Design and Characterization of Nanostructured Titanium Monoxide Films Decorated with Polyaniline Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization
2.2. Raman Spectroscopy
2.3. Electrochemical Measurements
3. Results
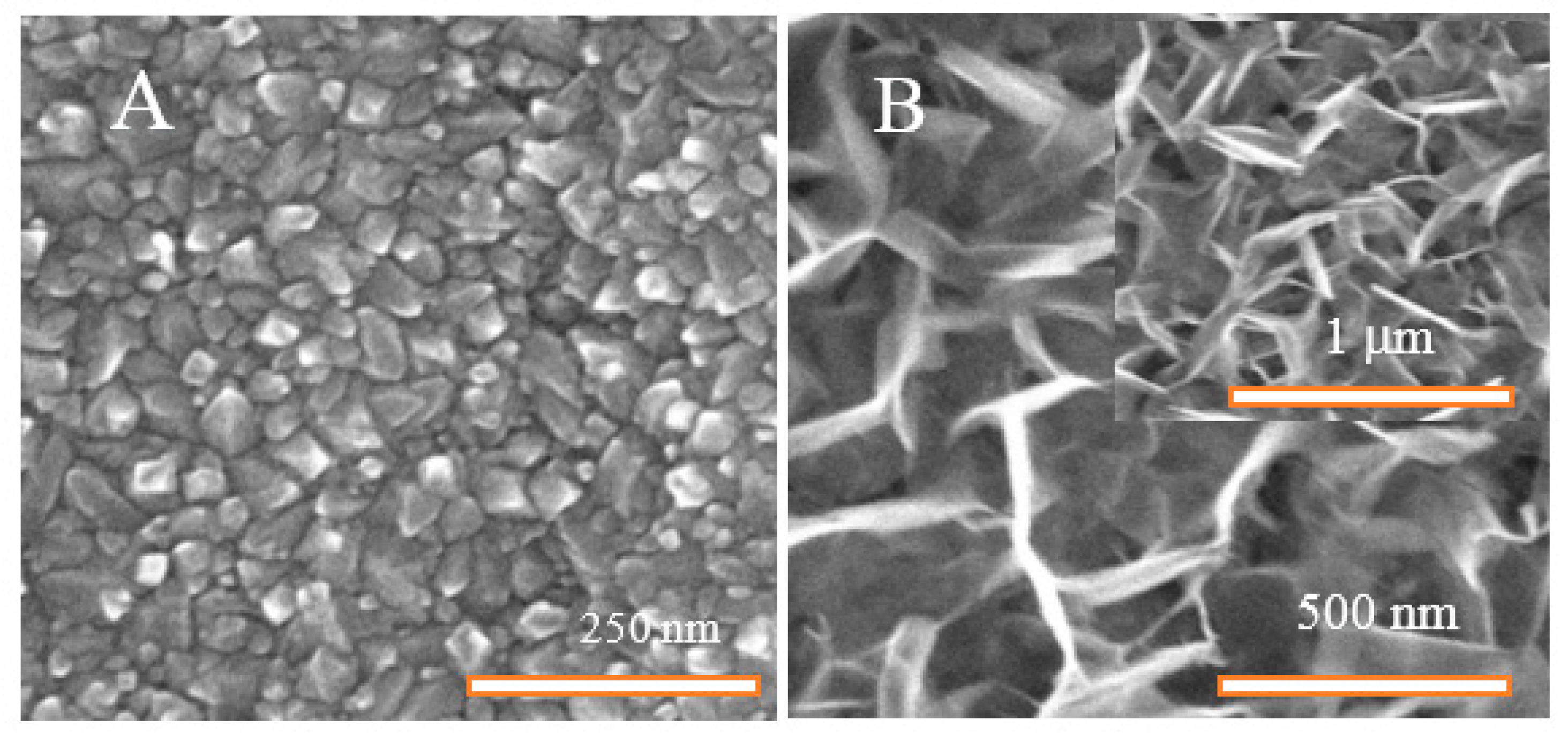
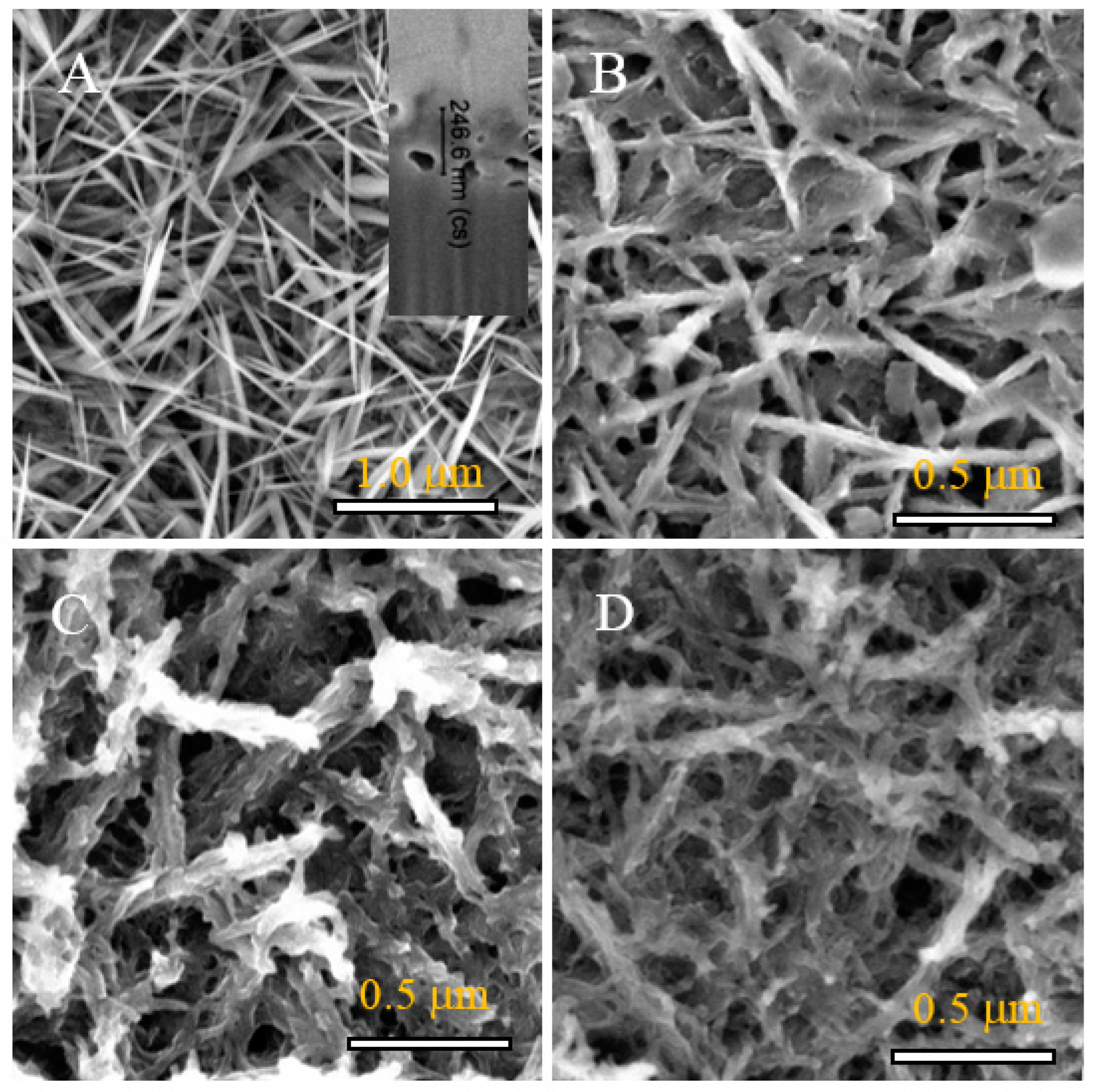
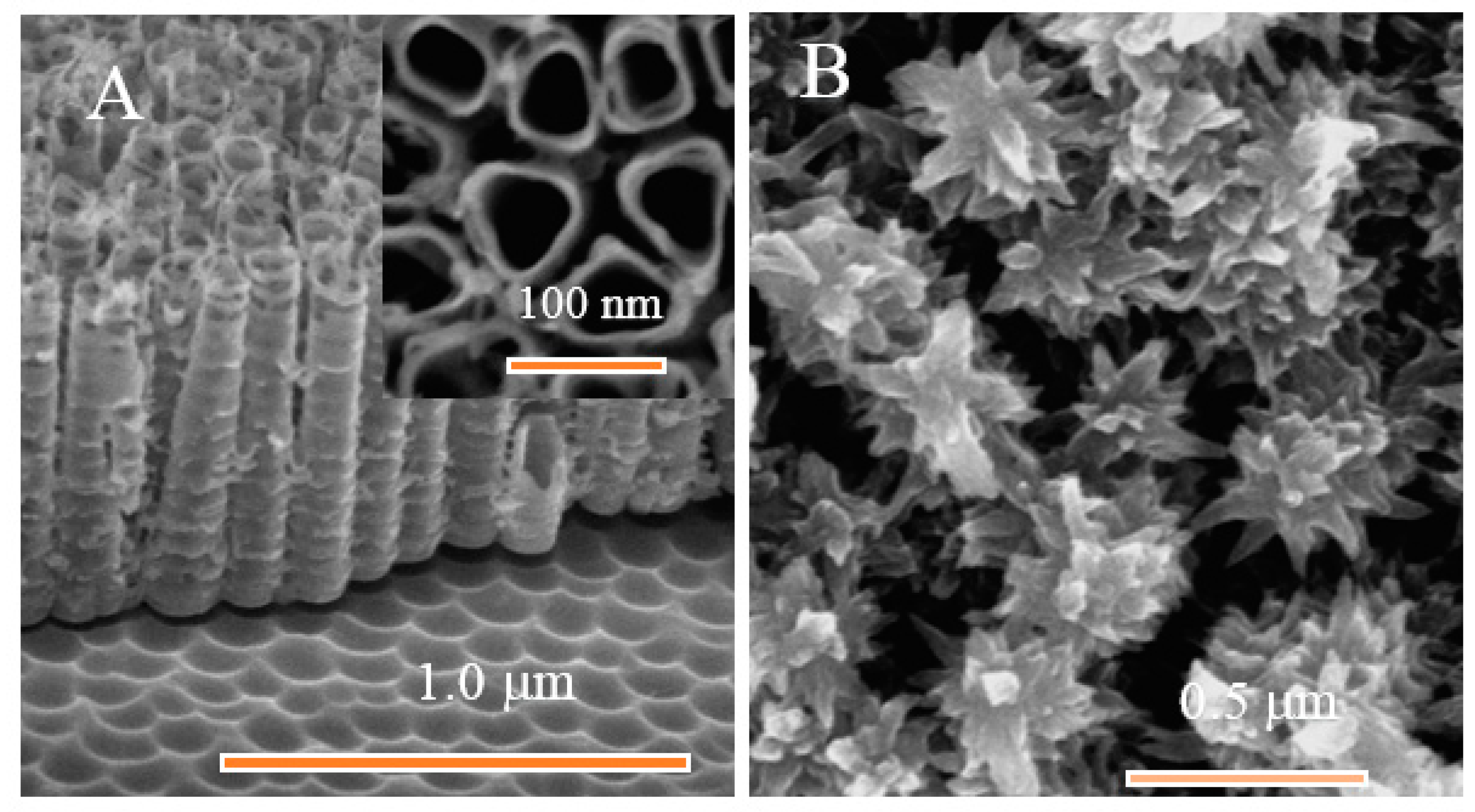
3.1. Structural Characterization
3.2. Raman Spectroscopy
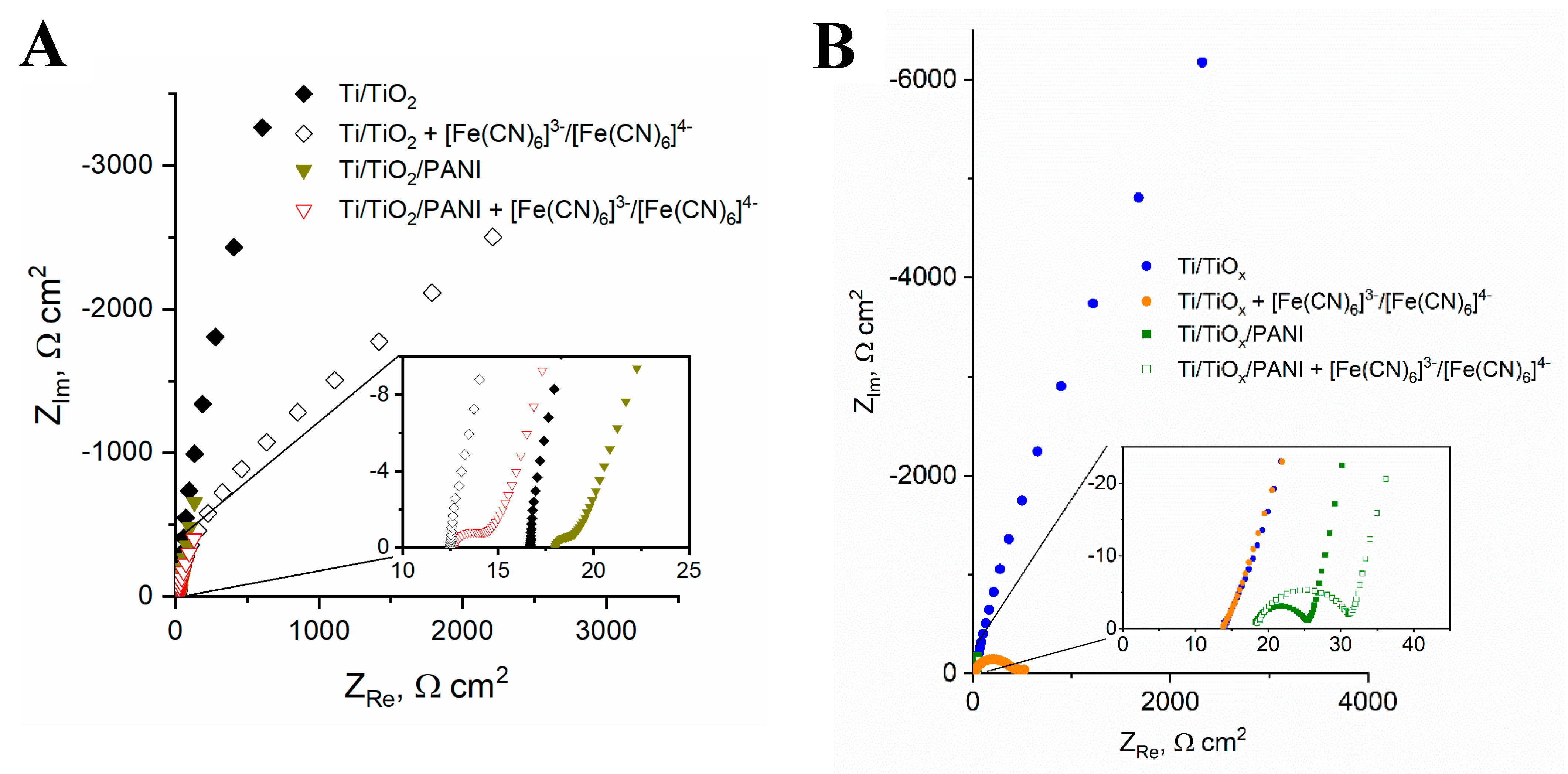
3.3. Electrochemical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geniès, E.; Boyle, A.; Lapkowski, M.; Tsintavis, C. Polyaniline: A historical survey. Synth. Met. 1990, 36, 139–182. [Google Scholar] [CrossRef]
- Mozafari, M.; Chauhan, N.P.S. Fundamentals and Emerging Applications of Polyaniline; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Zare, E.N.; Makvandi, P.; Ashtari, B.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Laourari, I.; Lakhdari, N.; Belgherbi, O.; Medjili, C.; Berkani, M.; Vasseghian, Y.; Golzadeh, N.; Lakhdari, D. Antimicrobial and antifungal properties of NiCu-PANI/PVA quaternary nanocomposite synthesized by chemical oxidative polymerization of polyaniline. Chemosphere 2021, 291, 132696. [Google Scholar] [CrossRef]
- You, C.; Wu, H.; Wang, M.; Wang, S.; Shi, T.; Luo, Y.; Sun, B.; Zhang, X.; Zhu, J. A strategy for photothermal conversion of polymeric nanoparticles by polyaniline for smart control of targeted drug delivery. Nanotechnology 2017, 28, 165102. [Google Scholar] [CrossRef]
- Nandihalli, N.; Liu, C.-J.; Mori, T. Polymer based thermoelectric nanocomposite materials and devices: Fabrication and characteristics. Nano Energy 2020, 78, 105186. [Google Scholar] [CrossRef]
