Abstract
This review reports recently published research related to the application of polysaccharide-based biodegradable and edible coatings (BECs) fortified with bioactive compounds obtained from plant essential oils (EOs) and phenolic compounds of plant extracts. Combinations of polysaccharides such as starches, pectin, alginate, cellulose derivatives, and chitosan with active compounds obtained from clove, lemon, cinnamon, lavender, oregano, and peppermint have been documented as potential candidates for biologically active coating materials for retardation of quality changes in fresh fruits. Additionally, polysaccharide-based active coatings supplemented with plant extracts such as cashew leaves, pomegranate peel, red roselle, apple fiber, and green tea extracts rich in phenolic compounds and their derivatives have been reported to be excellent substituents to replace chemically formulated wax coatings. Moreover, EOs and plant polyphenolics including alcohols, aldehydes, ketones phenols, organic acids, terpenes, and esters contain hydroxyl functional groups that contribute bioactivity to BECs against oxidation and reduction of microbial load in fresh fruits. Therefore, BECs enriched with active compounds from EOs and plant extracts minimize physiological and microbial deterioration by reducing moisture loss, softening of flesh, ripening, and decay caused by pathogenic bacterial strains, mold, or yeast rots, respectively. As a result, shelf life of fresh fruits can be extended by employing active polysaccharide coatings supplemented with EOs and plant extracts prior to postharvest storage.
Keywords:
coating; polysaccharide; bioactivity; essential oil; plant extract; polyphenols; antimicrobial; antioxidant 1. Introduction
Fresh fruits containing essential nutrients, vitamins, and minerals are consumed worldwide in part because of their strong antioxidant potential against chronic diseases [1]. Fresh fruit packaging materials after single use are disposed of in the environment. The application of synthetic and non-biodegradable polymer-tailored packaging materials for fresh fruit has raised potentially alarming consequences for the environment [2]. Conventional packaging materials such as glass, wood, aluminum, tin, and paper have been employed as fresh fruit containers to prevent mechanical damage during bulk transportation [3]. The innovative designs of synthetic packaging materials have been of great convenience to customers in supermarkets [4]. Synthetic packaging materials used for fruits may lack the optimum oxygen and moisture barrier properties to maintain their postharvest quality in the markets [5]. Additionally, the production of synthetic packaging materials may directly have an impact on the sustainability of non-renewable petroleum-based resources [6].
Fresh fruits typically have a short postharvest shelf life due to ongoing physiological and biochemical changes occurring in the living tissues until consumption [7]. Mechanical damages and pathological changes during improper handling and transportation have been associated with heavy economic losses [8]. Conventional synthetic waxes and chemical fungicides have been used as postharvest treatments to minimize losses in fresh fruits. These materials have been reported to cause health and environmental concerns [9]. Chemical-based coatings fortified with synthetic antimicrobial additives have been associated with the antimicrobial resistance of food borne pathogenic strains. Taking all the research challenges into consideration, the novel idea of active food coatings composed of polysaccharides supplemented with natural essential oils, phenolics, and active nanoparticles has been an effective adjunct to conventional postharvest treatments of fresh fruits [10]. Because several polysaccharides have limited barrier and mechanical properties even after the addition of bioactive compounds, the inclusion of inorganic clays [11] or nanoparticles [12] has been proposed.
During the past decade, several findings reported applications of natural and biodegradable edible coatings that have proven to be sustainable alternatives with excellent barrier properties compared to synthetic plastic packaging commonly used in the market [13]. Edible coating materials employed consisted of a wide range of plant or crustacean-based polysaccharides [14]. Hydrocolloid-based coating forming solutions prepared from starches, pectin, alginate, carboxymethyl cellulose, and chitosan have been applied to delay ripening and prevent senescence or detachment of fruit skin during postharvest storage [15]. In addition to plasticizers, emulsifiers, surfactants, and hydrophobic materials, the use of inorganic clays [11] or nanoparticles [12] have also been proposed.
Essential oils (EOs) have been incorporated as active ingredients in polysaccharide-based coating materials against oxidation of vital nutrients and bacterial and fungal growth. EOs from different herbs such as clove, lemon, cinnamon, tea tree, lavender, oregano, and peppermint are a source of diverse bioactive compounds with higher antimicrobial efficacy for the preparation of active food coating materials employed in fresh fruits [16]. Bioactivity of EOs has been documented because of antioxidant and antimicrobial functional groups present such as monoterpenes, flavonoids, aldehydes, isoflavones, carotenoids, and phenolic compounds that exhibit numerous nutraceutical properties [17]. EOs incorporated in the polysaccharide coating materials to extend the shelf life of fresh fruits have generated tremendous interest and are generally recognized as safe food coating additives [18]. EOs incorporated in polysaccharide-based coating materials may result in a hydrophobic film on the coated fruits to reduce loss of weight and firmness [19]. Biodegradable and edible coatings (BECs) containing EOs may also suppress several hormonal and enzymatic reactions triggered by contact with atmospheric oxygen during postharvest storage of fruit [20]. In addition to physiochemical quality preservation, EOs have been investigated to provide protection against a broad spectrum of food-borne spoilage and pathogenic microorganisms [21].
The use of plant extracts containing alcohols, aldehydes, ketones phenols, organic acids, esters, and terpenes as active coating additives has tremendous scope in the preservation of postharvest physical, oxidative, and microbial quality of fresh fruits [22,23]. Phytochemical polyphenols comprising multiple hydroxyl functional groups attached to benzene rings have been supplemented in different polysaccharide edible coatings [10]. Plant extracts such as pomegranate peel and pineapple extracts incorporated in cassava starch, alginate, and chitosan coatings have been documented to safeguard the postharvest quality of fruits [24]. Furthermore, the bioactive properties of plant-derived natural essential oils and polyphenols along with polysaccharides have been exploited in the preparation of emulsion-based active coatings to combat the postharvest losses of in fruits [25]. EOs and plant extracts containing bioactive compounds have been reported as potential substitutes for chemical additives to ensure food quality and safety of fruits during postharvest storage [26]. Therefore, this review reports the different polysaccharide-based coatings fortified with plant EOs and phytochemical extracts with bioactive properties to prolong the postharvest shelf life of fresh fruits. The review also emphasizes the beneficial effects of the aforementioned eco-friendly coatings on the physical, biochemical, and microbiological quality of fresh fruits. Thus, the heavy losses in the horticulture sector in the future could be prevented leading to the sustainable development of a green economy.
2. Postharvest Quality Constraints of Fresh Fruits
Fruits are commonly harvested on the basis of conventional extrinsic factors such as firmness, color, size, and shape. More recently, intrinsic factors such as nutritional and functional attributes have been considered, including minerals, vitamins, dietary fibers, and other polyphenolic constituents that exhibit beneficial health properties [27]. During postharvest handling, transportation, and bulk storage, fruits may be highly susceptible to biological and/or mechanical hazards that can affect both intrinsic and extrinsic factors [28]. In addition to improper postharvest handling of fruits, mechanical vibrations may affect the fruit quality during transportation, triggering heavy losses during longer storage periods. The quality problems that emerge in metabolically active fruits during postharvest storage include physiological deterioration and microbial deterioration as evidenced by moisture loss, softening of flesh, ripening, and decay caused by pathogenic bacterial strains, molds, or yeast rots [27,29].
Microbial and Biochemical Causes of Deterioration in Fresh Fruits
Fruits after harvesting from the field may be contaminated with pathogenic microbes, insects, and pests. Fresh fruits in unprocessed and raw form contain infectious germs on the skin of fruits that can lead to food borne diseases [30]. The microbial population is an important factor in considering the quality of the food product [31]. The low pH fruits, like ripe tomatoes, in a pH range (3.9–4.5) could inhibit the human intestinal pathogens such as Shigella and Escherichia coli O157:H7. Melons and soft fruits with a pH of 4–6 can favor the growth and survival of Botrytis cinerea and Penicillium species [32]. Pathogenic organisms are transmitted from the environment mostly during fruit harvesting from plants, post-harvest displacements, processing, and transport movements [33]. Several types of microorganisms, such as bacteria, yeasts, and fungi that cause deterioration may be transmitted during postharvest storage. Approximately 80–90% of microbial contamination in fresh fruits is due to Pseudomonas and Enterobacteriaceae (Klebsiella, Enterobacter, Citrobacter, Salmonella, Escherichia coli, Shigella, Proteus, Serratia, and other species) referred as Gram-negative bacteria [32,34]. Additionally, lactic acid bacteria, which are a natural flora of fruits, are corrosive and develop unpleasant odors [32]. Moreover, fresh fruits contaminated with fungi (Rhizopus, Penicillium, Aspergillus, and Eurotiumand Wallemia) and the yeast (Debaryomyces, Pichia, Candida, Hanseniaspora, Zygo saccharomyces), also have major role in the spoilage of fresh fruits during postharvest handling and storage [32]. The use of chemical disinfectants such as organic acids, chlorine dioxide, hydrogen peroxide, hypochlorite, sodium bisulfite, sulfur dioxide, and ozone has been proposed for reducing the bacterial population during postharvest storage [35]. Such chemical-based disinfectants have limited applications due to ill effects on human health and degradation of sensory quality in fruits [36].
Biochemical quality deterioration may depend on the storage temperature and metabolic processes occurring during respiration of living tissues in postharvest storage of fruits. Temperature is an important factor responsible for controlling metabolism of carbohydrates, lipids, and amino acids in respiring fruits. Temperate fruit crops are commonly stored at temperatures (0–1 °C) compared to the tropical or subtropical fruits that must be stored at higher temperatures (7–15 °C) to avoid losses due to chilling injury (CI) [37]. CI may alter the ripening process by damaging the external peel, inducing internal flesh browning, pitting, loss of firmness, and discoloration evidenced after the removal of fruits from cold temperature storage [37].
