Process Simulation and Abrasion Behavior of Jet Electrodeposited Ni–TiN Nanocoatings
Abstract
:1. Introduction
2. Experimental Procedure
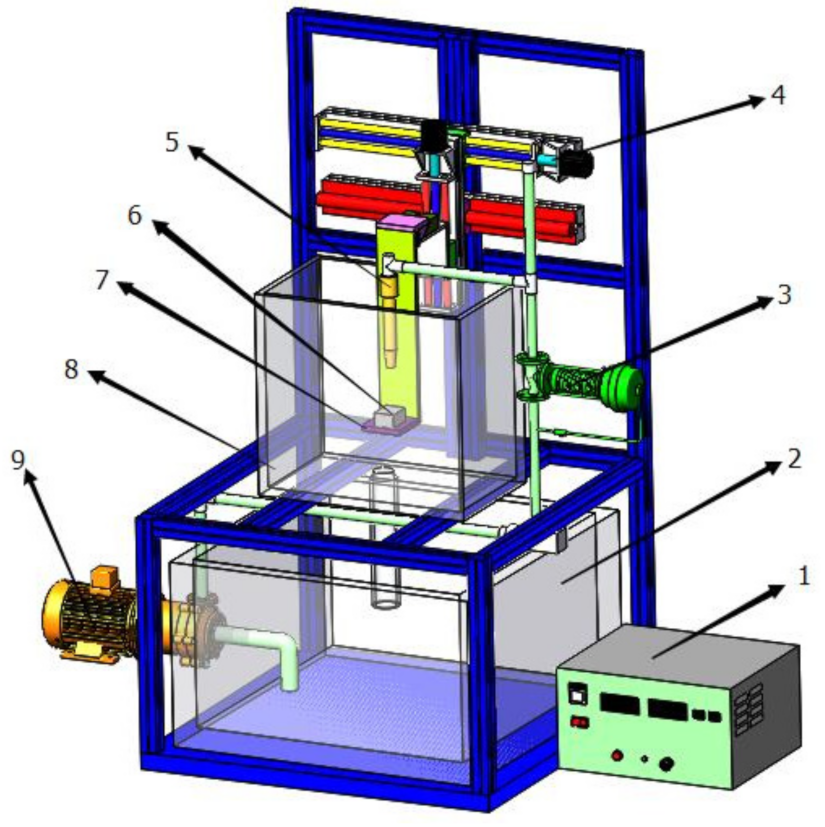
2.1. Preparation Process
2.2. Characterizations
3. Results and Discussion
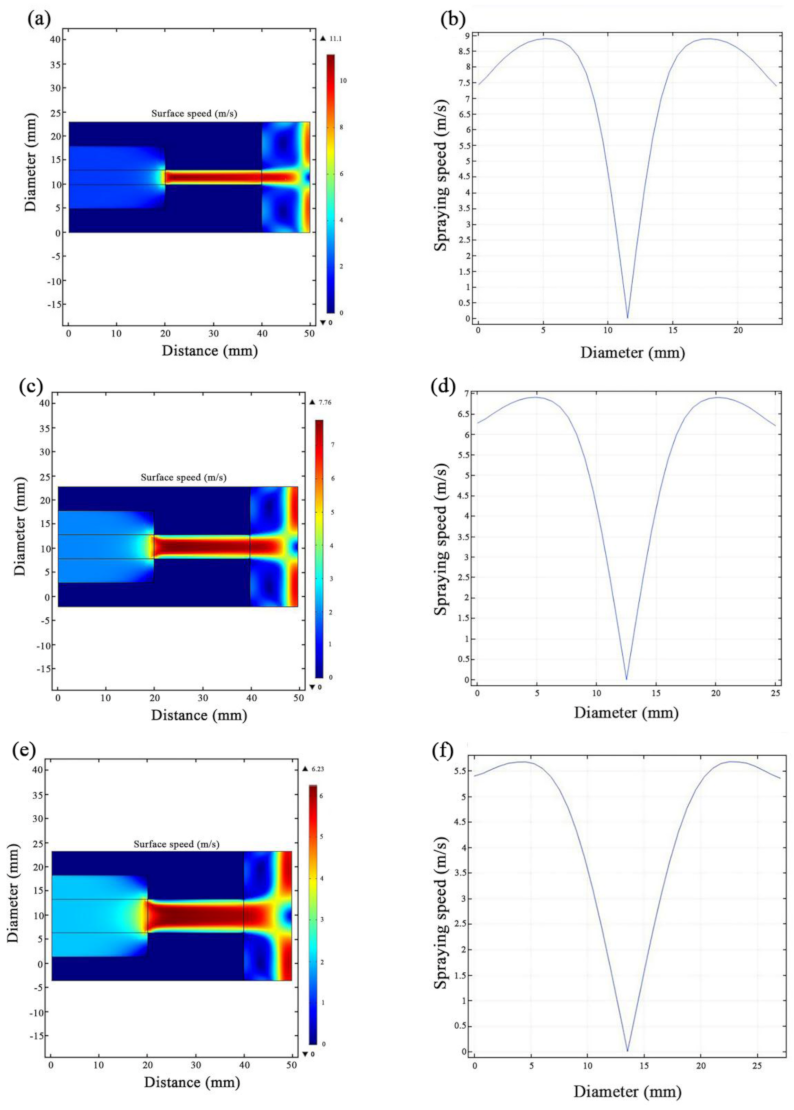