- Xu, D.; Fan, L.; Gao, L.; Xiong, Y.; Wang, Y.; Ye, Q.; Yu, A.; Dai, H.; Yin, Y.; Cai, J.; et al. Micro-Nanostructured Polyaniline Assembled in Cellulose Matrix via Interfacial Polymerization for Applications in Nerve Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 17090–17097. [Google Scholar] [CrossRef]
- Li, M.; Guo, Y.; Wei, Y.; MacDiarmid, A.G.; Lelkes, P.I. Electrospinning polyaniline-contained gelatin nanofibers for tissue engineering applications. Biomaterials 2006, 27, 2705–2715. [Google Scholar] [CrossRef]
- Samukaite-Bubniene, U.; Valiūnienė, A.; Bucinskas, V.; Genys, P.; Ratautaite, V.; Ramanaviciene, A.; Aksun, E.; Tereshchenko, A.; Zeybek, B.; Ramanavicius, A. Towards supercapacitors: Cyclic voltammetry and fast Fourier transform electrochemical impedance spectroscopy based evaluation of polypyrrole electrochemically deposited on the pencil graphite electrode. Colloids Surf. A Physicochem. Eng. Asp. 2021, 610, 125750. [Google Scholar] [CrossRef]
- Viter, R.; Kunene, K.; Genys, P.; Jevdokimovs, D.; Erts, D.; Sutka, A.; Bisetty, K.; Viksna, A.; Ramanaviciene, A.; Ramanavicius, A. Photoelectrochemical Bisphenol S Sensor Based on ZnO-Nanoroads Modified by Molecularly Imprinted Polypyrrole. Macromol. Chem. Phys. 2019, 221, 1900232. [Google Scholar] [CrossRef]
- Ratautaite, V.; Brazys, E.; Ramanaviciene, A.; Ramanavicius, A. Electrochemical sensors based on l-tryptophan molecularly imprinted polypyrrole and polyaniline. J. Electroanal. Chem. 2022, 917, 116389. [Google Scholar] [CrossRef]
- Ratautaite, V.; Boguzaite, R.; Brazys, E.; Ramanaviciene, A.; Ciplys, E.; Juozapaitis, M.; Slibinskas, R.; Bechelany, M.; Ramanavicius, A. Molecularly imprinted polypyrrole based sensor for the detection of SARS-CoV-2 spike glycoprotein. Electrochim. Acta 2022, 403, 139581. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Mažeikienė, R.; Niaura, G.; Malinauskas, A. A comparative multiwavelength Raman spectroelectrochemical study of polyaniline: A review. J. Solid State Electrochem. 2019, 23, 1631–1640. [Google Scholar] [CrossRef]
