Controllable Preparation of Fe3O4@RF and Its Evolution to Yolk–Shell-Structured Fe@C Composite Microspheres with High Microwave Absorbing Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Fe3O4@RF Microspheres
2.3. Fabrication of Fe@C Composite Microspheres
2.4. Analysis and Characterization
3. Results and Discussion
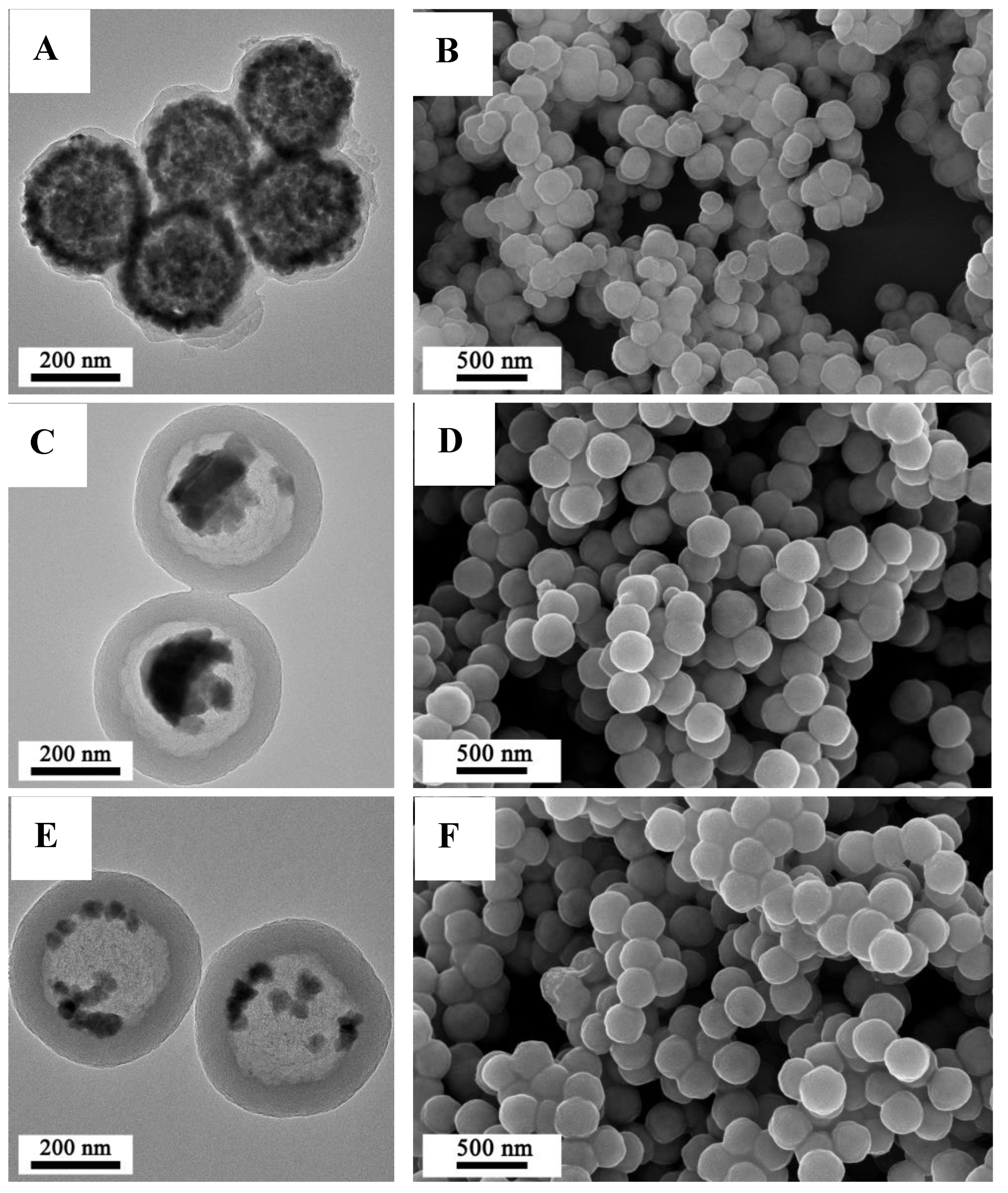
3.1. Shell Control of Fe3O4@RF Microspheres
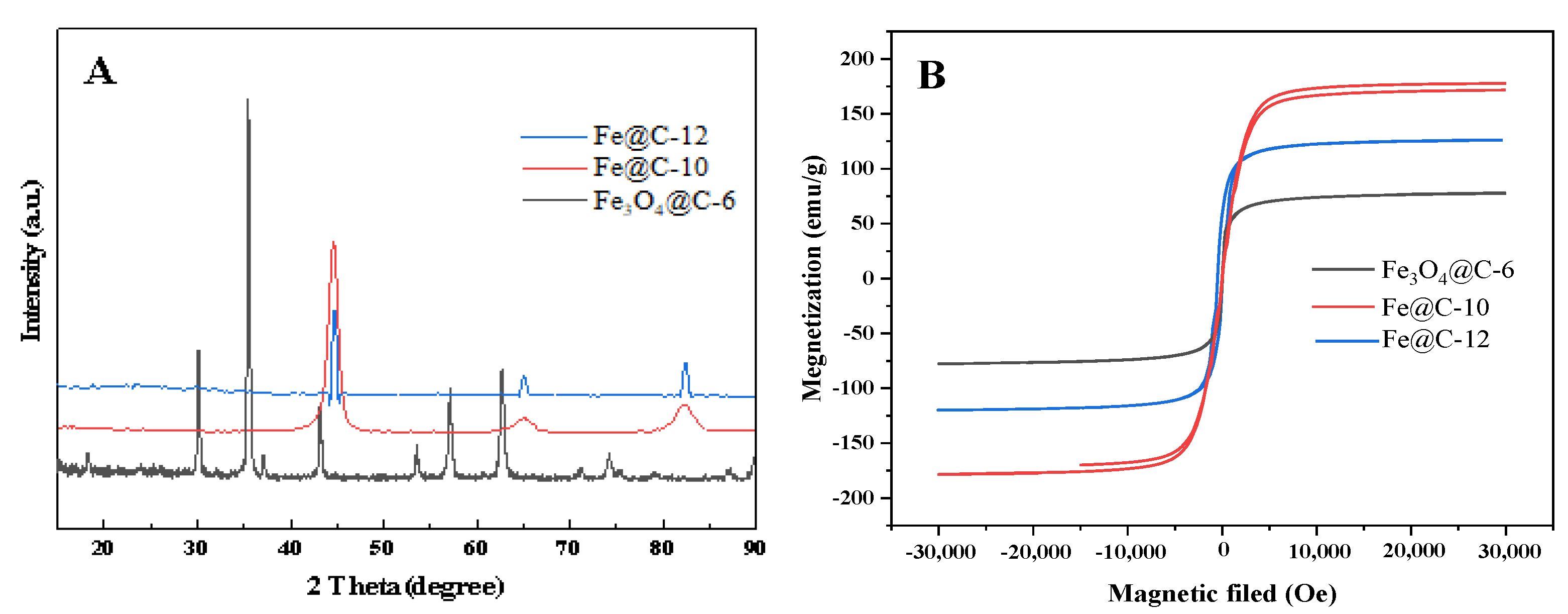
3.2. Characterization of Fe3O4@C Composite Microspheres
3.3. Morphology and Structure of Rattle-Shaped Fe@C Composite Microspheres
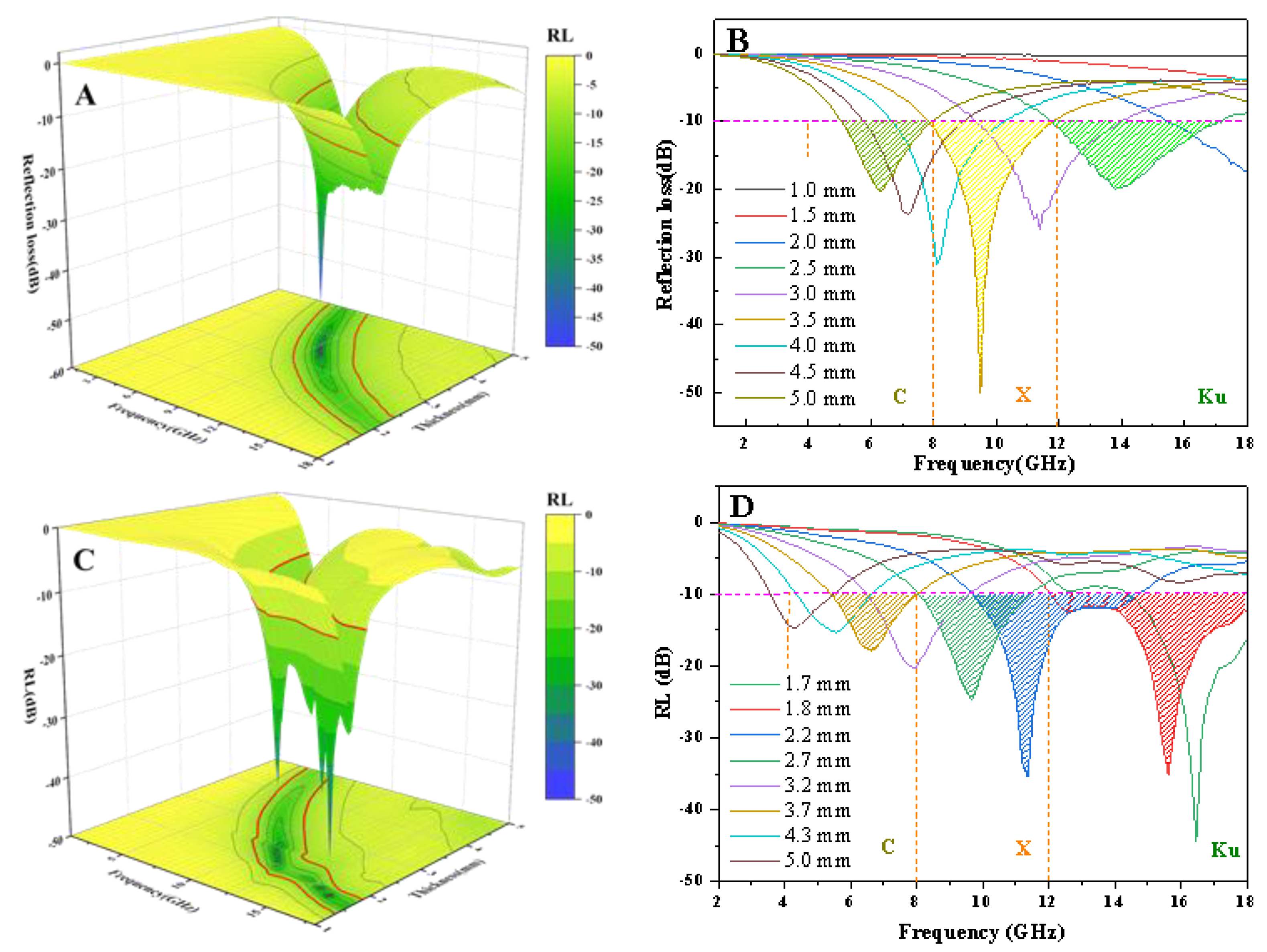
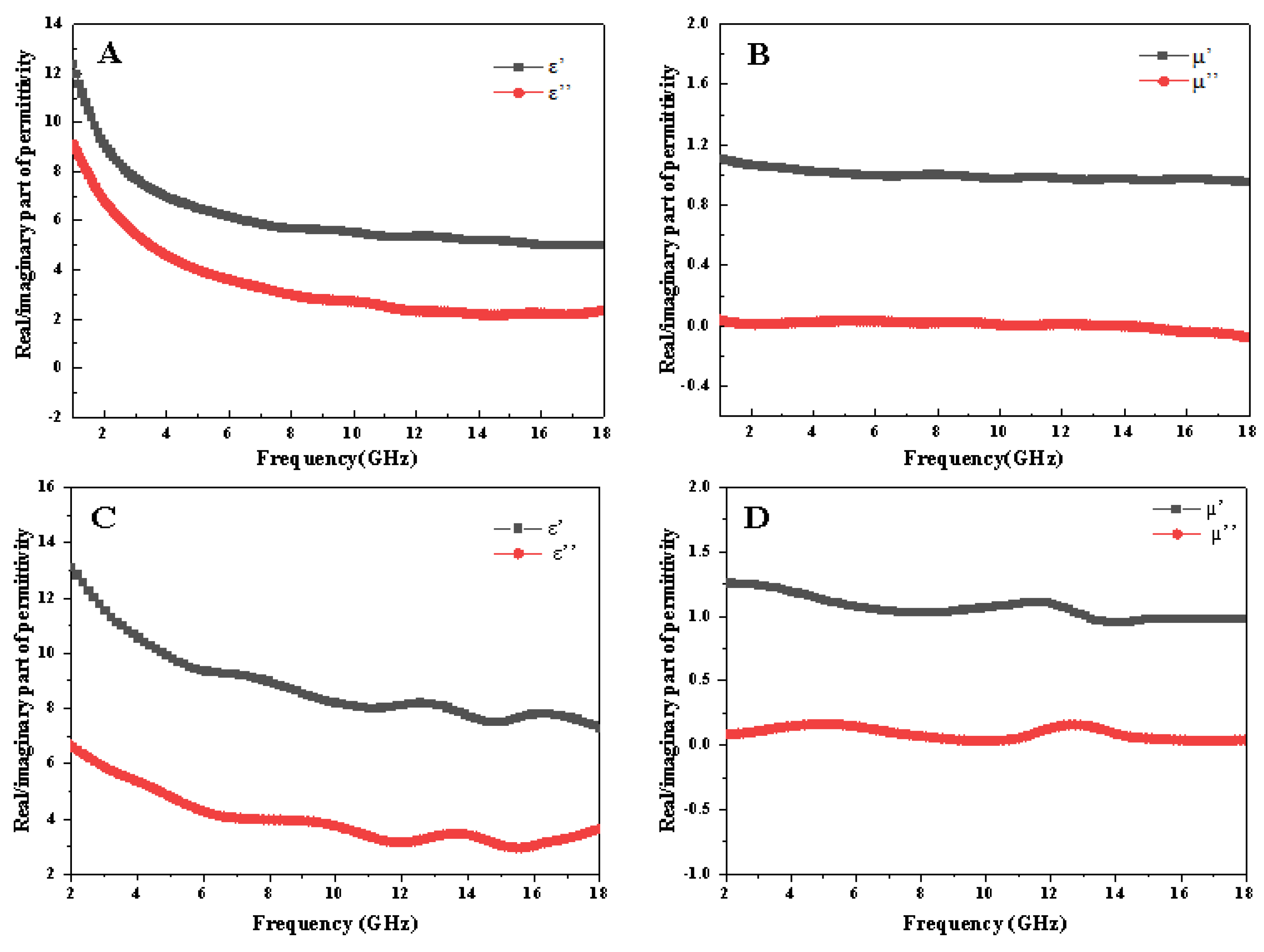
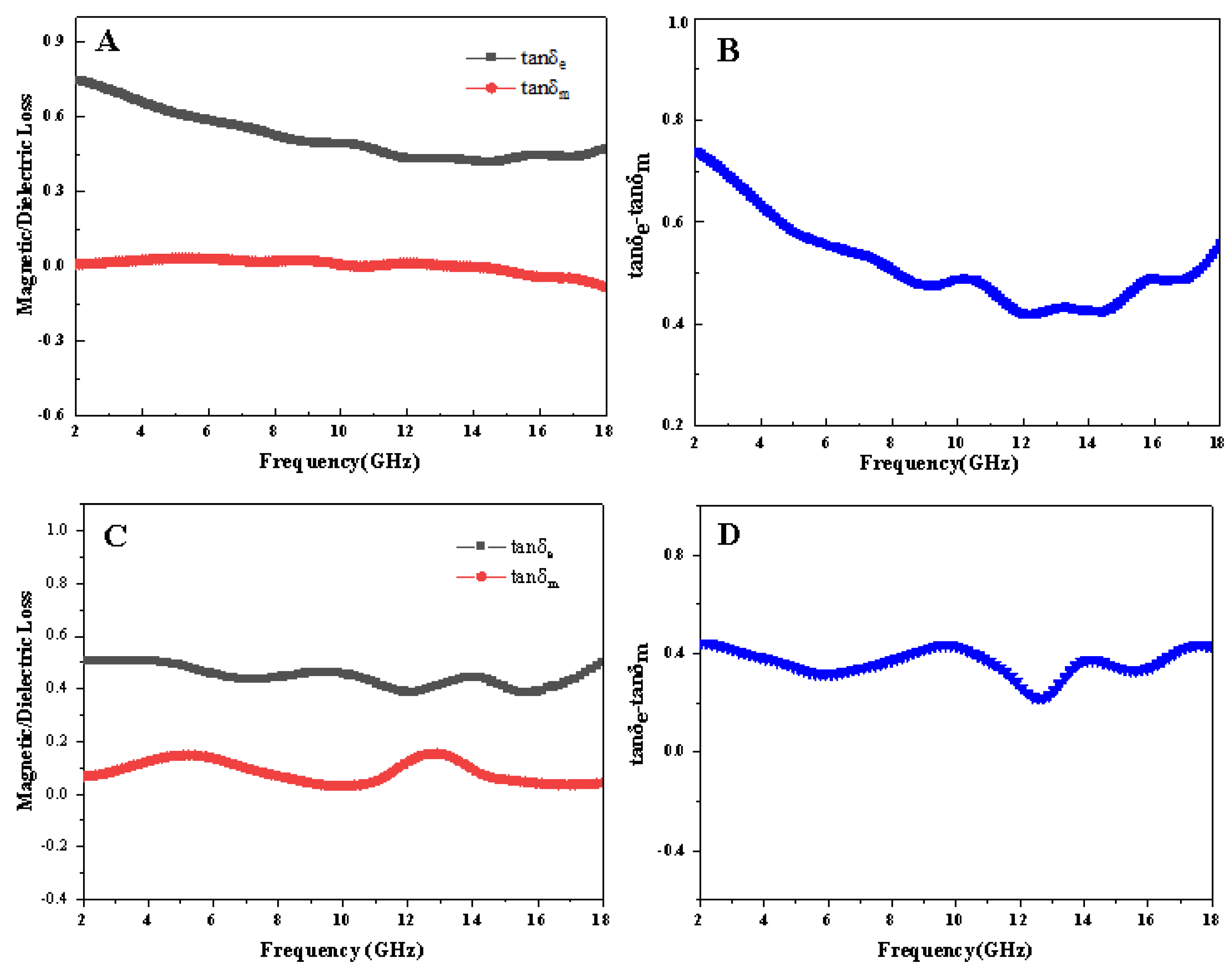
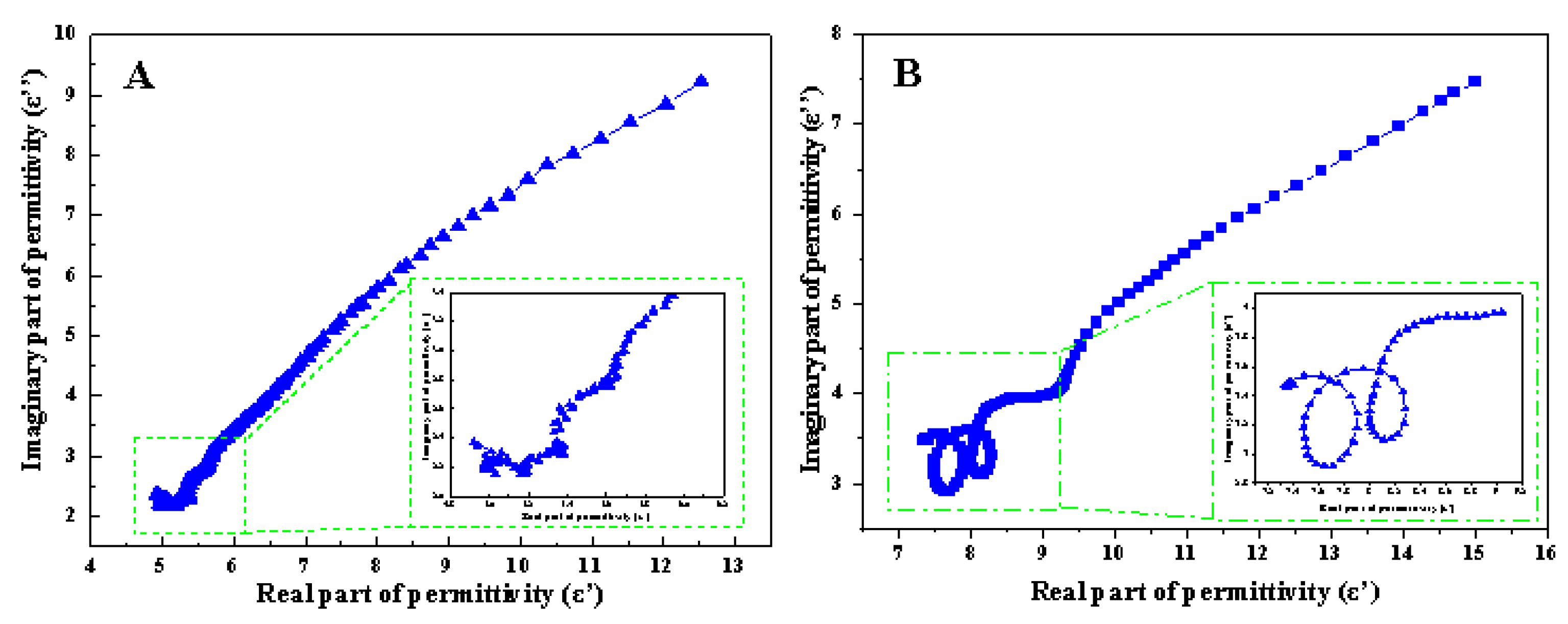
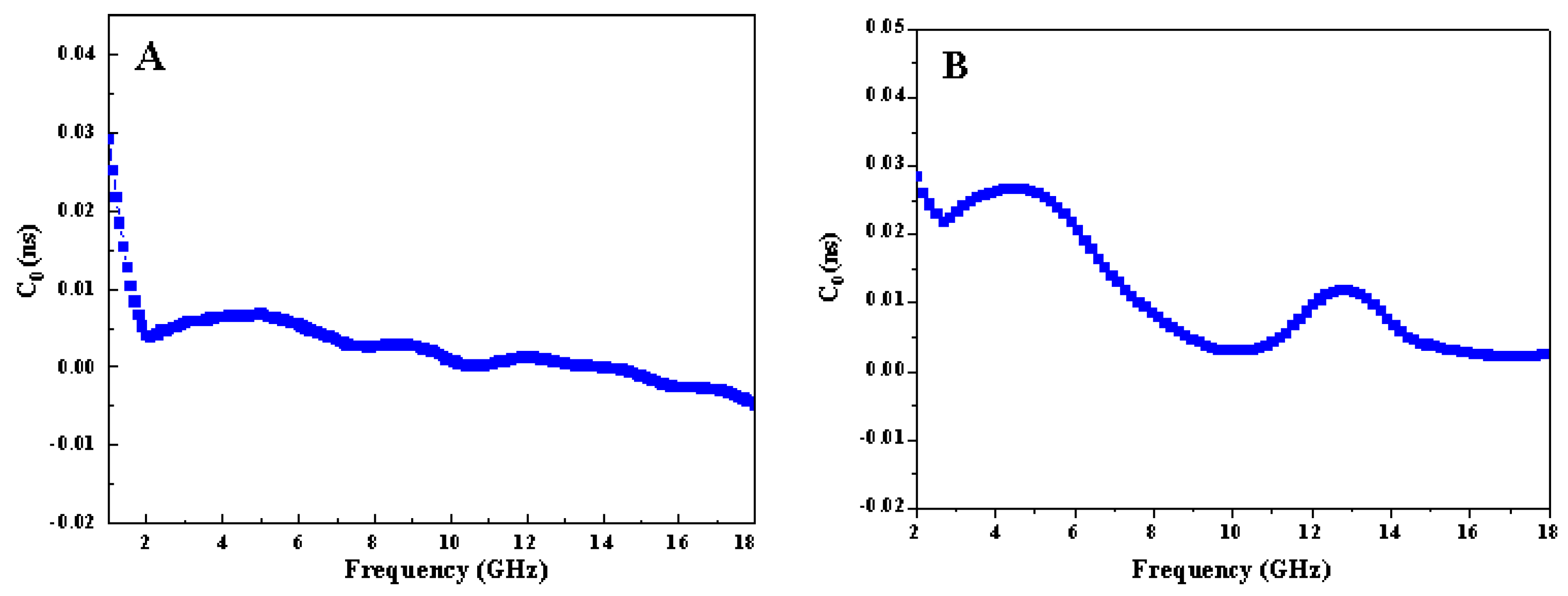
3.4. Evaluation of Microwave Absorption Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Shi, S.; Tang, Y.; Wang, G.; Zhou, M.; Zhao, G.; Zhou, X.; Lin, S.; Meng, F. Growth of NiAl Layered Double Hydroxide on Graphene toward Excellent Anticorrosive Microwave Absorption Application. Adv. Sci. 2021, 8, 2002658. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Wan, G.; Mou, P.; Teng, S.; Lin, S.; Wang, G. CNT@NiO/natural rubber with excellent impedance matching and low interfacial thermal resistance toward flexible and heat-conducting microwave absorption application. J. Mater. Chem. C 2021, 9, 869–880. [Google Scholar] [CrossRef]
- Hou, T.; Jia, Z.; Wang, B.; Li, H.; Liu, X.; Bi, L.; Wu, G. MXene-based accordion 2D hybrid structure with Co9S8/C/Ti3C2Tx as efficient electromagnetic wave absorber. Chem. Eng. J. 2021, 414, 128875. [Google Scholar] [CrossRef]