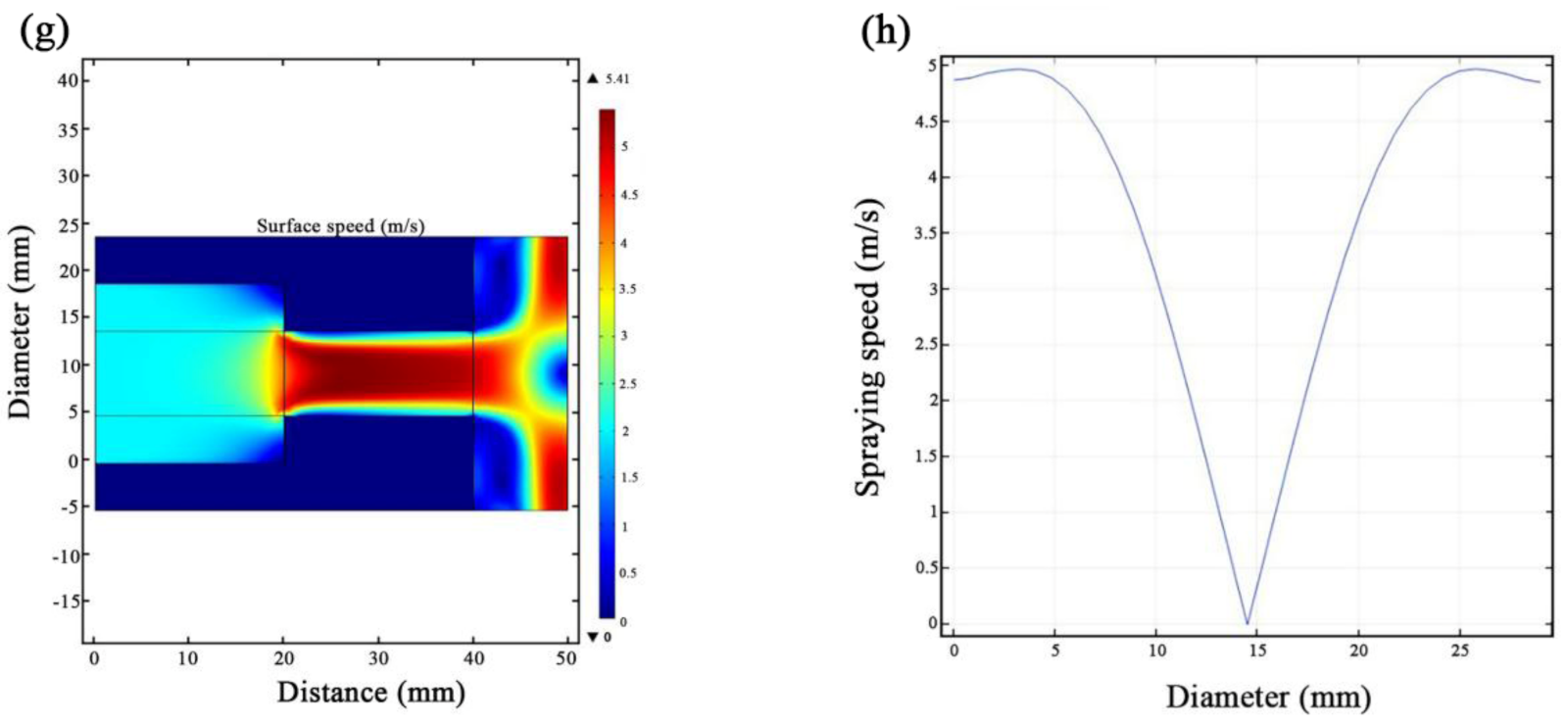
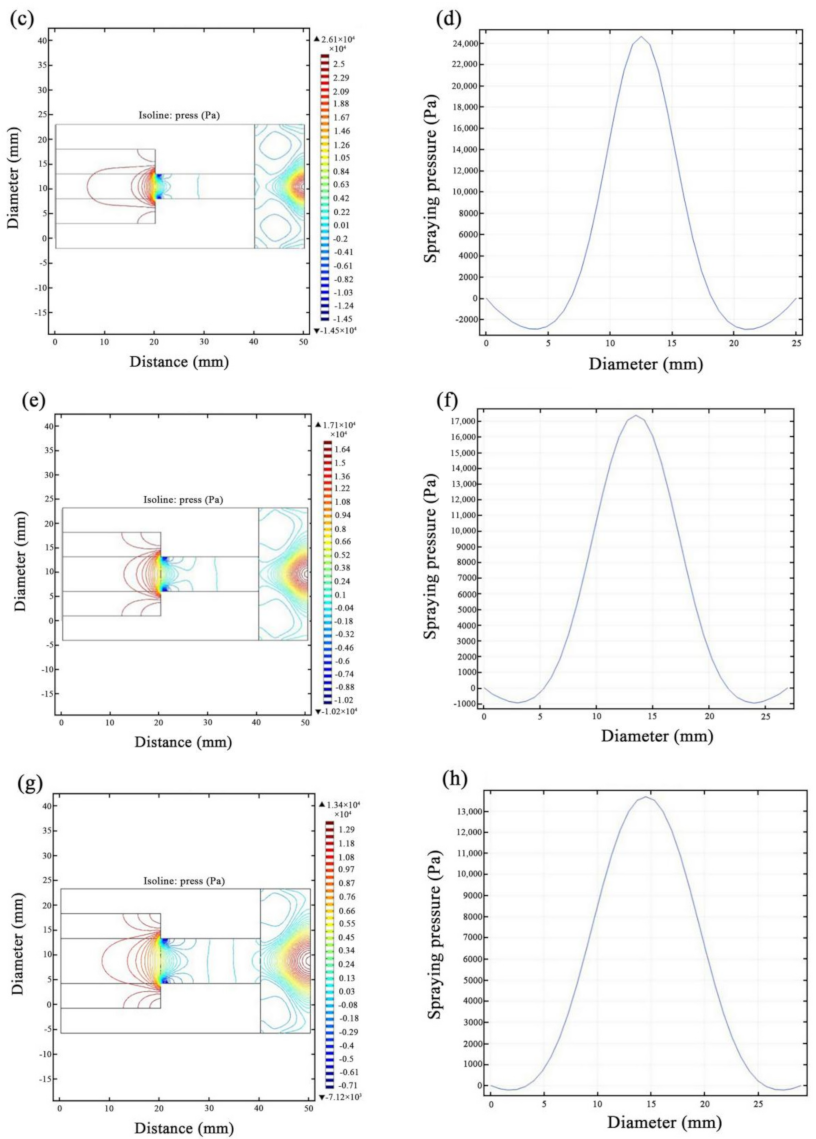
3.1. JED Simulations
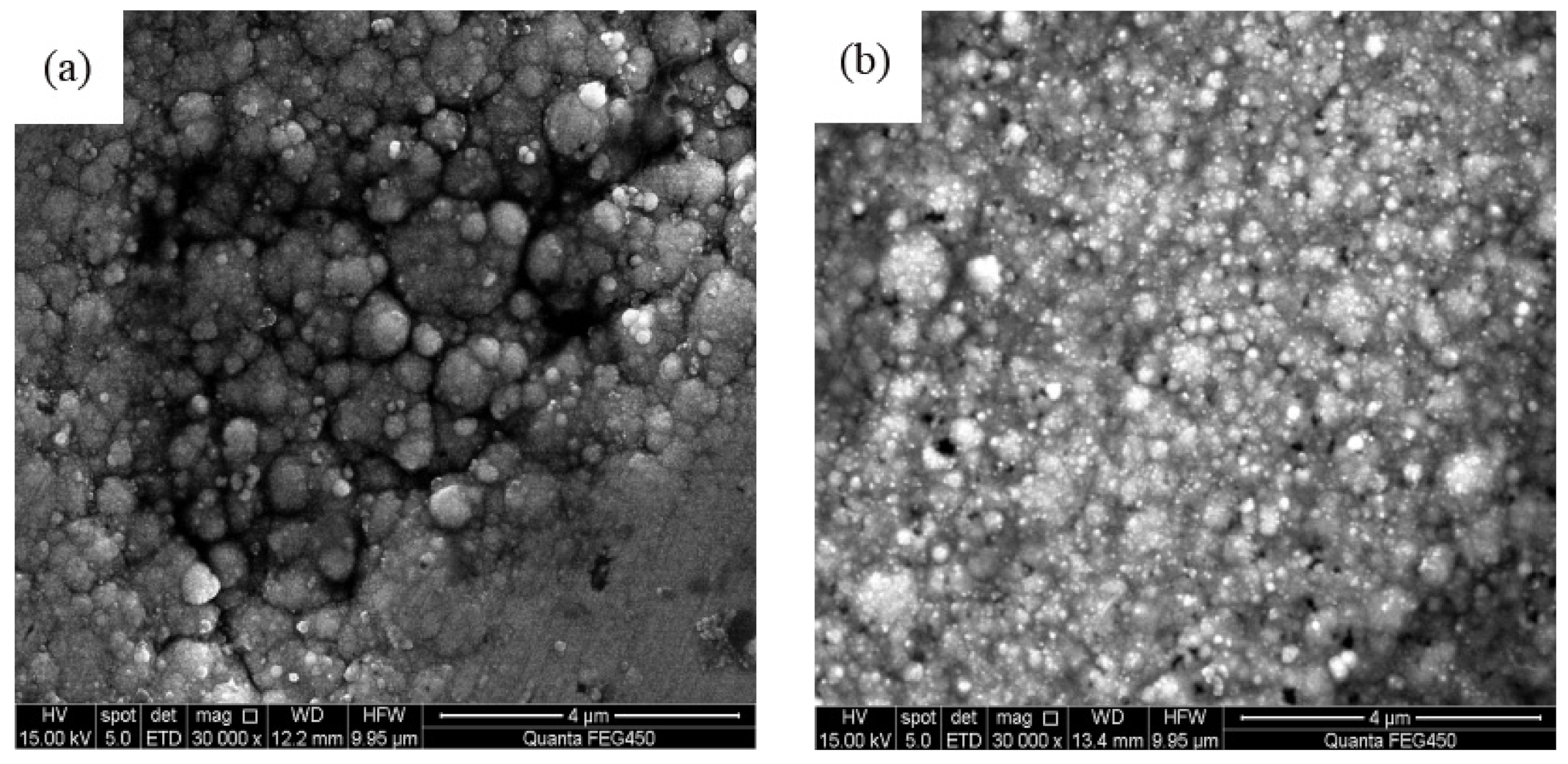
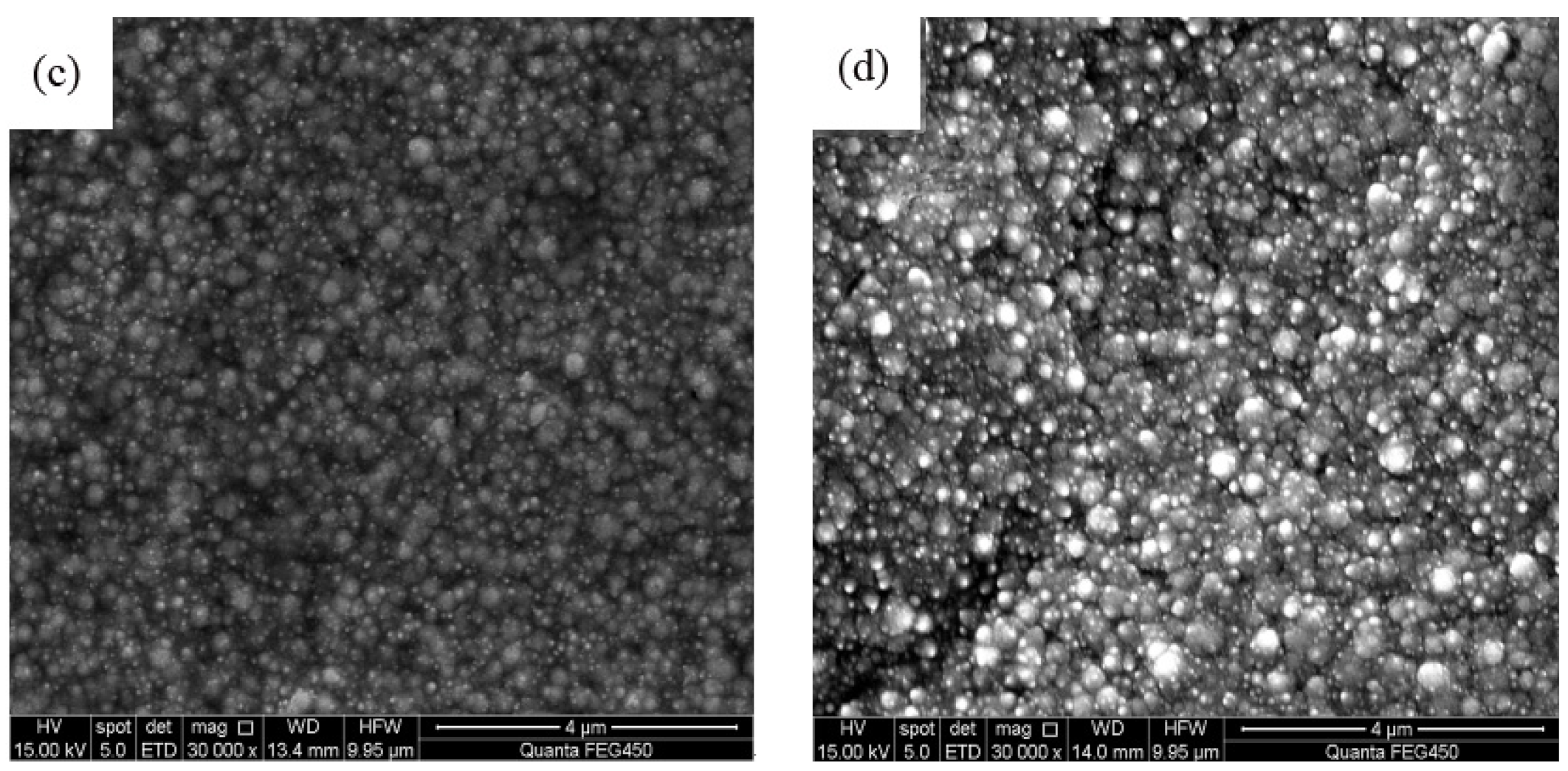
3.2. Analysis of Surface Morphology
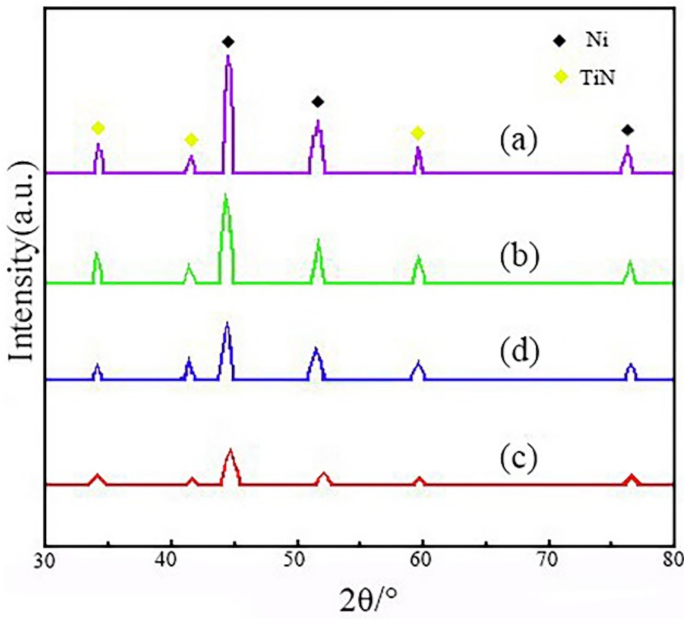