- Gicevicius, M.; Kucinski, J.; Ramanaviciene, A.; Ramanavicius, A. Tuning the optical pH sensing properties of polyaniline-based layer by electrochemical copolymerization of aniline with o-phenylenediamine. Dye. Pigment. 2019, 170, 107457. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y. Chemical Oxidative Polymerization of Polyaniline: A Practical Approach for Preparation of Smart Conductive Textiles. J. Chem. Educ. 2016, 93, 1606–1611. [Google Scholar] [CrossRef]
- Li, Z.; Yang, S.; Song, Y.; Xu, H.; Wang, Z.; Wang, W.; Zhao, Y. Performance evaluation of treating oil-containing restaurant wastewater in microbial fuel cell using in situ graphene/polyaniline modified titanium oxide anode. Environ. Technol. 2018, 41, 420–429. [Google Scholar] [CrossRef]
- Li, Z.; Yang, S.; Song, Y.; Xu, H.; Wang, Z.; Wang, W.; Dang, Z.; Zhao, Y. In-situ modified titanium suboxides with polyaniline/graphene as anode to enhance biovoltage production of microbial fuel cell. Int. J. Hydrog. Energy 2019, 44, 6862–6870. [Google Scholar] [CrossRef]
- Dahl, M.; Liu, Y.; Yin, Y. Composite Titanium Dioxide Nanomaterials. Chem. Rev. 2014, 114, 9853–9889. [Google Scholar] [CrossRef]
- Xu, B.; Sohn, H.Y.; Mohassab, Y.; Lan, Y. Structures, preparation and applications of titanium suboxides. RSC Adv. 2016, 6, 79706–79722. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Tereshchenko, A.; Karpicz, R.; Ratautaite, V.; Bubniene, U.; Maneikis, A.; Jagminas, A.; Ramanavicius, A. TiO2-x/TiO2-Structure Based ‘Self-Heated’ Sensor for the Determination of Some Reducing Gases. Sensors 2019, 20, 74. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Insights in the Application of Stoichiometric and Non-Stoichiometric Titanium Oxides for the Design of Sensors for the Determination of Gases and VOCs (TiO2−x and TinO2n−1 vs. TiO2). Sensors 2020, 20, 6833. [Google Scholar] [CrossRef] [PubMed]