- Xu, X.; Wang, G.; Wan, G.; Shi, S.; Hao, C.; Tang, Y.; Wang, G. Magnetic Ni/graphene connected with conductive carbon nano-onions or nanotubes by atomic layer deposition for lightweight and low-frequency microwave absorption. Chem. Eng. J. 2020, 382, 122980. [Google Scholar] [CrossRef]
- Ning, M.; Lu, M.; Li, J.; Chen, Z.; Dou, Y.; Wang, C.Z.; Rehman, F.; Cao, M.S.; Jin, H.B. Two-dimensional nanosheets of MoS2: A promising material with high dielectric properties and microwave absorption performance. Nanoscale 2015, 7, 15734–15740. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Z.; Xiu, T.; Zhu, B.; Khan, I.; Liu, P.; Zhang, Q.; Zhang, B. Fabrication of ultralight helical porous carbon fibers with CNTs-confined Ni nanoparticles for enhanced microwave absorption. Compos. Part B Eng. 2021, 215, 108814. [Google Scholar] [CrossRef]
- Matatagui, D.; Sánchez, J.L.; Pea, A.; Serrano, A.; Horrillo, M.D.C. Ultrasensitive NO2 gas sensor with insignificant NH3-interference based on a few-layered mesoporous graphene. Sens. Actuators B Chem. 2021, 335, 129657. [Google Scholar] [CrossRef]
- Xu, H.; Yin, X.; Zhu, M.; Han, M.; Hou, Z.; Li, X.; Zhang, L.; Cheng, L. Carbon Hollow Microspheres with a Designable Mesoporous Shell for High-Performance Electromagnetic Wave Absorption. ACS Appl. Mater. Interfaces 2017, 9, 6332–6341. [Google Scholar] [CrossRef]