3.3. Survey of Phase Structure
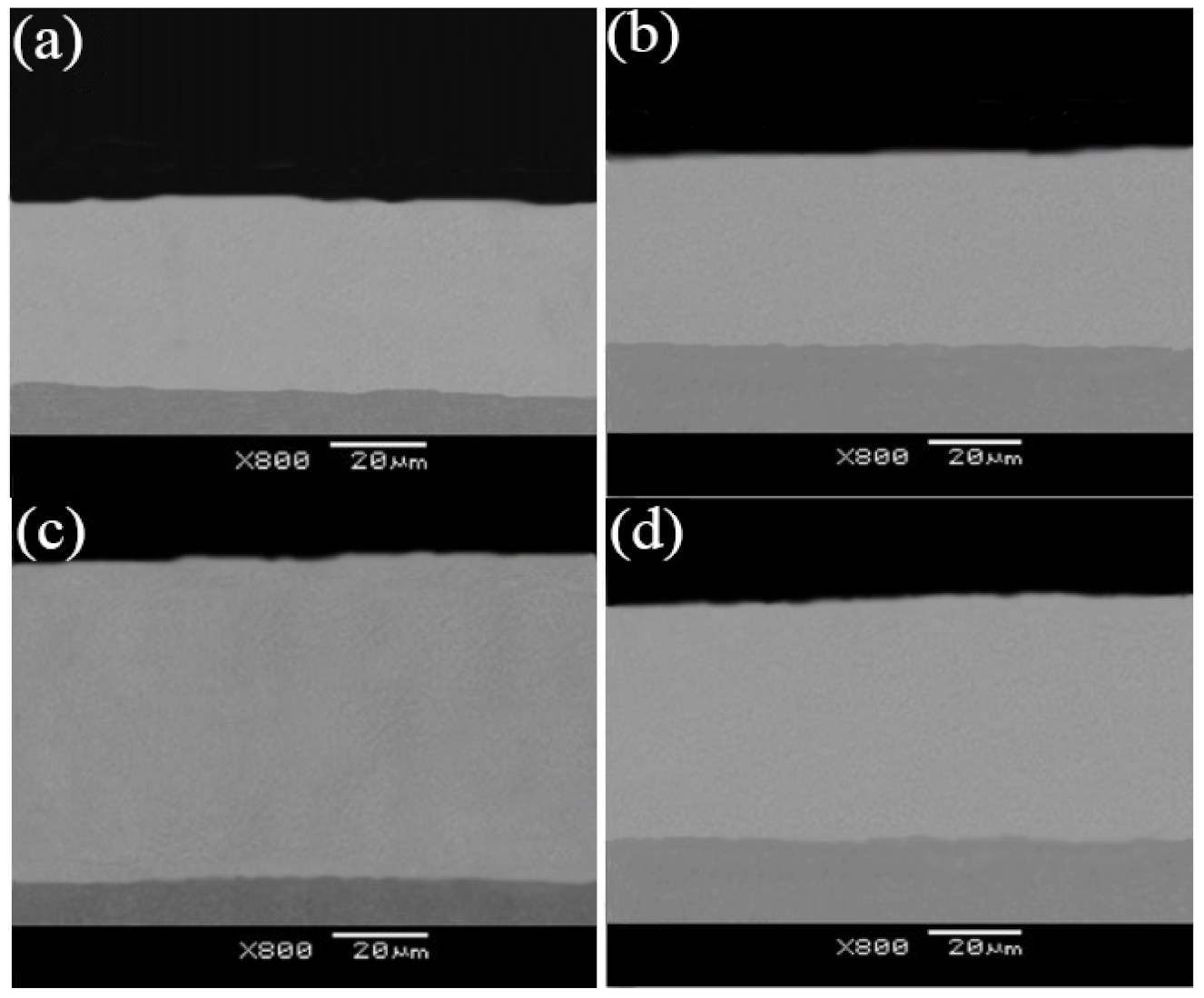
3.4. Abrasion Resistance
4. Conclusions
- The spraying speed and spraying pressure of the electrolyte generated from a Φ7 mm nozzle diameter was optimum and produced Ni–TiN CNCs of the best quality. The Ni–TiN CNCs produced under these conditions contained a dense and smooth microstructure, containing a large number of TiN nanoparticles. The Ni–TiN CNCs manufactured at the too-small or too-large nozzle diameters were composed of an uneven surface, coarse crystal grains and a low TiN particle content.
- The diffraction intensity of the (111) and (200) crystal planes of Ni–TiN CNCs prefabricated at a Φ7 mm nozzle diameter was lowest, which suggested that the crystal grains of Ni–TiN CNCs were smallest, with average diameters of nickel grains and TiN particles of 76 and 45 nm, respectively.