- Jagminas, A.; Naujokaitis, A.; Gaigalas, P.; Ramanavičius, S.; Kurtinaitienė, M.; Trusovas, R. Substrate Impact on the Structure and Electrocatalyst Properties of Molybdenum Disulfide for HER from Water. Metals 2020, 10, 1251. [Google Scholar] [CrossRef]
- Kim, G.; Kong, J.; Kim, J.; Kang, H.; Back, H.; Kim, H.; Lee, K. Overcoming the Light-Soaking Problem in Inverted Polymer Solar Cells by Introducing a Heavily Doped Titanium Sub-Oxide Functional Layer. Adv. Energy Mater. 2014, 5, 1401298. [Google Scholar] [CrossRef]
- Verrelli, E.; Tsoukalas, D. Cluster beam synthesis of metal and metal-oxide nanoparticles for emerging memories. Solid-State Electron. 2014, 101, 95–105. [Google Scholar] [CrossRef]
- Singh, A.; Kalra, V. TiO Phase Stabilized into Freestanding Nanofibers as Strong Polysulfide Immobilizer in Li–S Batteries: Evidence for Lewis Acid–Base Interactions. ACS Appl. Mater. Interfaces 2018, 10, 37937–37947. [Google Scholar] [CrossRef] [PubMed]
- Dronov, A.; Gavrilin, I.; Kirilenko, E.; Dronova, D.; Gavrilov, S. Investigation of anodic TiO2 nanotube composition with high spatial resolution AES and ToF SIMS. Appl. Surf. Sci. 2018, 434, 148–154. [Google Scholar] [CrossRef]
- Mahmood, P.H.; Amiri, O.; Ahmed, S.S.; Hama, J.R. Simple microwave synthesis of TiO2/NiS2 nanocomposite and TiO2/NiS2/Cu nanocomposite as an efficient visible driven photocatalyst. Ceram. Int. 2019, 45, 14167–14172. [Google Scholar] [CrossRef]
- Wiener, J.; Shahidi, S.; Goba, M. Laser deposition of TiO2 nanoparticles on glass fabric. Opt. Laser Technol. 2012, 45, 147–153. [Google Scholar] [CrossRef]