- Wang, J.; Huyan, Y.; Yang, Z.; Zhang, A.; Zhang, Q.; Zhang, B. Tubular carbon nanofibers: Synthesis, characterization and applications in microwave absorption. Carbon 2019, 152, 255–266. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Z.; Li, X.; Ren, J.; Wang, Y.; Zhang, Q.; Zhang, B. MOF-derived yolk-shell Co@ZnO/Ni@NC nanocage: Structure control and electromagnetic wave absorption performance. J. Colloid Interface Sci. 2021, 600, 99–110. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, S.; Wang, H.; Wang, G.; Sun, X.; Yin, P. Hollow Porous CoNi/C Composite Nanomaterials Derived from MOFs for Efficient and Lightweight Electromagnetic Wave Absorber. Carbon 2020, 167, 485–494. [Google Scholar] [CrossRef]
- Yang, K.; Cui, Y.; Liu, Z.; Liu, P.; Zhang, Q.; Zhang, B. Design of core-shell structure NC@MoS2 hierarchical nanotubes as high-performance electromagnetic wave absorber. Chem. Eng. J. 2021, 426, 131308. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Z.; Zhang, Y.; Liu, P.; Ahmad, M.; Zhang, Q.; Zhang, B. Wrinkled three-dimensional porous MXene/Ni composite microspheres for efficient broadband microwave absorption. Carbon 2021, 181, 58–68. [Google Scholar] [CrossRef]
- Xu, J.; Cui, Y.; Wang, J.; Fan, Y.; Shah, T.; Ahmad, M.; Zhang, Q.; Zhang, B. Fabrication of Wrinkled Carbon Microspheres and the Effect of Surface Roughness on the Microwave Absorbing Properties. Chem. Eng. J. 2020, 401, 126027. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, K.; Wang, J.; Shah, T.; Zhang, Q.; Zhang, B. Preparation of pleated RGO/MXene/Fe3O4 microsphere and its absorption properties for electromagnetic wave. Carbon 2020, 172, 1–14. [Google Scholar] [CrossRef]