Appropriate storage temperatures can extend storage life by approximately 2–4 weeks for crops such as apricots, sweet cherries, and peaches, and up to several months for apples, pears, and kiwifruits [37]. The general effect of low temperature storage upregulates stress-responsive genes, blocks signal transduction of ethylene production processes affecting metabolic changes in vital components of fruits [38,39]. Various commercially important fruits, such as apples, pears, kiwifruits, bananas, and nectarines, at physiological maturity are characterized by high starch content that is converted to sugars at low temperatures during postharvest storage [40]. Induction of chilling tolerance of nectarines stored at near freezing temperatures (−1.4 °C) was shown to reduced activities of sucrose metabolism-associated enzymes that resulted in higher sucrose contents [40]. Moreover, fatty acids are essential cell membrane components forming a selectively permeable barrier between the cells in a fruit matrix. Fruits are composed of different types of fatty acids that show active roles in the biochemical quality degradation during postharvest cold storage. Peaches containing plastidic glycerolipid and triacylglycerides (TAGs) are used as a source of energy during fruit senescence [41]. Phosphatidic acid (PA) is accumulated in pineapple fruit during blackheart development at 10 °C [42]. Increased levels of phospholipase D enzyme activities have been observed in cold stored pears [43,44]. Similarly, chilling injury of “Honeycrisp” apples with soggy flesh showed elevated contents of glycerol and TAGs [45]. During postharvest storage of fruits, proteins may be degraded into free amino acids due to the activation of proteolytic enzymes. Amino acids such as Glu, Gln, Asp, and Asn contents increased in tomatoes stored at 4 °C [46]. Similar results were also documented in kiwifruit that showed increased Thr, Ile, and Val contents [47].
Additionally, temperature fluctuations during turbulent transportation may lead to mechanical bruising of fruits without any postharvest coating, thereby accelerating their decay [48]. In this regard, it is of primary concern to apply different novel coating techniques to delay ripening and senescence in fruits [49]. The aim is to eradicate biochemical quality deterioration during defective cold chain management that may accelerate the rate of respiration in living tissues and induce undesirable ripening (the main cause of senescence), thereby shortening the shelf-life of fruits [50]. Fruit ripening increases the total soluble solids resulting in higher sugar content; it involves several metabolic processes that differ between ‘climacteric’ and ‘non-climacteric’ fruits [51]. During the ripening of climacteric fruit, respiration increases until it reaches a peak, which is accompanied by an increase in ethylene production. In contrast, respiration of non-climacteric fruit does not increase during ripening, and ethylene is not required in order to complete the ripening process [52]. Regardless of the type of ripening, this process, as well as other metabolic processes that lead to deterioration, are driven by respiration. After harvest, the fresh produce continues to respire, utilizing food reserves, taking in oxygen, and releasing carbon dioxide and heat from stored carbohydrates [37]. For that reason, postharvest active coating treatments are applied on the fruit surfaces through various methods to reduce respiration, delay deterioration processes, prolong shelf life, and help to maintain produce quality.
3. Application Methods of Polysaccharide-Based Active Edible Coatings in Fresh Fruits
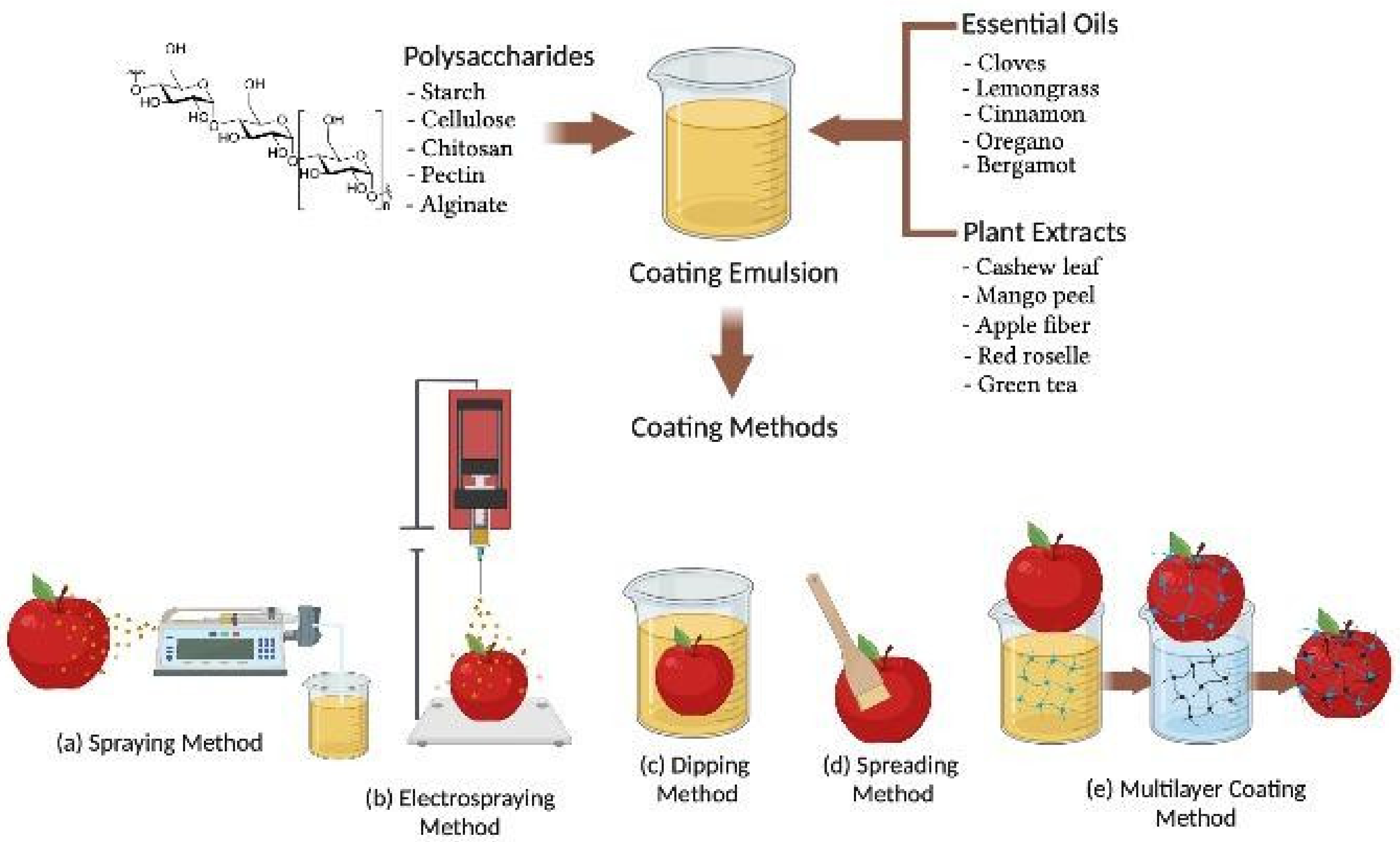
BECs can be applied to fresh fruits after harvesting from the plants or trees using various methods as shown in Figure 1. The selection of BECs mainly depends on the fruit surface hydrophobicity and roughness and the physical properties of the BEC such as surface tension, viscosity, density, coating emulsion stability, cost, and drying conditions for industrial application [53]. The various methods of BEC application for fresh fruits explained in this reviewed work include conventional spraying, electrospraying, dipping, spreading, brushing, and layer by layer deposition techniques, respectively (Figure 1). Spraying is a conventional technique for applying low viscous BEC solutions on the fresh fruit surface [54]. A homogenous spray with fine droplets may form a uniform layer on the fruit surface at a high-pressure atomization in the range of 60–80 psi (4.1–5.5 bar) [55]. The desirable layer of coating thickness mainly relies on the lower hydrodynamic diameter of the droplet and atomizer features (spray gun type, operating pressure, and nozzle temperature) as well as the humidity and flow rate of air or liquid in the BEC solution [56]. Conventional spraying methods applied on the rough surfaces of strawberry fruit have shown lower transfer efficiency and coating evenness compared to the electrospraying method of coating [57].
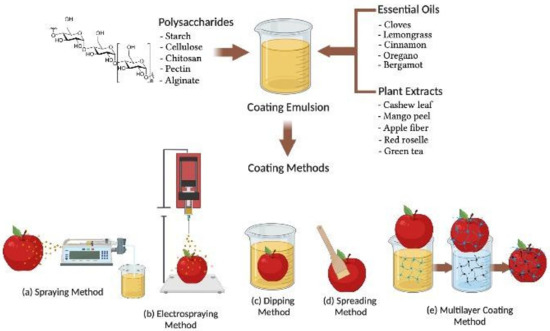
Figure 1.
Schematic representation of biodegradable and edible active coating applications via (a) spraying; (b) electrospraying; (c) dipping; (d) spreading; and (e) multilayer coating methods, employed in the postharvest treatment of fruits.
Electrospraying is a novel method of coating in which a coating material is atomized in the presence of a high-intensity electric field, which enables the formation of micrometric and sub-micrometric charged droplets with an extremely narrow size distribution [58,59]. The tip of an emitter causes the formation of a Taylor cone of the nascent charged droplets and destabilizes the liquid surface to generate a cluster of charged droplets [60]. Electrospraying promotes the efficient adhesion to the surface of fresh fruit compared to conventional spraying because of electrostatic interactions of micrometric-sized charged droplets [61]. The droplet size, deposition rate, and coating thickness during electrospraying depend on the conductivity, flow rate, and viscosity of the coating solution [57]. The electrospraying coating method was employed to obtain even distribution of charged coating material droplets containing micro to nano size magnetic cellulose with special affinity to orient under an electric field, forming a compact coating film [62].
BECs applied by the dipping method undergo in three steps. The first step is immersing fresh fruits in the coating solution and holding for 2 to 3 min so the coating material can adhere on the fruit [63]. The last two steps are deposition and drainage of extra adhered BEC solution followed by evaporation and drying of coated fruit either at ambient temperature or flushed with hot air to accelerate drying [64]. Coating thickness and morphology of the coating’s material deposited by the dipping method on the surface of fruits depends on various factors such as immersion time, withdrawal speed, dip-coating cycles, density, viscosity, surface tension, and drying conditions [65,66,67]. Hydroxypropyl methylcellulose in a dip-coating solution was analyzed for viscosity, density, and surface tension during coating of Fuji apples, after which the internal oxygen and carbon dioxide levels were measured at room temperature for 4 days. Results indicated that coating thickness varied with viscosity, concentration, density, and draining time of the biopolymer solution. Coating thickness relates to the square root of viscosity and the inverse square root of draining time, which agrees with the theoretical approach for flat plate dip-coating in low-capillary-number Newtonian liquids. These results indicate the possibility of controlling coating thickness and internal gas composition based on coating solution properties [67]. The dipping compared to conventional spraying or electrospraying is more beneficial for coating fruits with complex and rough surfaces, resulting in excellent uniformity [68]. Dipping generally forms a thick coating layer on the fruit surface and may effectively reduce microbial load, contamination, respiration rate, and mechanical damage and prevent physiological changes of coated fruits [69,70].
