Highly Bioactive Elastomeric Hybrid Nanoceramics for Guiding Bone Tissue Regeneration
Abstract
:1. Introduction
2. Experimental
2.1. Materials
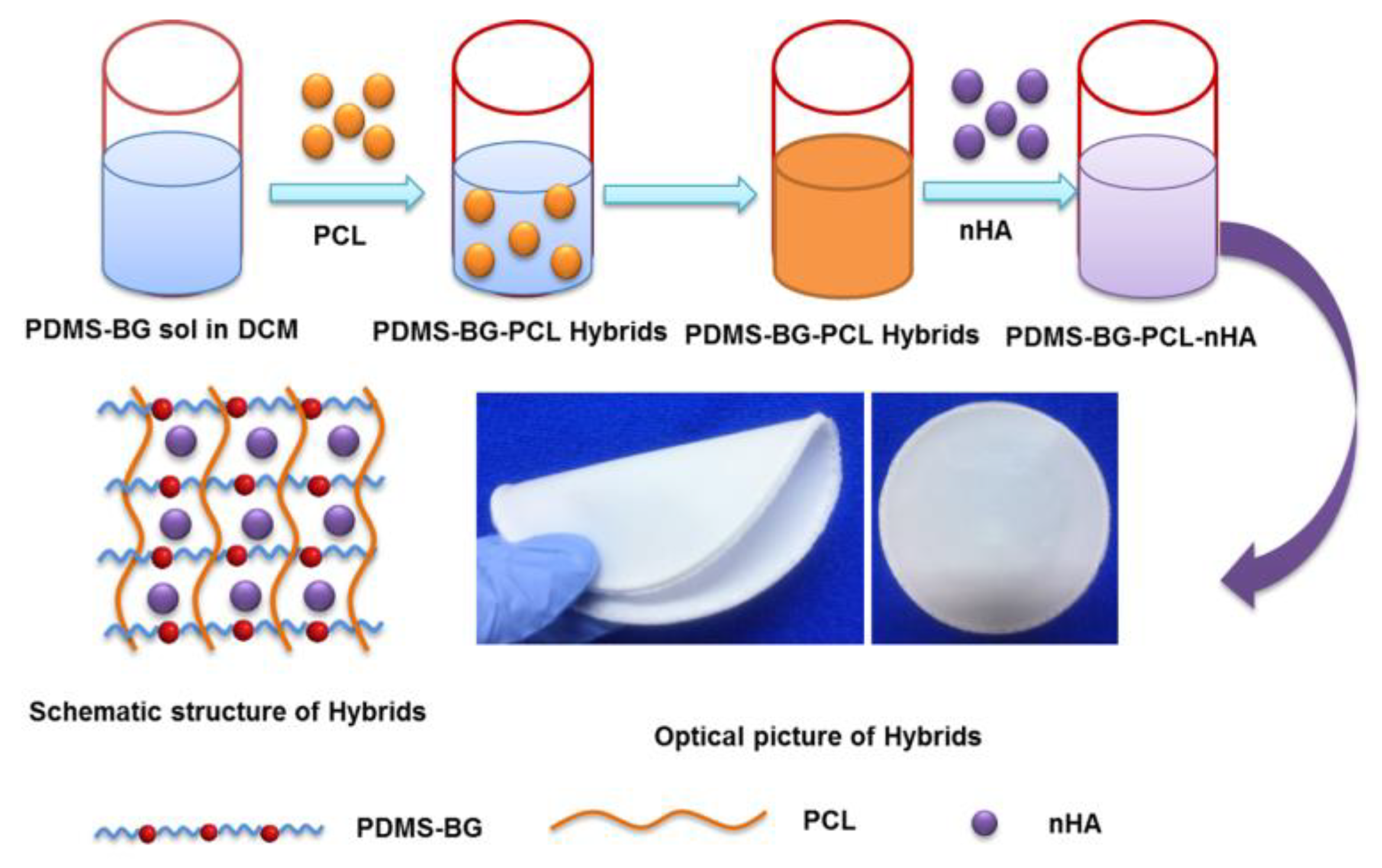
2.2. Synthesis of nHA-PBP Hybrid Membrane
2.3. Characterization of the Specimens
2.4. Mechanical Behavior Assessment of Hybrids
2.5. Biomineralization Activity
2.6. Cell Proliferation and Viability of the Hybrid Membranes
2.7. Statistics Analysis
3. Results and Discussion
3.1. Morphological Measurement
3.2. Mechanical Properties Assessment of the nHA-PBP Hybrid Membranes
3.3. Biomineralization Activity of the nHA-PBP Hybrid Membranes
3.4. Osteoblasts Biocompatibility Assessment of the nHA-PBP Hybrid Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaur, G.; Kumar, V.; Baino, F.; Mauro, J.C.; Pickrell, G.; Evans, L.; Bretcanu, O. Mechanical properties of bioactive glasses, ceramics, glass-ceramics and composites: State-of-the-art review and future challenges. Mater. Sci. Eng. C 2019, 104, 109895. [Google Scholar]
- Hum, J.; Boccaccini, A.R. Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: A review. J. Mater. Sci. Mater. Med. 2012, 23, 2317–2333. [Google Scholar] [CrossRef]
- Furlan, R.G.; Correr, W.R.; Russi, A.F.C.; da Costa Iemma, M.R.; Trovatti, E.; Pecoraro, É. Preparation and characterization of boron-based bioglass by sol–gel process. J. Sol-Gel Sci. Technol. 2018, 88, 181–191. [Google Scholar] [CrossRef] [Green Version]
- Kaur, G.; Pandey, O.P.; Singh, K.; Homa, D.; Scott, B.; Pickrell, G. A review of bioactive glasses: Their structure, properties, fabrication and apatite formation. J. Biomed. Mater. Res. A 2014, 102, 254–274. [Google Scholar] [CrossRef] [PubMed]
- Biswal, T. Biopolymers for tissue engineering applications: A review. Mater. Today 2021, 41, 397–402. [Google Scholar] [CrossRef]
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2019, 13, 189–201. [Google Scholar] [CrossRef] [Green Version]
- Ozdil, D.; Murat Aydin, H. Polymers for medical and tissue engineering applications. J. Chem. Technol. Biotechnol. 2014, 89, 1793–1810. [Google Scholar] [CrossRef]
- Chen, J.; Que, W.; Xing, Y.; Lei, B. Molecular level-based bioactive glass-poly (caprolactone) hybrids monoliths with porous structure for bone tissue repair. Ceram. Int. 2015, 41, 3330–3334. [Google Scholar] [CrossRef]