- Yang, K.; Cui, Y.; Li, Q.; Liu, P.; Zhang, Q.; Zhang, B. Bimetallic MOFs-derived yolk-shell structure ZnCo/NC@TiO2 and its microwave absorbing properties. Appl. Surf. Sci. 2021, 556, 149715. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, X.; Luo, H.; Deng, L.; Wei, S.; Zheng, Y.; Jia, Q.; Liu, J. Rapid and direct growth of bipyramid TiO2 from Ti3C2Tx MXene to prepare Ni/TiO2/C heterogeneous composites for high-performance microwave absorption. Chem. Eng. J. 2020, 382, 123095. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Y.; Li, D.; Zhong, B.; Wu, Z.; Zuo, S.; Yan, D.; Zhuo, R.; Feng, J.; Yan, P. Microwave absorption properties of 3D cross-linked Fe/C porous nanofibers prepared by electrospinning. Carbon 2018, 134, 264–273. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Wu, F.; Shah, T.; Ahmad, M.; Zhang, A.; Zhang, Q.; Zhang, B. Core-shell structured Fe/Fe3O4@TCNFs@TiO2 magnetic hybrid nanofibers: Preparation and electromagnetic parameters regulation for enhanced microwave absorption. Carbon 2020, 165, 275–285. [Google Scholar] [CrossRef]
- Wu, F.; Liu, P.; Wang, J.; Shah, T.; Ahmad, M.; Zhang, Q.; Zhang, B. Fabrication of Magnetic Tubular Fiber with Multi-Layer Heterostructure and Its Microwave Absorbing Properties. J. Colloid Interface Sci. 2020, 577, 242–255. [Google Scholar] [CrossRef]
- Li, X.; Deng, Z.; Li, Y.; Zhang, H.; Zhao, S.; Zhang, Y.; Wu, X.; Yu, Z. Controllable synthesis of hollow microspheres with Fe@Carbon dual-shells for broad bandwidth microwave absorption. Carbon 2019, 147, 172–181. [Google Scholar] [CrossRef]