The brushing method involves the use of a sterile brush for spreading high viscosity BECs on the fruit surface and depends on the wetting degree and the spreading rate parameters followed by a drying process [71]. Brushing of BECs is generally carried out manually by experienced operators and includes several factors to minimize manual error of BEC application and ingredient quality to achieve better coating layer uniformity [15]. The efficiency of BECs is also affected by the roughness of the fruit surface and geometry, viscosity, surface tension, density, drying temperature, and relative humidity [70]. The degree of spreading or wettability of BECs can be characterized on the surface of fruit by contact angle measurements that maintain mechanical equilibrium of the coating drops under the influence of mainly three surface tension forces—solid–liquid, liquid–vapor, and solid–vapor interfaces—to assess the adhesion properties of coating solutions on the fruit surfaces [70,72]. The ideal case of a contact angle value equal to 0° corresponds to a hydrophilic solid surface where total wetting conditions can be attained by an aqueous solution. A contact angle value between 0° and 180° suggests the occurrence of partial wetting, which is higher for a contact angle below 90°. The ideal case of a contact angle equal to 180° corresponds to a hydrophobic solid surface, where no wetting conditions occur when in contact with an aqueous medium. The contact angle can be measured directly on the food surface through the sessile drop method or atomic force microscopy to visualize the thickness and adherence of the coated surface [72,73].
BECs applied via the multilayer coating method include layer by layer deposition of coating solutions for better adhesion, especially on the surfaces of fresh-cut fruits [74]. Multilayer coating adhesion exhibits electrostatic interaction of the charged polyelectrolytes with that of the fruit surface [75,76]. The electrostatic interactions between the multilayer coatings of nano size dimensions may form chemical bonds, thereby providing effective control of physiological, mechanical, and functional properties on coated fruit [77]. In the multilayer coating method, coating materials containing oppositely charged polyelectrolytes are deposited through alternate dipping of the fruit in different coating solutions (Figure 1e). The dipping of fruit in many cycles creates a layer-by-layer deposition of a coating solution that mainly depends on the ionic strength, pH, and charge densities to form a bonded network via electrostatic forces of attraction [74]. Therefore, the application of the multilayer coating method has been reported in polysaccharides and charged polyelectrolytes capable of hydrogen and covalent bonding to increase compactness of the coating layers during postharvest storage of fruits [10,78].
4. Impact of Polysaccharide-Based Active Edible Coatings Fortified with Essential Oils and Plant Extracts on the Postharvest Quality of Fresh Fruits
The various carboxymethyl cellulose (CMC), chitosan, pectin, alginate, and starch-based active coatings supplemented with EOs and plant polyphenolic extracts have been applied over the past five years in published research work as an active coating material for fresh fruits. The aforementioned active BECs have shown promising results with a diverse combination of other plant-based gums (Table 1, Table 2 and Table 3).

Table 1.
Polysaccharide-based biodegradable and edible coatings for the quality preservation of fresh fruits during postharvest storage.

Table 2.
Polysaccharides combined with essential oils for the quality preservation of fresh fruits during postharvest storage.

Table 3.
Polysaccharides combined with plant polyphenolic extracts for the quality preservation of fresh fruits during postharvest storage.
4.1. CMC-Based Active Coatings
CMC is a cellulose derivative that is generally odorless and tasteless, flexible, transparent, and non-toxic and can be labelled as an edible coating [115]. CMC usually forms a clear, colorless and tasteless solution. It is cold water soluble and shows tolerance to high concentrations of sugar. I is available in a wide range of viscosities and has good heat stability and film forming properties [116]. Several studies have applied CMC or CMC in combination with other polysaccharides as BECs (Table 1). To provide bioactivity in the CMC coating material against physical, chemical, and microbial deterioration, EOs and plant extracts have been incorporated to form active BECs [117]. In some of the recent studies, garlic EO fortified in CMC coatings maintained higher concentrations of total phenols and anthocyanins in strawberries [118]. CMC coatings containing Mentha spicata EO inhibited Listeria monocytogenes and preserved physicochemical and organoleptic properties of strawberries [119]. CMC-based coatings incorporated with Zataria multiflora Boiss EO and grape seed extract (GSE) retarded changes in chemical, microbial, and sensory characteristics of coated fresh food during low temperature storage [120]. CMC coating enriched with clove EO delayed fungal growth and ripening and also reduced the rate of respiration and weight loss with enhanced commercial acceptability of ‘Xinyu’ mandarin oranges [121]. CMC reduced decay, weight loss, chilling injury, and hydrogen peroxide and malondialdehyde content in ‘Kinnow’ mandarin fruits during low temperature storage [122]. CMC along with pistachio (Pistacia atlantica L.) EO supplemented coating material showed higher anthocyanin, antioxidant capacity, phenol, tannin, and titratable acidity with a slight increment in TSS of grape cv. Rasheh during postharvest storage [123]. CMC Impatiens balsamina L. stem extract acted as an antimicrobial barrier to pathogen and gases, reduced the decay rate and weight loss, and inhibited the enzyme activities involved in the biochemical deterioration and softening in “Xinyu” tangerines [124]. CMC acted as a barrier to mold damage by forming a thick layer on the surface of oranges [125]. Methyl cellulose coating with thyme oil retained the higher antioxidant activity and reduced weight loss, total yeast, mold and total plate counts of mesophilic and psychrophilic microorganisms in “Acco” Pomegranate Arils [126].
4.2. Chitosan-Based Active Coatings
Chitosan is a renewable biopolymer derived from chitin. The cationic linear structure of chitosan composed of β-(1–4)-linked D-glucosamine (deacetylated unit) and N-acetyl-D-glucosamine (acetylated unit) is derived from crustaceans, fungi, and yeast [76]. Chitosan incorporated with Mentha spicata EO and coated on the surface of strawberries prevented growth of L. monocytogenes and retarded changes in physicochemical and organoleptic properties [119]. Chitosan with Origanum vulgare L. EO reduced the incidence of black mold and soft rot triggered by R. stolonifer and Aspergillus niger in cherry tomato fruit [127]. Chitosan with thymol EO prevented weight loss, retarded the rate of respiration, maintained TSS and the ratio of TSS to TA, lowered fungal decay incidence, and retained firmness, TA, anthocyanin, and sensory characteristics of fresh fig (Ficus carica L.) under low temperature storage [128]. Chitosan with Mentha piperita L. EO delayed changes in peel and pulp color and retained the catechins, procyanidins B1 and B2 in mango cultivar ‘Tommy Atkins’ during cold storage [129]. Chitosan applied with cinnamon EO reduced weight loss and preserved physical and biochemical quality of jujube fruits during 60 days of cold storage [130]. Clove EO fortified in chitosan inhibited activity of enzymes corresponding to browning of freshly cut lemons [131]. Chitosan–pullulan (50:50) edible coating prepared with pomegranate peel extract (0.02 g/mL) reduced weight loss and maintained TSS, pH, firmness, phenolic content, and antioxidant activity of mango fruits during 18 days of postharvest storage at 4 °C [132]. Chitosan coating incorporated with olive oil residues extracts (2% w/v) showed higher inhibition of Penicillium expansum compared with Rhizopus stolonifer in vitro and in vivo, thereby maintained the fresh quality of apple and strawberry fruits during postharvest storage [133]. Chitosan (1.5% w/v) enriched with hairy fig (Ficus hirta Vahl.) fruit extract coating applied to “Newhall” navel orange showed the lowest decay rate (5.2%), weight loss (5.16%), and malondialdehyde content while enhancing the activities of protective enzyme such as superoxide dismutase, peroxidase, chitinase, and β-1,3-glucanase during 120 days of cold storage [134]. Additionally, chitosan (1% w/v) and alginate (2% w/v) coatings in combination with pomegranate peel extract (1% w/v) recorded reduced losses in ascorbic acid (29%), total phenolics (8%), total flavonoids (12%), and antioxidant activity measured by DPPH (12%) and FRAP (9%) in coated guavas (cv Allahabad safeda) for 20 days at 10 °C [135]. Different chitosan-based coatings with bioactive properties applied to fruits are presented in Table 2 and Table 3.
4.3. Pectin-Based Active Coatings
Pectin is a complex network-forming biopolymer consisting of high molecular weight glycanogalacturonans in which 1,4-linked α-D-galacturonic acid molecules are linked to a small number of rhamnose and arabinose residues in the main chain and galactose and xylose in the side chains. Pectin is extracted from fruit peels and apples and is widely used as fruit coating material alone or in combination with other polysaccharides and EOs or plant extracts [136]. Apple pectin, cellulose nanocrystals, and lemongrass EO were documented to minimize weight loss and physiological and chemical attributes in coated strawberries (Fragaria Ananassa) [123]. Pectin coatings enriched with citral and eugenol EOs reduced microbial spoilage and maintained sensory attributes of raspberries [137]. Pectin enriched with lemon EO reduced loss of weight and retained higher antioxidant activity of strawberry fruit. Oregano (Lippia graveolens) EO added with pectin delayed the growth of A. alternata under in vitro conditions with an increase in total phenols and antioxidant activity in coated tomatoes [138]. Pectin-based coating incorporated with EO extracted from orange peel showed higher antibacterial and antifungal properties, reduced weight loss, and maintained TSS and ascorbic acid levels in coated oranges [139]. Pectin coating effectively delayed respiration and ripening processes, reduced weight loss, and restricted color change in coated lime (Citrus aurantifolium) [140]. Pectin-coated sapota fruits also recorded minimum weight loss and maintained acidity, TSS, pH, color, ascorbic acid content, and firmness up to 11 days of postharvest storage at room temperature [141].
