google Scholar] [CrossRef]
- Elyashevich, G.K.; Dmitriev, I.Y.; Rosova, E.Y. Electroconducting polypyrrole coatings as an electrode contact material on porous poly(vinylidene fluoride) piezofilm. Polym. Sci. A 2021, 63, 47–53. [Google Scholar] [CrossRef]
- Elyashevich, G.K.; Kuryndin, I.S.; Rosova, E.Y.; Saprykina, N.N. Polymer piezoelements based on porous polyvinylidene fluoride films and contact electrode polyaniline layers. Phys. Solid State 2020, 62, 566–573. [Google Scholar] [CrossRef]







| Process Parameters | Material of Support | ||
|---|---|---|---|
| PE | PP | PVDF | |
| 1. Melt draw ratio at extrusion | 76 | 76 | 76 |
| 2. Temperature of isometric annealing, °C | 126 | 170 | 168 |
| 3. Total degree of extension, % | 450 | 200 | 110 |
| 4. Temperature of thermal stabilization, °C | 105 | 130 | 100 |
| Porous Supports | Thickness, µm | P, % | S, m2/g | G, l/(m2·h atm) (Ethanol) | The Most Probable Size of Through Channels, nm | Maximal Size of Through Channels, nm | Number of Through Channels/cm2 |
|---|---|---|---|---|---|---|---|
| PE | 11 | 52 | 54 | 470 | 200 | 640 | 2 × 107 |
| PP | 21 | 50 | 104 | 260 | 120 | 260 | 4 × 107 |
| PVDF | 20 | 27 | 38 | 1.5 | <60 | 65 | – |
| Sample | Thickness, μm | Specific Surface, m2/g | Content of Conducting Polymer, % | σv, S/cm | σs, S/cm | References, σs, S/cm |
|---|---|---|---|---|---|---|
| PE support/PANI | 11 | 43 | 3.8 | 3.2 × 10−7 | 1.2 | 2 × 10−2 [44] |
| PP support/PANI | 21 | 68 | 4.7 | 6.7 × 10−7 | 0.8 | 0.46 × 10−2 [45] |
| PVDF support/PANI | 20 | 31 | 2.1 | 5.7 × 10−7 | 0.7 | 0.5 [46] |
| – | – | – | – | – | – | – |
| PE support/PPy | 12 | 23 | 42 | 1.6 × 10−4 | 9 | 3.25–4.46 [47] |
| PP support/PPy | 22 | 10 | 56 | 1.3 × 10−3 | 18 | – |
| PVDF support/PPy | 20 | 11 | 3.0 | 1.9 × 10−6 | 1.9 | – |
| Sample | PE | PP | PVDF |
|---|---|---|---|
| Porous support | 70° | 85° | 68° |
| Composite (porous support/PANI) | 59° | 59° | 60° |
| Composite (porous support/PPy) | 53° | 55° | 56° |
| Sample | Tensile Strength, MPa | Elastic Modulus, MPa | Elongation at Break, % |
|---|---|---|---|
| PE support | 150/8 | 700/600 | 40/5 |
| PE support/PANI | 135/9 | 850/780 | 45/4 |
| PE support/PPy | 147/10 | 2000/1550 | 38/2 |
| – | – | – | – |
| PP support | 149/9 | 820/350 | 78/9 |
| PP support/PANI | 147/9 | 830/400 | 75/8 |
| PP support/PPy | 140/9 | 2100/910 | 70/8 |
| – | – | – | – |
| PVDF support | 137/29 | 650/1380 | 44/4 |
| PVDF support/PANI | 140/30 | 720/1460 | 50/3 |
| PVDF support/PPy | 140/28 | 730/1500 | 42/3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elyashevich, G.K.; Gerasimov, D.I.; Kuryndin, I.S.; Lavrentyev, V.K.; Rosova, E.Y.; Vylegzhanina, M.E. Evolution of the Surface Structure and Functional Properties of the Electroconducting Polymer Coatings onto Porous Films. Coatings 2022, 12, 51. https://doi.org/10.3390/coatings12010051
Elyashevich GK, Gerasimov DI, Kuryndin IS, Lavrentyev VK, Rosova EY, Vylegzhanina ME. Evolution of the Surface Structure and Functional Properties of the Electroconducting Polymer Coatings onto Porous Films. Coatings. 2022; 12(1):51. https://doi.org/10.3390/coatings12010051
Chicago/Turabian StyleElyashevich, Galina Kazimirovna, Dmitry Igorevich Gerasimov, Ivan Sergeevich Kuryndin, Viktor Konstantinovich Lavrentyev, Elena Yurievna Rosova, and Milana Ernestovna Vylegzhanina. 2022. "Evolution of the Surface Structure and Functional Properties of the Electroconducting Polymer Coatings onto Porous Films" Coatings 12, no. 1: 51. https://doi.org/10.3390/coatings12010051
APA StyleElyashevich, G. K., Gerasimov, D. I., Kuryndin, I. S., Lavrentyev, V. K., Rosova, E. Y., & Vylegzhanina, M. E. (2022). Evolution of the Surface Structure and Functional Properties of the Electroconducting Polymer Coatings onto Porous Films. Coatings, 12(1), 51. https://doi.org/10.3390/coatings12010051