- Li, J.; Lu, S.; Huang, H.; Liu, D.; Zhuang, Z.; Zhong, C. ZIF-67 as Continuous Self-Sacrifice Template Derived NiCo2O4/Co, N-CNTs Nanocages as Efficient Bifunctional Electrocatalysts for Rechargeable Zn-Air Batteries. ACS Sustain. Chem. Eng. 2018, 6, 10021–10029. [Google Scholar] [CrossRef]
- Dai, W.; Chen, F.; Luo, H.; Xiong, Y.; Wang, X.; Cheng, Y.; Gong, R. Synthesis of yolk-shell structured carbonyl iron@void@nitrogen doped carbon for enhanced microwave absorption performance. J. Alloys Compd. 2020, 812, 152083. [Google Scholar] [CrossRef]
- Zhang, B.; Huyan, Y.; Wang, J.; Chen, X.; Zhang, H.; Zhang, Q. Fe3O4@SiO2@CCS porous magnetic microspheres as adsorbent for removal of organic dyes in aqueous phase. J. Alloys Compd. 2018, 735, 1986–1996. [Google Scholar] [CrossRef]
- Wu, T.; Liu, Y.; Zeng, X.; Cui, T.; Zhao, Y.; Li, Y.; Tong, G. Facile Hydrothermal Synthesis of Fe3O4/C Core-Shell Nanorings for Efficient Low-Frequency Microwave Absorption. ACS Appl. Mater. Interfaces 2016, 8, 7370–7380. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Huang, Y.; Yin, J.; Zhao, X.; Cheng, X.; He, S.; Li, X.; He, J.; Liu, J. Synthesis and Electromagnetic and Microwave Absorption Properties of Monodispersive Fe3O4/a-Fe2O3 Composites. ACS Appl. Nano Mater. 2018, 1, 3935–3944. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, D.; Tian, K.; Hu, W.; Wang, J.; Su, M.; Li, L. Facile Preparation of Core-Shell Fe3O4@Polypyrrole Composites with Superior Electromagnetic Wave Absorption Properties. J. Phys. Chem. C 2017, 121, 15784–15792. [Google Scholar] [CrossRef]