4.4. Alginate-Based Active Coatings
Alginate is a natural polysaccharide commonly obtained from algae and consists of unbranched, linear binary copolymers of β-d-mannuronic acid and α-l-guluronic acid residues linked by 1–4 glycosidic bonds [142]. Alginate combined with citral and eugenol EOs revealed lower microbial and higher sensory acceptability in coated raspberries [143]. Shirazi thyme EO incorporated into alginate increased phenolic content and antioxidant activity and reduced mold and yeast growth in fresh pistachio (Pistacia vera L.) [144]. Alginate mixed with thyme, cinnamon, and oregano EOs in which thyme EO with alginate effectively inhibited the microbial growth, respiration rate, weight loss, firmness, and browning of fresh cut ‘Red Fuji’ apples [145]. Lemon (Citrus lemon L.), orange (Citrus sinensis L.), and grapefruit (Citrus paradisi L.) coated with sodium alginate edible coating lowered rates of O2 consumption and CO2 production and yeast and mold counts. Lemon and orange EOs improved firmness and ascorbic acid content during storage of kiwifruit [146]. Ficus hirta fruit extract with alginate coating retarded the growth of blue mold increased antioxidant content, and activity of defense enzymes in Nanfeng mandarin [147]. Alginate coating incorporated with cinnamon EO effectively reduced the rate of respiration and weight loss, retained original color, increased lightness, and inhibited polyphenoloxidase and peroxidase activity in fresh-cut apple cv Golden Delicious [148]. Rhubarb extract with alginate inhibited Penicillium expansum and preserved the physiological and sensory attributes in coated peaches (Prunus persica) [149]. Sodium alginate with cinnamon EO (0.9%, v/v) inhibited the growth of A. carbonarius on coated sliced apples and pears [150]. Different alginate-based coatings with bioactive properties applied on fruits are presented in Table 2 and Table 3.
4.5. Starch-Based Active Coatings
Starch is the main component of plant crops such as maize, wheat, edible cassava, potato, amaranth, and quinoa mainly constituted of linear amylose and branched amylopectin fractions amounting to 98–99% of the dry weight [151]. The linear structure of amylose tends to orient itself in a parallel direction to facilitate the hydrogen bonding between hydroxyl groups that increases hydrophobicity in coating films [152]. Starches with higher amylose content have better film-forming properties, i.e., better mechanical strength, elongation, and gas barrier properties [153]. To produce starch-based coatings with a higher amylose content, it can be extracted via selective leaching of starch in hot water (50–70 °C) [154]. Different starches from pea (61–88%), corn (50–85%), potato (21–30%), and tapioca (17%) have been reported with higher amylose content for functionality, barrier, mechanical, and sorption properties of the starch-based coatings [10]. During the retrogradation of starch, the dissociated amylose and amylopectin chains in a gelatinized starch dispersion reunite to form more ordered structures that affect the permeability, solubility, and mechanical properties of starch coating films [155,156]. Additionally, starch-based edible coatings are odorless, tasteless, colorless, non-toxic, act as a good barrier to gases (carbon dioxide, oxygen), and show adequate durability and cohesive strength in coated foods [157]. Rice starch coated on apple (Malus L.) retained color, firmness, total soluble solids, titratable acidity, antioxidant activity, and reduced weight loss, respiration rate, and fruit greasiness [155]. Corn starch with Moringa oleifera extract decreased weight loss and retained firmness and ascorbic acid content in orange (Citrus sinensis L. Osbeck) [158]. The various starch-based coatings incorporated with EOs and plant extracts on the quality of fresh fruits during storage are presented in Table 1, Table 2 and Table 3.
Polysaccharide-based edible coating films added with bioactive compounds from plants have been documented to show excellent barrier, optical, and mechanical properties that play an important role in the postharvest shelf-life of fruits. Barrier properties of polysaccharide coating films include water vapor transmission rate (WVTR) and oxygen or carbon dioxide gas transmission rate (GTR). Chitosan films containing essential oils or other plant extracts addition of carvacrol (0.5, 1.0, and 1.5% v/v) significantly decreased the WVTR of chitosan film [159]. Several reports of decreased WVTR using EOs and plant extracts such as tea tree essential oil, carvacrol, cinnamon essential oil, and turmeric EO were attained in chitosan coating films, possibly due to the hydrophobicity of the EO particles and their ability to occupy the amorphous regions of the films [160,161,162,163]. A gellan gum-chitosan multilayer coating film incorporated with thyme essential oil (TEO) nano-emulsion showed improved elongation at break (EB) and UV blocking ability and increased the water vapor permeability (WVP) of the films with the addition of TEO [163]. The incorporation of turmeric essential oil in chitosan film notably inhibited Aspergillus flavus and prevented biosynthesis of aflatoxin [159]. Generally, the chitosan network interacts with essential oil components via hydrogen and covalent bonds, limiting the accessibility of hydrogen groups in forming hydrophilic bonds with water, which leads to a consequent reduction in affinity of chitosan film to water. The color and opacity of the coating films are important indices regarding the appearance and consumer acceptability of the coated fruits. The opacity of films has also been of interest, as an increase in opacity can be positively related to an improved light barrier property. In addition, the incorporation of rosemary essential oil reduced the light transmission in UV light of the chitosan films by more than 25% [164]. The introduction of thyme essential oil nano-emulsion obviously enhanced the UV blocking property and the yellowness index of chitosan films [165].
Mechanical properties of chitosan coating films have been directly related to the type of essential oil contained in the chitosan matrix. The Young’s modulus, strength, and maximum elongation of chitosan increased with higher olive oil concentrations (5, 10, and 15%, w/w) [166]. The tensile strength (TS) of chitosan composite film significantly increased with the incorporation of cinnamon essential oil (CEO) at levels ranging from 0.4%, to 2% (v/v). CEO generated a strong cross-linked effect with chitosan, which reduced the free volume and the molecular mobility of the polymer that forms a compact sheet-like structure resulting in increased TS and decreased elongation in break (EB) [167]. Additionally, intermolecular interaction and molecular compatibility between the functional group of citronella essential oil and cedarwood oil ingredients and hydroxyl and amino groups in the CH matrix could influence the mechanical properties of the films [163]. Therefore, organic compounds in essential oils consist primarily of hydrocarbon molecules such as alcohols, esters, terpenes, ketones, and phenols are categorized as benzene derivatives and terpenes [168]. The most common functional group in essential oils is aromatic that can interact with polysaccharides to exhibit efficient mechanical properties [169].
5. Conclusions
BECs fortified with EOs and plant extracts as active coating materials could extend the postharvest shelf life of coated fruits to achieve longer storage periods. This review compiled data from recent studies on active edible coatings in which the dipping method was the most reliable on both rough and smooth fruit surfaces compared to other coating methods. The dipping method is an inexpensive manual method and was recommended for small-scale or batch processes in industries for coating of fruits. Polysaccharides like alginate, pectin, CMC, and chitosan added with EOs and plant extracts have been employed over the past decade in fruits and have shown promising results related to the preservation of quality attributes such as firmness, weight loss, delayed ripening, and retardation of the biochemical and microbial changes in coated fruits. EOs and plant extracts containing bioactive compounds are safe additives compared to chemicals additives to be incorporated in BECs. Therefore, this review concludes that polysaccharides fortified with bioactive compounds from plant sources could be a potential means to extend shelf life of fresh fruits during postharvest storage.
Author Contributions
Conceptualization, K.A.S. and W.T.; methodology, K.A.S. and W.T.; software, K.A.S.; validation, W.T.; formal analysis, K.A.S. and W.T.; investigation, K.A.S. and W.T.; resources, W.T.; data curation, K.A.S. and K.N.; writing—original draft preparation, K.A.S.; writing review and editing, K.A.S., K.N., and W.T.; visualization, K.A.S. and W.T.; supervision, W.T.; project administration, W.T.; funding acquisition, W.T. All authors have read and agreed to the published version of the manuscript.
Funding
This reviewed research was funded under the program of postdoctoral fellowship grant (09/2021) awarded to Khursheed Ahmad Shiekh and the research financial supports to Wirongrong Tongdeesoontorn by Mae Fah Luang University, Chiang Rai, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Mae Fah Luang University for facilities and financial supports. The authors are also thankful for the technical and financial support of postdoctoral fellowship grant (09/2021) awarded to Khursheed Ahmad Shiekh by Mae Fah Luang University, Chiang Rai, Thailand. Authors are grateful to Gordon L. Robertson from School of Agriculture and Food Sciences, University of Queensland, Brisbane, Australia, for his noteworthy suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiang, H.; Zhang, W.; Li, X.; Xu, Y.; Cao, J.; Jiang, W. The anti-obesogenic effects of dietary berry fruits: A review. Food Res. Int. 2021, 147, 110539. [Google Scholar] [CrossRef]