- Zhou, B.; Jiang, X.; Liu, Z.; Shen, R.; Rogachev, A.V. Preparation and characterization of TiO2 thin film by thermal oxidation of sputtered Ti film. Mater. Sci. Semicond. Process. 2012, 16, 513–519. [Google Scholar] [CrossRef]
- Chen, B.T.D.; Dammann, J.F.; Boback, J.L. Nanomaterials. Chem. Soc. Rev. 2017, 44, 1861. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Shan, Y.; Lin, T.; Zhao, W.; Xu, J.; Tian, Z.; Zhang, H.; Zheng, C.; Huang, F. Hydrogenated blue titania with high solar absorption and greatly improved photocatalysis. Nanoscale 2016, 8, 4705–4712. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tian, Z.; Yin, G.; Lin, T.; Huang, F. Controllable reduced black titania with enhanced photoelectrochemical water splitting performance. Dalton Trans. 2016, 46, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Jagminas, A.; Ramanavičius, S.; Jasulaitiene, V.; Šimėnas, M. Hydrothermal synthesis and characterization of nanostructured titanium monoxide films. RSC Adv. 2019, 9, 40727–40735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, K.H.; Kar, A.K. Effect of band gap variation and sensitization process of polyaniline (PANI)-TiO2 p-n heterojunction photocatalysts on the enhancement of photocatalytic degradation of toxic methylene blue with UV irradiation. J. Environ. Chem. Eng. 2020, 8, 104181. [Google Scholar] [CrossRef]
- Zhao, Y.-N.; Lee, U.; Suh, M.-K.; Kwon, Y.-U. Synthesis and Characterization of Highly Crystalline Anatase Nanowire Arrays. Bull. Korean Chem. Soc. 2004, 25, 1341–1345. [Google Scholar]
- Zárate, R.; Fuentes, S.; Wiff, J.; Fuenzalida, V.; Cabrera, A. Chemical composition and phase identification of sodium titanate nanostructures grown from titania by hydrothermal processing. J. Phys. Chem. Solids 2007, 68, 628–637. [Google Scholar] [CrossRef]