- Deng, Z.; He, S.; Wang, W.; Xu, M.; Zheng, H.; Yan, J.; Zhang, W.; Yun, J.; Zhao, W.; Gan, P. Construction of Hierarchical SnO2@Fe3O4 Nanostructures for Efficient Microwave Absorption. J. Magn. Magn. Mater. 2020, 498. [Google Scholar] [CrossRef]
- Cui, Y.; Wu, F.; Wang, J.; Wang, Y.; Shah, T.; Liu, P.; Zhang, Q.; Zhang, B. Three dimensional porous MXene/CNTs microspheres: Preparation, characterization and microwave absorbing properties. Compos. Part A Appl. Sci. Manuf. 2021, 145, 106378. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, Y.; Li, Q.; Zhang, Q.; Zhang, B. Fabrication of folded MXene/MoS2 composite microspheres with optimal composition and their microwave absorbing properties. J. Colloid Interface Sci. 2021, 607, 633–644. [Google Scholar] [CrossRef]
- Wang, J.; Wu, F.; Cui, Y.; Zhang, A.; Zhang, Q.; Zhang, B. Efficient synthesis of N-doped porous carbon nanoribbon composites with selective microwave absorption performance in common wavebands. Carbon 2021, 175, 164–175. [Google Scholar] [CrossRef]
- Xu, W.; Wang, G.; Yin, P. Designed Fabrication of Reduced Graphene Oxides/Ni Hybrids for Effective Electromagnetic Absorption and Shielding. Carbon 2018, 139, 759–767. [Google Scholar] [CrossRef]
- Gueye, B.; Jesús, S.; Navarro, E.; Serrano, A.; Marín, P. Control of the Length of Fe73.5Si13.5Nb3Cu1B9 Microwires to Be Used for Magnetic and Microwave Absorbing Purposes. ACS Appl. Mater. Interfaces 2020, 12, 15644–15656. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Shen, Y.; Du, X.; Chong, J. Microwave Absorption Properties of Magnetite Particles Extracted from Nickel Slag. Materials 2020, 13, 2162. [Google Scholar] [CrossRef]