- Chen, Y.; Awasthi, A.K.; Wei, F.; Tan, Q.; Li, J. Single-use plastics: Production, usage, disposal, and adverse impacts. Sci. Total Environ. 2021, 752, 141772. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to fuel: A review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Marsh, K.; Bugusu, B. Food packaging—roles, materials, and invironmental issues. J. Food Sci. 2007, 72, R39–R55. [Google Scholar] [CrossRef]
- Sangroniz, A.; Zhu, J.-B.; Tang, X.; Etxeberria, A.; Chen, E.Y.X.; Sardon, H. Packaging materials with desired mechanical and barrier properties and full chemical recyclability. Nat. Commun. 2019, 10, 3559. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Palou, L.; Valencia-Chamorro, S.A.; Pérez-Gago, M.B. Antifungal edible coatings for fresh citrus fruit: A review. Coatings 2015, 5, 962–986. [Google Scholar] [CrossRef]
- Iordachescu, G. Postharvest losses in transportation and storage for fresh fruits and vegetables sector. J. Int. Sci. Publ. 2019, 7, 244–251. [Google Scholar]
- Bayer, I.S. Superhydrophobic Coatings from Ecofriendly Materials and Processes: A Review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as edible films and coatings: Characteristics and influence on fruit and vegetable quality—A review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Pinto, L.; Bonifacio, M.A.; De Giglio, E.; Santovito, E.; Cometa, S.; Bevilacqua, A.; Baruzzi, F. Biopolymer hybrid materials: Development, characterization, and food packaging applications. Food Packag. Shelf Life 2021, 28, 100676. [Google Scholar] [CrossRef]
- Anugrah, D.S.; Alexander, H.; Pramitasari, R.; Hudiyanti, D.; Sagita, C.P. A review of polysaccharide-zinc oxide nanocomposites as safe coating for fruits preservation. Coatings 2020, 10, 988. [Google Scholar] [CrossRef]
- Galus, S.; Arik Kibar, E.A.; Gniewosz, M.; Kraśniewska, K. Novel materials in the preparation of edible films and coatings—A review. Coatings 2020, 10, 674. [Google Scholar] [CrossRef]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A review of crustacean and fungal chitin in wound treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Tahir, H.E.; Xiaobo, Z.; Mahunu, G.K.; Arslan, M.; Abdalhai, M.; Zhihua, L. Recent developments in gum edible coating applications for fruits and vegetables preservation: A review. Carbohydr. Polym. 2019, 224, 115141. [Google Scholar] [CrossRef]
- Anis, A.; Pal, K.; Al-Zahrani, S.M. Essential oil-containing polysaccharide-based edible films and coatings for food security applications. Polymers 2021, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Abifarin, T.O.; Otunola, G.A.; Afolayan, A.J. Chemical composition of essential oils obtained from Heteromorpha arborescens (Spreng.) cham. and schltdl leaves using two extraction methods. Sci. World J. 2020, 2020, 9232810. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2020, 343, 128403. [Google Scholar] [CrossRef]
- Botelho, L.N.S.; Rocha, D.A.; Braga, M.A.; Silva, A.; de Abreu, C.M.P. Quality of guava cv. ‘Pedro Sato’ treated with cassava starch and cinnamon essential oil. Sci. Hortic. 2016, 209, 214–220. [Google Scholar] [CrossRef]
- Jianglian, D.; Shaoying, Z. Application of chitosan based coating in fruit and vegetable preservation: A review. J. Food Process. Technol. 2013, 4, 227. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils-present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Ahmad Shiekh, K.; Odunayo Olatunde, O.; Zhang, B.; Huda, N.; Benjakul, S. Pulsed electric field assisted process for extraction of bioactive compounds from custard apple (Annona squamosa) leaves. Food Chem. 2021, 359, 129976. [Google Scholar] [CrossRef]
- Zhang, H.; Birch, E.; Pei, J.; Ma, Z.F.; Bekhit, A. Phytochemical compounds and biological activity in Asparagus roots: A review. Int. J. Food Sci. Technol. 2018, 54, 966–977. [Google Scholar] [CrossRef]
- Paolucci, M.; Di Stasio, M.; Sorrentino, A.; La Cara, F.; Volpe, M.G. Active edible polysaccharide-based coating for preservation of fresh figs (Ficus carica L.). Foods 2020, 9, 1793. [Google Scholar] [CrossRef]
- Ramos, M.; Mellinas, C.; Solaberrieta, I.; Garrigós, M.C.; Jiménez, A. Emulsions Incorporated in polysaccharide-based active coatings for fresh and minimally processed vegetables. Foods 2021, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.D.; Gago, C.M.; Cavaco, A.M.; Miguel, M.G. Edible coatings enriched with essential oils and their compounds for fresh and fresh-cut fruit. Recent Pat. Food Nutr. Agric. 2012, 4, 114–122. [Google Scholar] [CrossRef]
- Ziv, C.; Fallik, E. Postharvest Storage Techniques and Quality Evaluation of Fruits and Vegetables for Reducing Food Loss. Agronomy 2021, 11, 1133. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y. Towards a new definition of quality for fresh fruits and vegetables. Sci. Hortic. 2018, 234, 463–469. [Google Scholar] [CrossRef]
- Wenneker, M.; Thomma, B.P.H.J. Latent postharvest pathogens of pome fruit and their management: From single measures to a systems intervention approach. Eur. J. Plant Pathol. 2020, 156, 663–681. [Google Scholar] [CrossRef]
- Machado-Moreira, B.; Richards, K.; Brennan, F.; Abram, F.; Burgess, C.M. Microbial contamination of fresh produce: What, where, and how? Compr. Rev. Food Sci. Food Saf. 2019, 18, 1727–1750. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, A.L.Q. Influence of gamma radiation treatment on the profile of phenolic compounds and on the quality parameters of strawberries cv. Albion during storage. Res. Soc. Dev. 2020, 9, e991975147. [Google Scholar] [CrossRef]
- Mostafidi, M.; Sanjabi, M.R.; Shirkhan, F.; Zahedi, M.T. A review of recent trends in the development of the microbial safety of fruits and vegetables. Trends Food Sci. Technol. 2020, 103, 321–332. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Drosinos, E.H.; Skandamis, P.N. Quantitative Microbiology in Food Processing; Microbial Ecology of Fruits and Fruit-Based Products; John Wiley & Sons, Ltd.: Chichester, UK; Hoboken, NJ, USA, 2017; pp. 358–381. [Google Scholar]
- Serradilla, M.J.; Villalobos, M.D.C.; Hernández, A.; Martín, A.; Lozano, M.; Córdoba, M.D.G. Study of microbiological quality of controlled atmosphere packaged ‘Ambrunés’ sweet cherries and subsequent shelf-life. Int. J. Food Microbiol. 2013, 166, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Putnik, P.; Roohinejad, S.; Greiner, R.; Granato, D.; Bekhit, A.E.-D.A.; Bursać Kovačević, D. Prediction and modeling of microbial growth in minimally processed fresh-cut apples packaged in a modified atmosphere: A review. Food Control 2017, 80, 411–419. [Google Scholar] [CrossRef]
- Luesuwan, S.; Naradisorn, M.; Shiekh, K.A.; Rachtanapun, P.; Tongdeesoontorn, W. Effect of active packaging material fortified with clove essential oil on fungal growth and post-harvest quality changes in table grape during cold storage. Polymers 2021, 13, 3445. [Google Scholar] [CrossRef]
- Brizzolara, S.; Manganaris, G.A.; Fotopoulos, V.; Watkins, C.B.; Tonutti, P. Primary metabolism in fresh fruits during storage. Front. Plant Sci. 2020, 11, 80. [Google Scholar] [CrossRef]
- Yun, Z.; Jin, S.; Ding, Y.; Wang, Z.; Gao, H.; Pan, Z.; Xu, J.; Cheng, Y.; Deng, X. Comparative transcriptomics and proteomics analysis of citrus fruit, to improve understanding of the effect of low temperature on maintaining fruit quality during lengthy post-harvest storage. J. Exp. Bot. 2012, 63, 2873–2893. [Google Scholar] [CrossRef]
- Lin, S.; Wu, T.; Lin, H.; Zhang, Y.; Xu, S.; Wang, J.; Wu, B.; Chen, Y.; Lin, S.; Lin, D.; et al. De Novo analysis reveals transcriptomic responses in Eriobotrya japonica fruits during postharvest cold storage. Genes 2018, 9, 639. [Google Scholar] [CrossRef]
- Zhao, H.; Jiao, W.; Cui, K.; Fan, X.; Shu, C.; Zhang, W.; Cao, J.; Jiang, W. Near-freezing temperature storage enhances chilling tolerance in nectarine fruit through its regulation of soluble sugars and energy metabolism. Food Chem. 2019, 289, 426–435. [Google Scholar] [CrossRef]
- Bustamante, C.A.; Brotman, Y.; Monti, L.L.; Gabilondo, J.; Budde, C.O.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Differential lipidome remodeling during postharvest of peach varieties with different susceptibility to chilling injury. Physiol. Plant. 2018, 163, 2–17. [Google Scholar] [CrossRef]
- Zhou, Y.; Pan, X.; Qu, H.; Underhill, S.J. Low temperature alters plasma membrane lipid composition and ATPase activity of pineapple fruit during blackheart development. J. Bioenerg. Biomembr. 2014, 46, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Sheng, L.; Zhou, X.; Liu, Z.-Y.; Wang, J.-W.; Wang, L.; Zhang, Q.; Ji, S.-J. Changed activities of enzymes crucial to membrane lipid metabolism accompany pericarp browning in ‘Nanguo’ pears during refrigeration and subsequent shelf life at room temperature. Postharvest Biol. Technol. 2016, 117, 1–8. [Google Scholar] [CrossRef]
- Shi, F.; Zhou, X.; Tan, Z.; Yao, M.-M.; Wei, B.-D.; Ji, S.-J. Membrane lipid metabolism changes and aroma ester loss in low-temperature stored Nanguo pears. Food Chem. 2017, 245, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Leisso, R.S.; Buchanan, D.A.; Lee, J.; Mattheis, J.P.; Sater, C.; Hanrahan, I.; Watkins, C.B.; Gapper, N.; Johnston, J.W.; Schaffer, R.J.; et al. Chilling-related cell damage of apple (Malus domestica Borkh.) fruit cortical tissue impacts antioxidant, lipid and phenolic metabolism. Physiol. Plant. 2015, 153, 204–220. [Google Scholar] [CrossRef]
- Gonzalez, C.; Zanor, M.; Ré, M.; Otaiza, S.; Asis, R.; Valle, E.; Boggio, S. Chilling tolerance of Micro-Tom fruit involves changes in the primary metabolite levels and in the stress response. Postharvest Biol. Technol. 2019, 148, 58–67. [Google Scholar] [CrossRef]