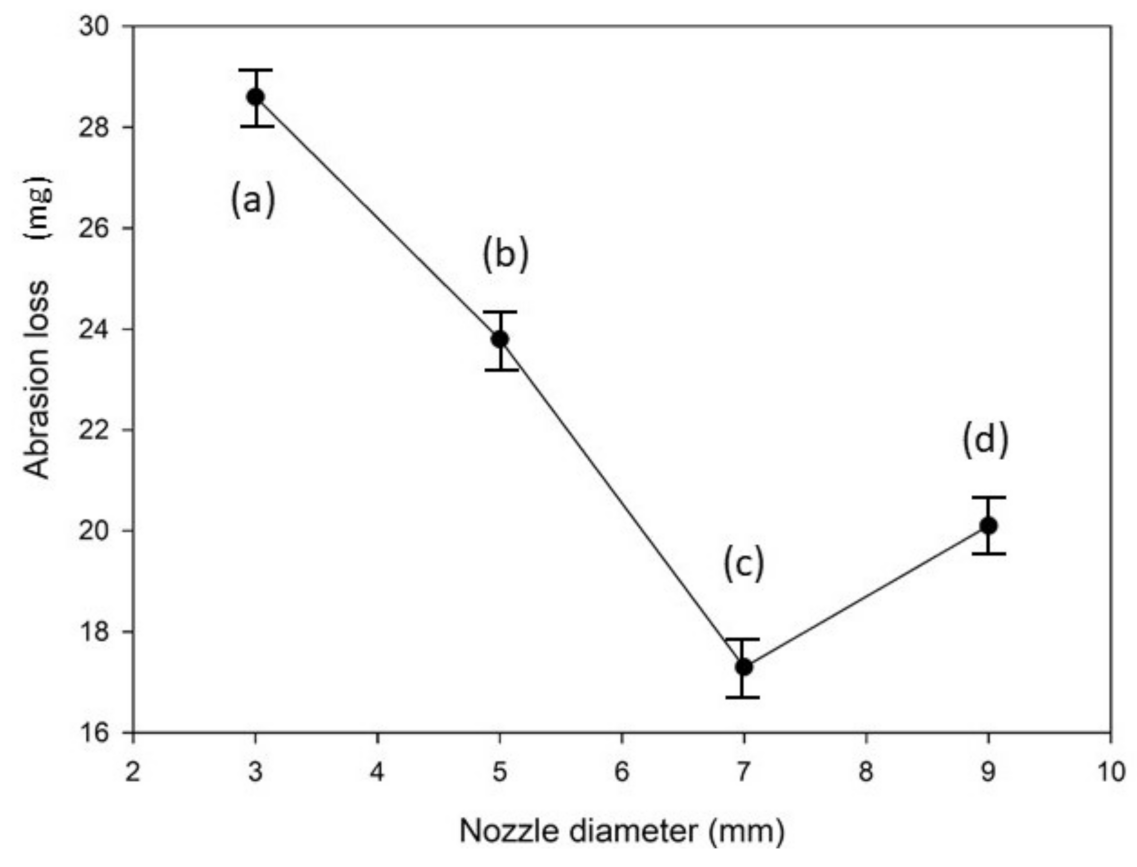
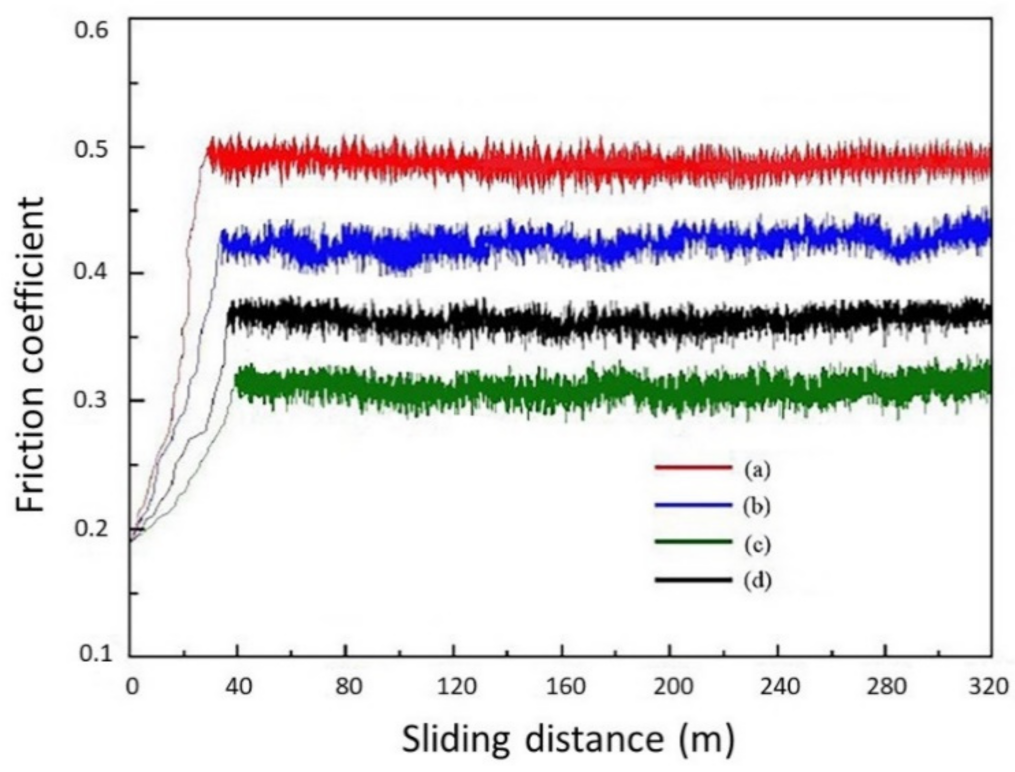
- The diverse nozzle diameters used to fabricate the CNCs impacted their abrasion resistance. The micro-hardness and the adhesion force of the Ni–TiN CNCs prepared at a Φ7 mm nozzle diameter were the largest, at 647 Hv and 198.2 N, respectively. Meanwhile, the abrasion loss and the average frictional coefficient was 17.3 mg and 0.312, respectively, which meant that these CNCs possessed the best abrasion resistance among the fabricated coatings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashemi, S.H.; Ashrafi, A. Characterisations of low phosphorus electroless Ni and composite electroless Ni–P-SiC coatings on A356 aluminium alloy. Trans. IMF 2018, 96, 52–56. [Google Scholar] [CrossRef]
- Ma, C.; Yu, W.; Jiang, M.; Cui, W.; Xia, F. Jet pulse electrodeposition and characterization of Ni–AlN nanocoatings in presence of ultrasound. Ceram. Int. 2018, 44, 5163–5170. [Google Scholar] [CrossRef]
- Kaushal, S.; Gupta, D.; Bhowmick, H. On development and wear behavior of microwave-processed functionally graded Ni-SiC clads on SS-304 substrate. J. Mater. Eng. Perform. 2018, 27, 777–786. [Google Scholar] [CrossRef]
- Mousavi, R.; Bahrololoom, M.E.; Deflorian, F. Preparation, corrosion, and wear resistance of Ni–Mo/Al composite coating reinforced with Al particles. Mater. Des. 2016, 110, 456–465. [Google Scholar] [CrossRef]
- Sun, C.; Liu, X.; Zhou, C.; Wang, C.; Cao, H. Preparation and wear properties of magnetic assisted pulse electrodeposited Ni–SiC nanocoatings. Ceram. Int. 2018, 45, 1348–1355. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.G.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Liu, X.; Zhai, H.; Liu, Z.; Tian, J. Preparation of Ni-coated Si3N4 powders via electroless plating method. Ceram. Int. 2009, 35, 3407–3410. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, Y.; Zhang, G.; Ke, Z.; Wu, H.; Yang, Z.; Zhou, W. Microstructure and mechanical properties of Ti(C,N)-based cermets fabricated using Ni–coated mixed powders. Ceram. Int. 2020, 46, 16944–16948. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.; Xu, M.; Wang, Z.; Tian, Z. Mechanical properties and corrosion resistance of Ni–Co-SiC composite coatings by magnetic field-induced jet electrodeposition. J. Alloys Compd. 2019, 791, 847–855. [Google Scholar] [CrossRef]
- Ma, C.; Zhao, D.; Xia, F.; Xia, H.; Williams, T.; Xing, H. Ultrasonic-assisted electrodeposition of Ni-Al2O3 nanocomposites at various ultrasonic powers. Ceram. Int. 2020, 46, 6115–6123. [Google Scholar] [CrossRef]
- Xia, F.; Jiao, J.; Ma, C.; Wu, M. Forecast the microhardnesses of the Ni–TiN nanocoatings by AR model. Adv. Funct. Mater. 2012, 43, 140–143. [Google Scholar]