- Wang, K.; Chen, Y.; Tian, R.; Li, H.; Zhou, Y.; Duan, H.; Liu, H. Porous Co-C Core-Shell Nanocomposites Derived from Co-MOF-74 with Enhanced Electromagnetic Wave Absorption Performance. ACS Appl. Mater. Interfaces 2018, 10, 11333–11342. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, K.Y.Q.; Shah, T.; Ahmad, M.; Zhang, Q.; Zhang, B. Biomass-derived 3D magnetic porous carbon fibers with a helical/chiral structure toward superior microwave absorption. Carbon 2021, 173, 918–931. [Google Scholar] [CrossRef]
- Liao, Q.; He, M.; Zhou, Y.; Nie, S.; Wang, Y.; Hu, S.; Yang, H.; Li, H.; Tong, Y. Highly Cuboid-Shaped Heterobimetallic Metal-Organic Frameworks Derived from Porous Co/ZnO/C Microrods with Improved Electromagnetic Wave Absorption Capabilities. ACS Appl. Mater. Interfaces 2018, 10, 29136–29144. [Google Scholar] [CrossRef]
- Wu, F.; Li, Q.; Liu, Z.; Shah, T.; Ahmad, M.; Zhang, Q.; Zhang, B. Fabrication of binary MOF-derived hybrid nanoflowers via selective assembly and their microwave absorbing properties. Carbon 2021, 182, 484–496. [Google Scholar] [CrossRef]
- Zhao, J.; Quan, X.; Chen, S.; Liu, Y.; Yu, H. Cobalt Nanoparticles Encapsulated in Porous Carbons Derived from Core-Shell ZIF-67@ZIF-8 as Efficient Electrocatalysts for Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 28685–28694. [Google Scholar] [CrossRef]
- Liang, L.; Han, G.; Li, Y.; Zhao, B.; Zhou, B.; Feng, Y.; Ma, J.; Wang, Y.; Zhang, R.; Liu, C. Promising Ti3C2Tx MXene/Ni chain hybrid with excellent electromagnetic wave absorption and shielding capacity. ACS Appl. Mater. Interfaces 2019, 11, 25399–25409. [Google Scholar] [CrossRef]