- Salzano, A.M.; Renzone, G.; Sobolev, A.P.; Carbone, V.; Petriccione, M.; Capitani, D.; Vitale, M.; Novi, G.; Zambrano, N.; Pasquariello, M.S.; et al. Unveiling kiwifruit metabolite and protein changes in the course of postharvest cold storage. Front. Plant Sci. 2019, 10, 71. [Google Scholar] [CrossRef]
- Pathare, P.B.; Al-Dairi, M. Bruise damage and quality changes in impact-bruised, stored tomatoes. Horticulturae 2021, 7, 113. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, H.; Zhang, J.; Sheng, Z.; Cao, J.; Jiang, W. Different molecular weights chitosan coatings delay the senescence of postharvest nectarine fruit in relation to changes of redox state and respiratory pathway metabolism. Food Chem. 2019, 289, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Amwoka, E.M.; Ambuko, J.L.; Jesang’, H.M.; Owino, W.O. Effectiveness of selected cold chain management practices to extend shelf life of mango fruit. Adv. Agric. 2021, 2021, 8859144. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Brummell, D.A.; Atkinson, R.G.; Burdon, J.N.; Patterson, K.J.; Schaffer, R.J. Fruit growth, ripening and postharvest physiology. In Plant in Action; Plant and Food Research: Palmerston North, New Zealand; Auckland, New Zealand, 2016. [Google Scholar]
- Iñiguez-Moreno, M.; Ragazzo-Sánchez, J.A.; Calderón-Santoyo, M. An extensive review of natural polymers used as coatings for postharvest shelf-life extension: Trends and challenges. Polymers 2021, 13, 3271. [Google Scholar] [CrossRef]
- Darmawati, E.; Nava, N.; Suyatma, N. Aloe vera as a coating material for tropical fruits using spray method. IOP Conf. Ser. Earth Environ. Sci. 2019, 309, 012011. [Google Scholar] [CrossRef]
- Andrade, R.; Skurtys, O.; Osorio, F. Atomizing spray systems for application of edible coatings. Compr. Rev. Food Sci. Food Saf. 2012, 11, 323–337. [Google Scholar] [CrossRef]
- Pirozzi, A.; Ferrari, G.; Donsì, F. The use of nanocellulose in edible coatings for the preservation of perishable fruits and vegetables. Coatings 2021, 11, 990. [Google Scholar] [CrossRef]
- Peretto, G.; Du, W.-X.; Avena-Bustillos, R.; Berrios, J.; Sambo, P.; McHugh, T. Electrostatic and conventional spraying of alginate-based edible coating with natural antimicrobials for preserving fresh strawberry quality. Food Bioprocess Technol. 2017, 10, 165–174. [Google Scholar] [CrossRef]
- Cakmak, H.; Kumcuoglu, S.; Tavman, S. Electrospray coating of minimally processed strawberries and evaluation of the shelf-life quality properties. J. Food Process Eng. 2019, 42, e13082. [Google Scholar] [CrossRef]
- Khan, M.; Schutyser, M.; Schroën, K.; Boom, R. Barrier properties and storage stability of edible coatings prepared with electrospraying. Innov. Food Sci. Emerg. Technol. 2014, 23, 182–187. [Google Scholar] [CrossRef]
- Lu, H.; Li, S.; Du, H.; Lu, Y.; Huang, X. Secondary breakup characteristics and mechanism of single electrified al/n-decane nanofluid fuel droplet in electrostatic field. Appl. Sci. 2020, 10, 5332. [Google Scholar] [CrossRef]
- Mahalakshmi, L.; Leena, M.M.; Moses, J.A.; Anandharamakrishnan, C. Micro- and nano-encapsulation of β-carotene in zein protein: Size-dependent release and absorption behavior. Food Funct. 2020, 11, 1647–1660. [Google Scholar] [CrossRef]
- Dhar, P.; Kumar, A.; Katiyar, V. Magnetic cellulose nanocrystal based anisotropic polylactic acid nanocomposite films: Influence on electrical, magnetic, thermal, and mechanical properties. ACS Appl. Mater. Interfaces 2016, 8, 18393–18409. [Google Scholar] [CrossRef] [PubMed]
- Mannozzi, C.; Glicerina, V.; Tylewicz, U.; Castagnini, J.M.; Canali, G.; Dalla Rosa, M.; Romani, S. Influence of two different coating application methods on the maintenance of the nutritional quality of fresh-cut melon during storage. Appl. Sci. 2021, 11, 8510. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Lindner, M.; Rothkopf, I.; Schmid, M.; Müller, K. The development of a uniform alginate-based coating for cantaloupe and strawberries and the characterization of water barrier properties. Foods 2019, 8, 203. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yan, X. Dip-coating for fibrous materials: Mechanism, methods and applications. J. Sol-Gel Sci. Technol. 2017, 81, 378–404. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Nassef, A.M.; Al-Dhaifallah, M.; Abdelkareem, M.A.; Rezk, H. The effect of a new coating on the drying performance of fruit and vegetables products: Experimental investigation and artificial neural network modeling. Foods 2020, 9, 308. [Google Scholar] [CrossRef]
- Cisneros-Zevallos, L.; Krochta, J.M. Dependence of coating thickness on viscosity of coating solution applied to fruits and vegetables by dipping method. J. Food Sci. 2003, 68, 503–510. [Google Scholar] [CrossRef]
- Atieno, L.; Owino, W.; Ateka, E.M.; Ambuko, J. Influence of coating application methods on the postharvest quality of cassava. Int. J. Food Sci. 2019, 2019, 2148914. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.G.; Lasekan, O.; Saari, N.; Khairunniza-Bejo, S. The effect of the application of edible coatings on or before ultraviolet treatment on postharvested longan fruits. J. Food Qual. 2017, 2017, 5454263. [Google Scholar] [CrossRef]
- Pirozzi, A.; Pataro, G.; Donsì, F.; Ferrari, G. Edible coating and pulsed light to increase the shelf life of food products. Food Eng. Rev. 2021, 13, 544–569. [Google Scholar] [CrossRef]
- Vaishali; Sharma, H.; Shami, V.; Samsher; Chaudhary, V.; Sunil, E.; Kumar, M. Importance of edible coating on fruits and vegetables: A review. J. Pharm. Phytochem. 2019, 8, 4104–4110. [Google Scholar]
- Osorio, F.; Valdés, G.; Skurtys, O.; Andrade, R.; Villalobos-Carvajal, R.; Silva-Weiss, A.; Silva-Vera, W.; Giménez, B.; Zamorano, M.; Lopez, J. Surface free energy utilization to evaluate wettability of hydrocolloid suspension on different vegetable epicarps. Coatings 2018, 8, 16. [Google Scholar] [CrossRef]
- Sapper, M.; Bonet, M.; Chiralt, A. Wettability of starch-gellan coatings on fruits, as affected by the incorporation of essential oil and/or surfactants. LWT Food Sci. Technol. 2019, 116, 108574. [Google Scholar] [CrossRef]
- Poverenov, E.; Danino, S.; Horev, B.; Granit, R.; Vinokur, Y.; Rodov, V. Layer-by-layer electrostatic deposition of edible coating on fresh cut melon model: Anticipated and unexpected effects of alginate–chitosan combination. Food Bioprocess Technol. 2014, 7, 1424–1432. [Google Scholar] [CrossRef]
- McShane, M.; Lvov, Y. Electrostatic Self-Assembly: Layer-by-Layer. In Dekker Encyclopedia of Nanoscience and Nanotechnology, 3rd ed.; Schwarz, J.A., Lyshevski, S.E., Contescu, C.I., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2014; pp. 1342–1358. [Google Scholar]
- Adiletta, G.; Di Matteo, M.; Petriccione, M. Multifunctional Role of Chitosan Edible Coatings on Antioxidant Systems in Fruit Crops: A Review. Int. J. Mol. Sci. 2021, 22, 2633. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Zhao, H. Application of pullulan and chitosan multilayer coatings in fresh papayas. Coatings 2019, 9, 745. [Google Scholar] [CrossRef]
- Yan, J.; Luo, Z.; Ban, Z.; Lu, H.; Li, D.; Yang, D.; Soleimani Aghdam, M.; Li, L. The effect of the layer-by-layer (LBL) edible coating on strawberry quality and metabolites during storage. Postharvest Biol. Technol. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Menezes, J.; Athmaselvi, K. Polysaccharide based edible coating on sapota fruit. Int. Agrophys. 2016, 30, 551–557. [Google Scholar] [CrossRef]
- Li, L.; Sun, J.; Gao, H.; Shen, Y.; Li, C.; Yi, P.; He, X.; Ling, D.; Sheng, J.; Li, J.; et al. Effects of polysaccharide-based edible coatings on quality and antioxidant enzyme system of strawberry during cold storage. Int. J. Polym. Sci. 2017, 2017, 9746174. [Google Scholar] [CrossRef]
- Shao, X.; Cao, B.; Xu, F.; Xie, S.; Yu, D.; Wang, H. Effect of postharvest application of chitosan combined with clove oil against citrus green mold. Postharvest Biol. Technol. 2015, 99, 37–43. [Google Scholar] [CrossRef]
- Arnon, H.; Granit, R.; Porat, R.; Poverenov, E. Development of polysaccharides-based edible coatings for citrus fruits: A layer-by-layer approach. Food Chem. 2015, 166, 465–472. [Google Scholar] [CrossRef]
- Panahirad, S.; Naghshiband-Hassani, R.; Bergin, S.; Katam, R.; Mahna, N. Improvement of postharvest quality of plum (prunus domestica l.) Using polysaccharide-based edible coatings. Plants 2020, 9, 1148. [Google Scholar] [CrossRef] [PubMed]
- Gol, N.B.; Vyas, P.B.; Ramana Rao, T.V. Evaluation of polysaccharide-based edible coatings for their ability to preserve the postharvest quality of indian blackberry (Syzygium cumini L.). Int. J. Fruit Sci. 2015, 15, 198–222. [Google Scholar] [CrossRef]
- Salinas-Roca, B.; Guerreiro, A.; Welti-Chanes, J.; Antunes, M.D.C.; Martín-Belloso, O. Improving quality of fresh-cut mango using polysaccharide-based edible coatings. Int. J. Food Sci. Technol. 2018, 53, 938–945. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Li, C.; Tao, J.; Zhang, H. Peach gum polysaccharides-based edible coatings extend shelf life of cherry tomatoes. 3 Biotech 2017, 7, 168. [Google Scholar] [CrossRef] [PubMed]
- Krishna, G.V.S.R. Evaluation of pullulan-based edible active coating methods on Rastali and Chakkarakeli bananas and their shelf-life extension parameters studies. J. Food Process. Preserv. 2020, 44, e14378. [Google Scholar] [CrossRef]
- Inthamat, P.; Hamauzu, Y.; Tongdeesoontorn, W. Storage life extension of Japanese cucumber fruit using edible coatings from lemon basil seed and Chinese quince seed mucilage. Agric. Sci. J. 2016, 47, 381–384. [Google Scholar]
- Pangesti, A.; Tongdeesoontorn, W.; Syarief, R. Application of Carboxymethyl Cellulose (CMC) from Pineapple Core as Edible Coating for Cherry Tomatoes During Storage. In Proceedings of the 22nd Tri-University International Joint Seminar and Symposium at Jiangsu University, Zhenjiang, China, 19–22 October 2015. [Google Scholar]