- Wang, C.; Li, K.; Huo, C.; He, Q.; Shi, X. Oxidation behavior and microstructural evolution of plasma sprayed La2O3-MoSi2-SiC coating on carbon/carbon composites. Surf. Coat. Technol. 2018, 348, 81–90. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Bathini, L.; Sundararajan, G. Tribological behavior of pulsed electrodeposited Ni-W/SiC nanocomposites. J. Mater. Eng. Perform. 2018, 27, 5236–5245. [Google Scholar] [CrossRef]
- Yu, F.; Guo, H.; Cao, J.; Sun, C. Preparation and characterization of nickel-coated copper powder. Electroplat. Finish. 2011, 30, 21–23. (In Chinese) [Google Scholar]
- Ghaziof, S.; Gao, W. The effect of pulse electroplating on Zn-Ni alloy and Zn-Ni–Al2O3 composite coatings. J. Alloys Compd. 2015, 622, 918–924. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Latha, S.M.; Ramakrishna, M.; Rao, D.S.; Sundararajan, G. Pulsed electrodeposition and mechanical properties of Ni-W/SiC nano-composite coatings. Mater. Des. 2016, 112, 140–150. [Google Scholar] [CrossRef]
- Wu, M.; Jia, W.; Lv, P. Electrodepositing Ni-TiN nanocomposite layers with applying action of ultrasonic waves. Procedia Eng. 2017, 174, 717–723. [Google Scholar] [CrossRef]
- Dehgahi, S.; Amini, R.; Alizadeh, M. Corrosion, passivation and wear behaviors of electrodeposited Ni–Al2O3–SiC nano-composite coatings. Surf. Coat. Technol. 2016, 304, 502–511. [Google Scholar] [CrossRef]
- Xia, F.; Li, C.; Ma, C.; Li, Q.; Xing, H. Effect of pulse current density on microstructure and wear property of Ni-TiN nanocoatings deposited via pulse electrodeposition. Appl. Surf. Sci. 2021, 538, 148139. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Jiang, Y.; Lin, H.; Feng, J. Preparation and properties of TaSi2-MoSi2-ZrO2-borosilicate glass coating on porous SiC ceramic composites for thermal protection. Ceram. Int. 2018, 44, 19143–19150. [Google Scholar] [CrossRef]
- Xia, F.; Jia, W.; Ma, C.; Wang, J. Synthesis of Ni–TiN composites through ultrasonic pulse electrodeposition with excellent corrosion and wear resistance. Ceram. Int. 2018, 44, 766–773. [Google Scholar] [CrossRef]
- Ma, C.; Jiang, M.; Xia, F. Preparation and characterization of Ni–TiN thin films electrodeposited with nickel baths of different TiN nanoparticle concentration. Surf. Rev. Lett. 2016, 24, 17500631. [Google Scholar] [CrossRef]
- Li, B.; Zhang, W.; Zhang, W. Preparation of Ni–W/SiC nanocomposite coatings by electrochemical deposition. J. Alloys Compd. 2017, 702, 38–50. [Google Scholar] [CrossRef]
- Fan, H.; Liu, M.; Zhao, Y. The Al2O3 nano-particles influenced on morphology and microstructure of Cu-Al2O3 composite coating by jet electrodeposition. Key Eng. Mater. 2018, 764, 164–173. [Google Scholar] [CrossRef]
- Xia, F.; Jia, W.; Ma, C.; Yang, R.; Wang, Y.; Potts, M. Synthesis and characterization of Ni-doped TiN thin films deposited by jet electrodeposition. Appl. Surf. Sci. 2018, 434, 228–233. [Google Scholar] [CrossRef]