- Jagminas, A.; Balčiūnaitė, A.; Niaura, G.; Tamašauskaitė-Tamašiūnaitė, L. Electrochemical synthesis and characterisation of polyaniline in TiO2nanotubes. Trans. IMF 2012, 90, 311–315. [Google Scholar] [CrossRef]
- Morávková, Z.; Dmitrieva, E. Structural changes in polyaniline near the middle oxidation peak studied by in situ Raman spectroelectrochemistry. J. Raman Spectrosc. 2017, 48, 1229–1234. [Google Scholar] [CrossRef]
- Mažeikienė, R.; Niaura, G.; Malinauskas, A. Raman spectroelectrochemical study of polyaniline at UV, blue, and green laser line excitation in solutions of different pH. Synth. Met. 2018, 243, 97–106. [Google Scholar] [CrossRef]
- Mažeikienė, R.; Niaura, G.; Malinauskas, A. Study of deprotonation processes of polyaniline by differential multiwavelength Raman spectroscopy in an electrochemical system. Chemija 2019, 30, 219–226. [Google Scholar] [CrossRef]
- Niaura, G.; Mažeikienė, R.; Malinauskas, A. Structural changes in conducting form of polyaniline upon ring sulfonation as deduced by near infrared resonance Raman spectroscopy. Synth. Met. 2004, 145, 105–112. [Google Scholar] [CrossRef]
- Cochet, M.; Louarn, G.; Quillard, S.; Buisson, J.P.; Lefrant, S. Theoretical and experimental vibrational study of emeraldine in salt form. Part II. J. Raman Spectrosc. 2000, 31, 1041–1049. [Google Scholar] [CrossRef]
- Šeděnková, I.; Prokeš, J.; Trchová, M.; Stejskal, J. Conformational transition in polyaniline films—Spectroscopic and conductivity studies of ageing. Polym. Degrad. Stab. 2008, 93, 428–435. [Google Scholar] [CrossRef]
- Yoon, S.-B.; Yoon, E.-H.; Kim, K.-B. Electrochemical properties of leucoemeraldine, emeraldine, and pernigraniline forms of polyaniline/multi-wall carbon nanotube nanocomposites for supercapacitor applications. J. Power Sources 2011, 196, 10791–10797. [Google Scholar] [CrossRef]