- Thakur, R.; Pristijono, P.; Bowyer, M.; Singh, S.P.; Scarlett, C.J.; Stathopoulos, C.E.; Vuong, Q.V. A starch edible surface coating delays banana fruit ripening. LWT Food Sci. Technol. 2019, 100, 341–347. [Google Scholar] [CrossRef]
- Chiumarelli, M.; Pereira, L.M.; Ferrari, C.C.; Sarantópoulos, C.I.G.L.; Hubinger, M.D. Cassava starch coating and citric acid to preserve quality parameters of fresh-cut “Tommy Atkins” mango. J. Food Sci. 2010, 75, E297–E304. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, G.; Dantas, R.; Sousa, A.; Soares, L.; Raylson, D.; Rosana, S.; Lima, R.; Rejane, M.; Beaudry, R.; Silva, S. Impact of cassava starch-alginate based coatings added with ascorbic acid and elicitor on quality and sensory attributes during pineapple storage. Afr. J. Agric. Res. 2017, 12, 664–673. [Google Scholar] [CrossRef][Green Version]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The use of polysaccharide-based edible coatings enriched with essential oils to improve shelf-life of strawberries. Postharvest Biol. Technol. 2015, 110, 51–60. [Google Scholar] [CrossRef]
- Murmu, S.; Mishra, H.N. The effect of edible coating based on arabic gum, sodium caseinate and essential oil of cinnamon and lemon grass on guava. Food Chem. 2017, 245, 820–828. [Google Scholar] [CrossRef]
- Hashemi, M.; Dastjerdi, A.M.; Mirdehghan, S.H.; Shakerardekani, A.; Golding, J.B. Incorporation of Zataria multiflora Boiss essential oil into gum Arabic edible coating to maintain the quality properties of fresh in-hull pistachio (Pistacia vera L.). Food Packag. Shelf Life 2021, 30, 100724. [Google Scholar] [CrossRef]
- Aboryia, M.; Omar, A. Effectiveness of some edible coatings on storage ability of Zaghloul date palm fruits. J. Plant Prod. 2020, 11, 1477–1485. [Google Scholar] [CrossRef]
- Etemadipoor, R.; Mirzaalian Dastjerdi, A.; Ramezanian, A.; Ehteshami, S. Ameliorative effect of gum arabic, oleic acid and/or cinnamon essential oil on chilling injury and quality loss of guava fruit. Sci. Hortic. 2020, 266, 109255. [Google Scholar] [CrossRef]
- Rastegar, S.; Atrash, S. Effect of alginate coating incorporated with Spirulina, Aloe vera and guar gum on physicochemical, respiration rate and color changes of mango fruits during cold storage. J. Food Meas. Charact. 2020, 15, 265–275. [Google Scholar] [CrossRef]
- Etemadipoor, R.; Ramezanian, A.; Dastjerdi, A.; Shamili, M. The potential of gum arabic enriched with cinnamon essential oil for improving the qualitative characteristics and storability of guava (Psidium guajava L.) fruit. Sci. Hortic. 2019, 51, 101–107. [Google Scholar] [CrossRef]
- Andrade, S.C.A.; Baretto, T.A.; Arcanjo, N.M.O.; Madruga, M.S.; Meireles, B.; Cordeiro, Â.M.T.; Barbosa de Lima, M.A.; de Souza, E.L.; Magnani, M. Control of Rhizopus soft rot and quality responses in plums (Prunus domestica L.) coated with gum arabic, oregano and rosemary essential oils. J. Food Process. Preserv. 2017, 41, e13251. [Google Scholar] [CrossRef]
- Oriani, V.B.; Molina, G.; Chiumarelli, M.; Pastore, G.M.; Hubinger, M.D. Properties of cassava starch-based edible coating containing essential oils. J. Food Sci. 2014, 79, E189–E194. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, A.B.; Blank, A.F.; de Aquino Santana, L.C.L. Impact of edible chitosan–cassava starch coatings enriched with Lippia gracilis Schauer genotype mixtures on the shelf life of guavas (Psidium guajava L.) during storage at room temperature. Food Chem. 2015, 171, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.C.M.; Molina, G.; Pelissari, F.M. Effect of edible coating from cassava starch and babassu flour (Orbignya phalerata) on Brazilian cerrado fruits quality. Food Bioprocess Technol. 2020, 13, 172–179. [Google Scholar] [CrossRef]
- Yang, Z.; Xiaobo, Z.; Zhihua, L.; Xiaowei, H.; Xiaodong, Z.; Zhang, W.; Shi, J.; Tahir, H.E. Improved postharvest quality of cold stored blueberry by edible coating based on composite gum arabic/roselle extract. Food Bioprocess Technol. 2019, 12, 1537–1547. [Google Scholar] [CrossRef]
- Moreira, M.R.; Cassani, L.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of polysaccharide-based edible coatings enriched with dietary fiber on quality attributes of fresh-cut apples. J. Food Sci. Technol. 2015, 52, 7795–7805. [Google Scholar] [CrossRef] [PubMed]
- Nair, M.S.; Saxena, A.; Kaur, C. Characterization and antifungal activity of pomegranate peel extract and its use in polysaccharide-based edible coatings to extend the shelf-life of capsicum (Capsicum annuum L.). Food Bioprocess Technol. 2018, 11, 1317–1327. [Google Scholar] [CrossRef]
- Sabaghi, M.; Maghsoudlou, Y.; Khomeiri, M.; Ziaiifar, A. Active edible coating from chitosan incorporating green tea extract as an antioxidant and antifungal on fresh walnut kernel. Postharvest Biol. Technol. 2015, 110, 224–228. [Google Scholar] [CrossRef]
- Alvarez, M.V.; Ponce, A.G.; Moreira, M.R. Influence of polysaccharide-based edible coatings as carriers of prebiotic fibers on quality attributes of ready-to-eat fresh blueberries. J. Sci. Food Agric. 2018, 98, 2587–2597. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Deng, J.; Wang, F.; Liu, Y.; Jiao, J.; Wang, L.; Zhang, J. Individual and combined effects of bamboo vinegar and peach gum on postharvest grey mould caused by Botrytis cinerea in blueberry. Postharvest Biol. Technol. 2019, 155, 86–93. [Google Scholar] [CrossRef]
- Moreno, M.; Bojorges, H.; Falcó, I.; Sanchez, G.; López-Carballo, G.; López-Rubio, A.; Zampini, C.; Isla, M.; Fabra, M. Active properties of edible marine polysaccharide-based coatings containing Larrea nitida polyphenols enriched extract. Food Hydrocoll. 2019, 102, 105595. [Google Scholar] [CrossRef]
- Mooksupang Liangpanth, W.T. Application of active edible coating from chitosan incorporated with Cashew (Anacardium occidentale) leaf extracts for extending shelf life of lime fruits. J. Food Sci. Agric. Technol. 2019, 5, 30–40. [Google Scholar]
- Araújo, J.M.S.; de Siqueira, A.C.P.; Blank, A.F.; Narain, N.; de Aquino Santana, L.C.L. A cassava starch–chitosan edible coating enriched with Lippia sidoides Cham. essential oil and pomegranate peel extract for preservation of Italian tomatoes (Lycopersicon esculentum Mill.) stored at room temperature. Food Bioprocess Technol. 2018, 11, 1750–1760. [Google Scholar] [CrossRef]
- Thomas, A.B.; Nassur, R.D.C.M.R.; Boas, A.C.V.; Lima, L.C.O. Cassava starch edible coating incorporated with propolis on bioactive compounds in strawberries. Cienc. Agrotecnol. 2016, 40, 87–96. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of polysaccharide-based materials as advanced food packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef] [PubMed]
- Adden, R.; Hübner-Keese, B.; Förtsch, S.; Knarr, M. Cellulosics. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2021; Chapter 15; pp. 481–508. [Google Scholar]
- Panahirad, S.; Dadpour, M.; Peighambardoust, S.H.; Soltanzadeh, M.; Gullón, B.; Alirezalu, K.; Lorenzo, J.M. Applications of carboxymethyl cellulose- and pectin-based active edible coatings in preservation of fruits and vegetables: A review. Trends Food Sci. Technol. 2021, 110, 663–673. [Google Scholar] [CrossRef]
- Dong, F.; Wang, X. Effects of carboxymethyl cellulose incorporated with garlic essential oil composite coatings for improving quality of strawberries. Int. J. Biol. Macromol. 2017, 104, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, Y. Application of carboxymethyl cellulose and chitosan coatings containing Mentha spicata essential oil in fresh strawberries. Int. J. Biol. Macromol. 2018, 112, 264–272. [Google Scholar] [CrossRef]
- Raeisi, M.; Tajik, H.; Aliakbarlu, J.; Mirhosseini, H.; Hosseini, S.M.H. Effect of carboxymethyl cellulose-based coatings incorporated with Zataria multiflora Boiss. essential oil and grape seed extract on the shelf life of rainbow trout fillets. Lebensm. Wiss. Technol. 2015, 64, 898–904. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zheng, J.-P.; Wan, C.; Chen, M.; Chen, J.-Y. Effect of carboxymethyl cellulose coating enriched with clove oil on postharvest quality of ‘Xinyu’ Mandarin oranges. Fruits 2016, 71, 319–327. [Google Scholar] [CrossRef]
- Ali, S.; Anjum, M.A.; Ejaz, S.; Hussain, S.; Ercisli, S.; Saleem, M.S.; Sardar, H. Carboxymethyl cellulose coating delays chilling injury development and maintains eating quality of ‘Kinnow’ mandarin fruits during low temperature storage. Int. J. Biol. Macromol. 2021, 168, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.-S.-V.-D.; Prado, N.-S.; Melo, P.-G.-D.; Arantes, D.-C.; Andrade, M.-Z.; Otaguro, H.; Pasquini, D. Edible coatings based on apple pectin, cellulose nanocrystals, and essential oil of lemongrass: Improving the quality and shelf life of strawberries (Fragaria Ananassa). J. Renew. Mater. 2019, 7, 73–87. [Google Scholar] [CrossRef]
- Ghaderi, N.; Shokri, B.; Javadi, T. The effect of carboxymethyl cellulose and pistachio (Pistacia atlantica L.) essential oil coating on fruit quality of cold-stored grape cv. Rasheh. Iran. J. Hortic. Sci. 2017, 48, 63–78. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Zeng, R.; Wan, C.; Chen, M.; Chen, J. Physiological and biochemical responses in cold-stored citrus fruits to carboxymethyl cellulose coating containing ethanol extract of Impatiens balsamina L. stems. J. Food Process. Preserv. 2017, 41, e12999. [Google Scholar] [CrossRef]
- Amiri, S.; Nicknam, Z.; Radi, M.; Sayadi, M.; Bagheri, F.; Karimi Khorrami, N.; Abedi, E. Postharvest quality of orange fruit as influenced by salicylic acid, acetic acid, and carboxymethyl cellulose coating. J. Food Meas. Charact. 2021, 15, 3912–3930. [Google Scholar] [CrossRef]