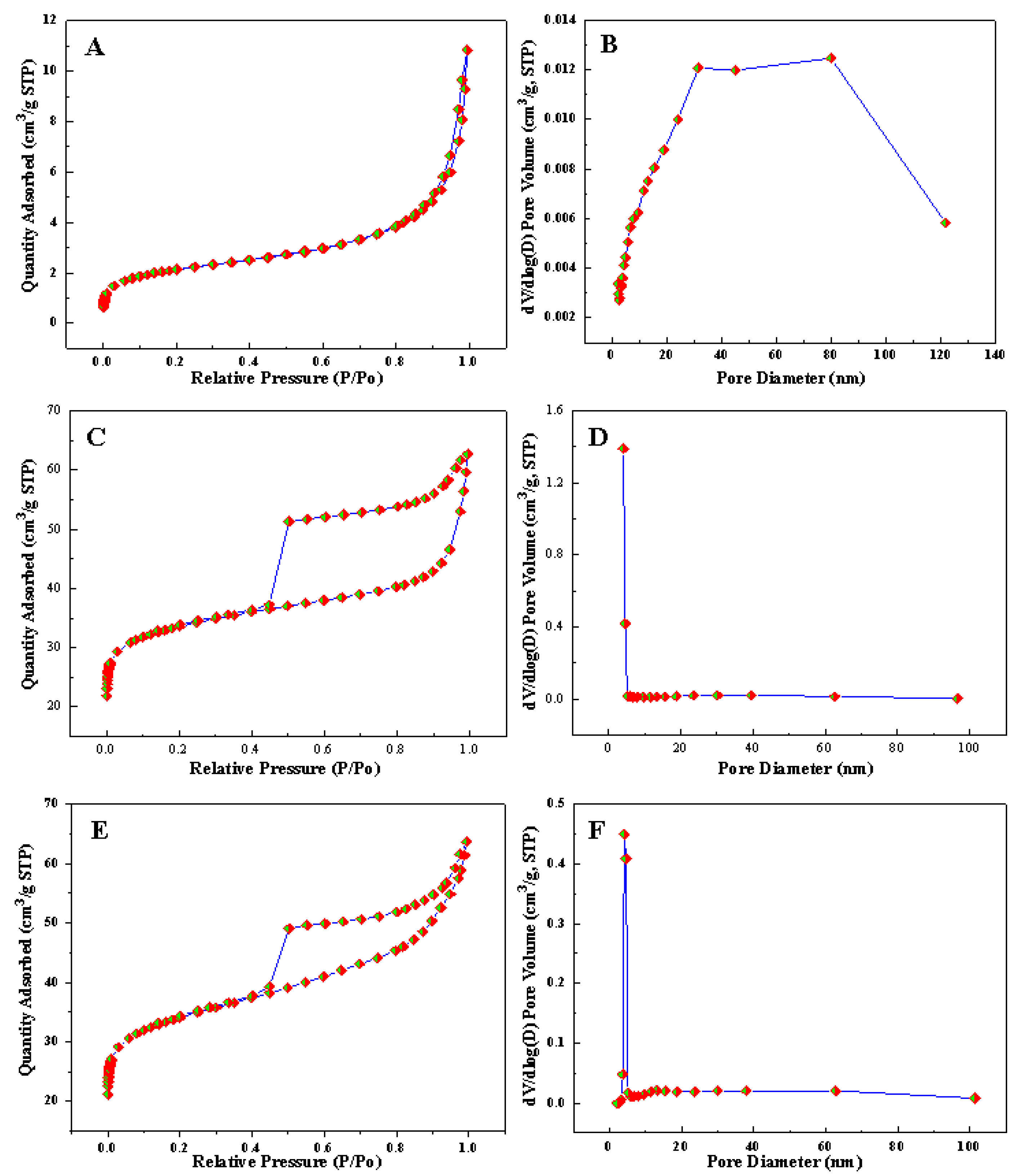

| Sample | BET (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| Fe3O4@RF-12 | 7.60 | 0.0168 | 14.71 |
| Fe3O4@C-700 | 114.25 | 0.0970 | 5.13 |
| Fe@C-12 | 116.95 | 0.0986 | 5.71 |
| Materials | Thickness (mm) | Effective Bandwidth (GHz) | RLmin (dB) | Reference |
|---|---|---|---|---|
| FeCPNFs | 2.0 | 3.0 | −26.1 | [18] |
| Fe/Fe3O4@TCNFs@TiO2 | 1.6 | 3.7 | −44.8 | [19] |
| TCF@Fe3O4@NCLs | 3.3 | 4.6 | −43.6 | [20] |
| Fe@C (HFC) | 4.5 | 8.1 | −54.4 | [21] |
| Fe73.5Si13.5Nb3Cu1B9 | 0.14 | 4.4 | −45.97 | [33] |
| Nickel Slag | 5.0 | 1.7 | −34.00 | [34] |
| Fe3O4@C-700 | 1.8 | 6.0 | −35.12 | This work |
| Fe@C-12 | 3.5 | 4.1 | −50.15 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lou, P.; Yang, L. Controllable Preparation of Fe3O4@RF and Its Evolution to Yolk–Shell-Structured Fe@C Composite Microspheres with High Microwave Absorbing Performance. Coatings 2022, 12, 62. https://doi.org/10.3390/coatings12010062
Li X, Lou P, Yang L. Controllable Preparation of Fe3O4@RF and Its Evolution to Yolk–Shell-Structured Fe@C Composite Microspheres with High Microwave Absorbing Performance. Coatings. 2022; 12(1):62. https://doi.org/10.3390/coatings12010062
Chicago/Turabian StyleLi, Xue, Peng Lou, and Longquan Yang. 2022. "Controllable Preparation of Fe3O4@RF and Its Evolution to Yolk–Shell-Structured Fe@C Composite Microspheres with High Microwave Absorbing Performance" Coatings 12, no. 1: 62. https://doi.org/10.3390/coatings12010062