- Li, X.; Rafie, A.; Smolin, Y.Y.; Simotwo, S.; Kalra, V.; Lau, K.K. Engineering conformal nanoporous polyaniline via oxidative chemical vapor deposition and its potential application in supercapacitors. Chem. Eng. Sci. 2019, 194, 156–164. [Google Scholar] [CrossRef]
- Lasia, A. Modeling of Impedance of Porous Electrodes. In Modeling and Numerical Simulations; Springer: Berlin, Germany, 2008; pp. 67–137. [Google Scholar]
- Bieńkowski, K.; Strawski, M.; Szklarczyk, M. The determination of the thickness of electrodeposited polymeric films by AFM and electrochemical techniques. J. Electroanal. Chem. 2011, 662, 196–203. [Google Scholar] [CrossRef]
- Gholivand, M.B.; Heydari, H.; Abdolmaleki, A.; Hosseini, H. Nanostructured CuO/PANI composite as supercapacitor electrode material. Mater. Sci. Semicond. Process. 2015, 30, 157–161. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabirovas, T.; Ramanavicius, S.; Naujokaitis, A.; Niaura, G.; Jagminas, A. Design and Characterization of Nanostructured Titanium Monoxide Films Decorated with Polyaniline Species. Coatings 2022, 12, 1615. https://doi.org/10.3390/coatings12111615
Sabirovas T, Ramanavicius S, Naujokaitis A, Niaura G, Jagminas A. Design and Characterization of Nanostructured Titanium Monoxide Films Decorated with Polyaniline Species. Coatings. 2022; 12(11):1615. https://doi.org/10.3390/coatings12111615
Chicago/Turabian StyleSabirovas, Tomas, Simonas Ramanavicius, Arnas Naujokaitis, Gediminas Niaura, and Arunas Jagminas. 2022. "Design and Characterization of Nanostructured Titanium Monoxide Films Decorated with Polyaniline Species" Coatings 12, no. 11: 1615. https://doi.org/10.3390/coatings12111615