- Xia, F.; Jia, W.; Jiang, M.; Cui, W.; Wang, J. Microstructure and corrosion properties of Ni-TiN nanocoatings prepared by jet pulse electrodeposition. Ceram. Int. 2017, 43, 14623–14628. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, K.; Li, Q.; Ma, C.; Xia, F. Jet pulse electrodeposited Ni–SiC thin coatings by using experimental system designed for potential industrial application. Int. J. Electrochem. Sci. 2021, 16, 21044. [Google Scholar] [CrossRef]
- Su, B.; Choy, K.L. Synthesis, microstructure and optical properties of ZnS films formed by electrostatic assisted aerosol jet deposition. J. Mater. Chem. 2000, 10, 949–952. [Google Scholar] [CrossRef]
- Xia, F.; Tian, J.; Wang, W.; He, Y. Effect of plating parameters on the properties of pulse electrodeposited Ni–TiN thin films. Ceram. Int. 2016, 42, 13268–13272. [Google Scholar] [CrossRef]
- Jiang, W.; Shen, L.; Qiu, M.; Wang, X.; Fan, M.; Tian, Z. Preparation of Ni-SiC composite coatings by magnetic field-enhanced jet electrodeposition. J. Alloys Compd. 2018, 762, 115–124. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, Q. Study on magnetic field assisted electrodeposition of Ni–SiC nanocoatings on the surface of automobile cylinder liner. J. Funct. Mater. 2019, 50, 3081–3089. (In Chinese) [Google Scholar]
- Liu, H.; Wang, H.; Yu, W.; He, Y.; Xia, F.; Ma, C.; Shakoor, A. Effect of TiN concentration on microstructure and properties of Ni/W–TiN composites obtained by pulse current electrodeposition. Ceram. Int. 2021, 47, 24331–24339. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Li, Q.; Chen, S.; Ma, C. Microstructure and performance of magnetic field assisted, pulse-electrodeposited Ni–TiN thin coatings with various TiN grain sizes. Ceram. Int. 2021, 47, 18532–18539. [Google Scholar] [CrossRef]
- Jiang, S.; Gao, S.; Kong, J.; Jin, X. Study on the synthesis of β-SiC nanoparticles from diamond-wire silicon cutting waste. RSC Adv. 2019, 9, 23785–23790. [Google Scholar] [CrossRef] [Green Version]


| Composition | Parameters |
|---|---|
| NiSO4·6H2O | 200 g/L |
| NiCl2·6H2O | 26 g/L |
| C3H6O4·6H2O | 3 g/L |
| TiN nanoparticles | 8 g/L |
| Saccharin sodium | 0.15 g/L |
| Cetyltrimethyl Ammonium Bromide (CTAB) | 0.05 g/L |
| Surfactant (Alkyl polyglycoside) | 150 mg/L |
| Temperature | 50 °C |
| pH | 4.3 |
| Poles distance | 10 mm |
| Plating time | 50 min |
| Current density | 0.5 A/dm2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Z.; Yi, S.; Cao, L.; Tang, S.; Li, Q. Process Simulation and Abrasion Behavior of Jet Electrodeposited Ni–TiN Nanocoatings. Coatings 2022, 12, 86. https://doi.org/10.3390/coatings12010086
Yang Z, Yi S, Cao L, Tang S, Li Q. Process Simulation and Abrasion Behavior of Jet Electrodeposited Ni–TiN Nanocoatings. Coatings. 2022; 12(1):86. https://doi.org/10.3390/coatings12010086
Chicago/Turabian StyleYang, Zhongguo, Shujuan Yi, Longkui Cao, Songhao Tang, and Qiang Li. 2022. "Process Simulation and Abrasion Behavior of Jet Electrodeposited Ni–TiN Nanocoatings" Coatings 12, no. 1: 86. https://doi.org/10.3390/coatings12010086