- Barreto, T.A.; Andrade, S.C.; Maciel, J.F.; Arcanjo, N.M.; Madruga, M.S.; Meireles, B.; Cordeiro, Â.M.; Souza, E.L.; Magnani, M.A. Chitosan coating containing essential oil from Origanum vulgare l. To control postharvest mold infections and keep the quality of cherry tomato fruit. Front. Microbiol. 2016, 7, 1724. [Google Scholar] [CrossRef] [PubMed]
- Saki, M.; ValizadehKaji, B.; Abbasifar, A.; Shahrjerdi, I. Effect of chitosan coating combined with thymol essential oil on physicochemical and qualitative properties of fresh fig (Ficus carica L.) fruit during cold storage. J. Food Meas. Charact. 2019, 13, 1147–1158. [Google Scholar] [CrossRef]
- De Oliveira, K.Á.R.; da Conceição, M.L.; de Oliveira, S.P.A.; Lima, M.D.S.; de Sousa Galvão, M.; Madruga, M.S.; Magnani, M.; de Souza, E.L. Postharvest quality improvements in mango cultivar Tommy Atkins by chitosan coating with Mentha piperita L. essential oil. J. Hortic. Sci. Biotechnol. 2020, 95, 260–272. [Google Scholar] [CrossRef]
- Xing, Y.; Lin, H.; Cao, D.; Han, W.; Wang, R.; Che, Z.; Li, X. Effect of chitosan coating with cinnamon oil on the quality and physiological attributes of China jujube fruits. BioMed Res. Int. 2015, 2015, 835151. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shui, Y.; Li, S.; Xing, Y.; Xu, Q.; Li, X.; Lin, H.; Wang, Q.; Yang, H.; Li, W.; et al. Quality of fresh cut lemon during different temperature as affected by chitosan coating with clove oil. Int. J. Food Prop. 2020, 23, 1214–1230. [Google Scholar] [CrossRef]
- Kumar, N.; Pratibha; Neeraj; Petkoska, A.T.; Al-Hilifi, S.A.; Fawole, O.A. Effect of chitosan–pullulan composite edible coating functionalized with pomegranate peel extract on the shelf life of mango (Mangifera indica). Coatings 2021, 11, 764. [Google Scholar] [CrossRef]
- Khalifa, I.; Barakat, H.; El-Mansy, H.A.; Soliman, S.A. Improving the shelf-life stability of apple and strawberry fruits applying chitosan-incorporated olive oil processing residues coating. Food Packag. Shelf Life 2016, 9, 10–19. [Google Scholar] [CrossRef]
- Chen, C.; Cai, N.; Chen, J.; Peng, X.; Wan, C. Chitosan-based coating enriched with hairy fig (Ficus hirta vahl.) Fruit extract for “Newhall” navel orange preservation. Coatings 2018, 8, 445. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of chitosan and alginate based coatings enriched with pomegranate peel extract to extend the postharvest quality of guava (Psidium guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Burgos, N.; Jimenez, A.; Garrigós, M. Natural pectin polysaccharides as edible coatings. Coatings 2015, 5, 865–886. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. Raspberry fresh fruit quality as affected by pectin- and alginate-based edible coatings enriched with essential oils. Sci. Hortic. 2015, 194, 138–146. [Google Scholar] [CrossRef]
- Abdi, S.; Roein, Z.; Erfanimoghadam, J.; Aziznia, S. Application of pectin coating containing essential oil for increasing quality of strawberry fruit. Chemistry 2017, 5, 83–94. [Google Scholar]
- Radi, M.; Akhavan-Darabi, S.; Akhavan, H.-R.; Amiri, S. The use of orange peel essential oil microemulsion and nanoemulsion in pectin-based coating to extend the shelf life of fresh-cut orange. J. Food Process. Preserv. 2018, 42, e13441. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S. Application and evaluation of a pectin-based edible coating process for quality change kinetics and shelf-life extension of lime fruit (Citrus aurantifolium). Coatings 2019, 9, 285. [Google Scholar] [CrossRef]
- Menezes, J.; Athmaselvi, K.A. Study on effect of pectin based edible coating on the shelf life of sapota fruits. Biosci. Biotechnol. Res. Asia 2016, 13, 1195–1199. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Guerreiro, A.; Gago, C.; Miguel, M.; Faleiro, M.L.; Antunes, M. The influence of edible coatings enriched with citral and eugenol on the raspberry storage ability, nutritional and sensory quality. Food Packag. Shelf Life 2016, 9, 20–28. [Google Scholar] [CrossRef]
- Hashemi, M.; Dastjerdi, A.M.; Shakerardekani, A.; Mirdehghan, S.H. Effect of alginate coating enriched with Shirazi thyme essential oil on quality of the fresh pistachio (Pistacia vera L.). J. Food Sci. Technol. 2021, 58, 34–43. [Google Scholar] [CrossRef]
- Sarengaowa; Hu, W.; Jiang, A.; Xiu, Z.; Feng, K. Effect of thyme oil-alginate-based coating on quality and microbial safety of fresh-cut apples. J. Sci. Food Agric. 2018, 98, 2302–2311. [Google Scholar] [CrossRef] [PubMed]
- Chiabrando, V.; Giacalone, G. Effects of citrus essential oils incorporated in alginate coating on quality of fresh-cut Jintao kiwifruit. J. Food Nutr. Res. 2019, 58, 177–186. [Google Scholar]
- Chen, C.; Peng, X.; Zeng, R.; Chen, M.; Wan, C.; Chen, J. Ficus hirta fruits extract incorporated into an alginate-based edible coating for Nanfeng mandarin preservation. Sci. Hortic. 2016, 202, 41–48. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Effect of essential oils incorporated into an alginate-based edible coating on fresh-cut apple quality during storage. Qual. Assur. Saf. Crops Foods 2014, 1, 251–259. [Google Scholar] [CrossRef]
- Li, X.-Y.; Du, X.-L.; Liu, Y.; Tong, L.-J.; Wang, Q.; Li, J.-L. Rhubarb extract incorporated into an alginate-based edible coating for peach preservation. Sci. Hortic. 2019, 257, 108685. [Google Scholar] [CrossRef]
- Kapetanakou, A.E.; Nestora, S.; Evageliou, V.; Skandamis, P.N. Sodium alginate–cinnamon essential oil coated apples and pears: Variability of Aspergillus carbonarius growth and ochratoxin-A production. Food Res. Int. 2019, 119, 876–885. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Ferreira, D.C.; Louzada, L.B.; dos Santos, F.; Corrêa, A.C.; Moreira, F.K.V.; Mattoso, L.H. Starch-Based Edible Films and Coatings: An Eco-friendly Alternative for Food Packaging. In Starches for Food Application; Academic Press: Cambridge, MA, USA, 2018; pp. 359–420. [Google Scholar]
- Guimarães, I.C.; dos Reis, K.C.; Menezes, E.G.T.; Rodrigues, A.C.; da Silva, T.F.; de Oliveira, I.R.N.; Vilas Boas, E.V.D.B. Cellulose microfibrillated suspension of carrots obtained by mechanical defibrillation and their application in edible starch films. Ind. Crops Prod. 2016, 89, 285–294. [Google Scholar] [CrossRef]
- Liu, Z. 19—Edible films and coatings from starches. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 318–337. [Google Scholar]
- Torres, F.G.; Troncoso, O.P.; Torres, C.; Díaz, D.A.; Amaya, E. Biodegradability and mechanical properties of starch films from Andean crops. Int. J. Biol. Macromol. 2011, 48, 603–606. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-based films: Major factors affecting their properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch retrogradation: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and science behind modified starch edible films and coatings: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- Adetunji, C.; Fawole, O.; Arowora, K.; Nwaubani, S.; Oloke, J.; Adepoju, A.; Ajani, A. Performance of edible coatings from carboxymethylcellulose (CMC) and corn starch (CS) incorporated with Moringa oleifera extract on Citrus sinensis Stored at ambient temperature. Agrosearch 2013, 13, 77–86. [Google Scholar] [CrossRef]
- López-Mata, M.A.; Ruiz-Cruz, S.; Silva-Beltrán, N.P.; Ornelas-Paz Jde, J.; Zamudio-Flores, P.B.; Burruel-Ibarra, S.E. Physicochemical, antimicrobial and antioxidant properties of chitosan films incorporated with carvacrol. Molecules 2013, 18, 13735–13753. [Google Scholar] [CrossRef]
- Li, Z.; Lin, S.; An, S.; Liu, L.; Hu, Y.; Wan, L. Preparation, characterization and anti-aflatoxigenic activity of chitosan packaging films incorporated with turmeric essential oil. Int. J. Biol. Macromol. 2019, 131, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yin, L.; Li, Y. Combined effects of lemon essential oil and surfactants on physical and structural properties of chitosan films. Int. J. Food Sci. Technol. 2013, 48, 44–50. [Google Scholar] [CrossRef]
- Sánchez González, L.; Gonzalez-Martinez, C.; Chiralt, A.; Cháfer, M. Physical and antimicrobial properties of chitosan-tea tree essential oil. J. Food Eng. 2010, 98, 443–452. [Google Scholar] [CrossRef]
- Shen, Z.; Kamdem, D.P. Development and characterization of biodegradable chitosan films containing two essential oils. Int. J. Biol. Macromol. 2015, 74, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, D.; Jin, T.Z.; Chen, W.; He, Q.; Zou, Z.; Zhao, H.; Ye, X.; Guo, M. Preparation and characterization of gellan gum-chitosan polyelectrolyte complex films with the incorporation of thyme essential oil nanoemulsion. Food Hydrocoll. 2021, 114, e106570. [Google Scholar] [CrossRef]
- Abdollahi, M.; Rezaei, M.; Farzi, G. Improvement of active chitosan film properties with rosemary essential oil for food packaging. Int. J. Food Sci. Technol. 2012, 47, 847–853. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and characterization of edible chitosan/olive oil emulsion films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Zhang, X.; Ismail, B.B.; Cheng, H.; Jin, T.Z.; Qian, M.; Arabi, S.A.; Liu, D.; Guo, M. Emerging chitosan-essential oil films and coatings for food preservation—A review of advances and applications. Carbohydr. Polym. 2021, 273, e118616. [Google Scholar] [CrossRef]
- Wani, A.R.; Yadav, K.; Khursheed, A.; Rather, M.A. An updated and comprehensive review of the antiviral potential of essential oils and their chemical constituents with special focus on their mechanism of action against various influenza and coronaviruses. Microb. Pathog. 2021, 152, e104620. [Google Scholar] [CrossRef] [PubMed]
- Moalla, S.; Ammar, I.; Fauconnier, M.-L.; Danthine, S.; Blecker, C.; Besbes, S.; Attia, H. Development and characterization of chitosan films carrying Artemisia campestris antioxidants for potential use as active food packaging materials. Int. J. Biol. Macromol. 2021, 183, 254–266. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).