- Lei, B.; Shin, K.H.; Noh, D.Y.; Jo, I.H.; Koh, Y.H.; Kim, H.E.; Kim, S.E. Sol–gel derived nanoscale bioactive glass (NBG) particles reinforced poly (ε-caprolactone) composites for bone tissue engineering. Mater. Sci. Eng. C 2013, 33, 1102–1108. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Que, W.; Xing, Y.; Lei, B. Content-dependent biomineralization activity and mechanical properties based on polydimethylsiloxane–bioactive glass–poly(caprolactone) hybrids monoliths for bone tissue regeneration. RSC Adv. 2015, 5, 61309–61317. [Google Scholar] [CrossRef]
- Pires, L.S.O.; Fernandes, M.H.F.V.; de Oliveira, J.M.M. Crystallization kinetics of PCL and PCL–glass composites for additive manufacturing. J. Therm. Anal. Calorim. 2018, 134, 2115–2125. [Google Scholar] [CrossRef]
- Mohammadkhah, A.; Marquardt, L.M.; Sakiyama-Elbert, S.E.; Day, D.E.; Harkins, A.B. Fabrication and characterization of poly-(ε)-caprolactone and bioactive glass composites for tissue engineering applications. Mater. Sci. Eng. C 2015, 49, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, C. Bioactive inorganic particles-based biomaterials for skin tissue engineering. Exploration 2022, 2, 20210083. [Google Scholar] [CrossRef]
- Sohrabi, M.; Eftekhari Yekta, B.; Rezaie, H.; Naimi-Jamal, M.R.; Kumar, A.; Cochis, A.; Miola, M.; Rimondini, L. Enhancing Mechanical Properties and Biological Performances of Injectable Bioactive Glass by Gelatin and Chitosan for Bone Small Defect Repair. Biomedicine 2020, 8, 616. [Google Scholar] [CrossRef] [PubMed]
- Erol-Taygun, M.; Unalan, I.; Idris, M.I.B.; Mano, J.F.; Boccaccini, A.R. Bioactıve Glass-Polymer Nanocomposites for Bone Tıssue Regeneration Applicatıons: A Revıew. Adv. Eng. Mater. 2019, 21, 1900287. [Google Scholar] [CrossRef]
- Chen, J.; Que, W.; Xing, Y.; Lei, B. Highly bioactive polysiloxane modified bioactive glass-poly (ethylene glycol) hybrids monoliths with controlled surface structure for bone tissue regeneration. Appl. Surf. Sci. 2015, 332, 542–548. [Google Scholar] [CrossRef]
- Wu, W.; Wang, W.; Li, J. Star polymers: Advances in biomedical applications. Progress Polym. Sci. 2015, 46, 55–85. [Google Scholar] [CrossRef]
- Gao, W.; Xiao, Y. Advances in cell membrane-encapsulated biomaterials for tissue repair and regeneration. Appl. Mater. Today 2022, 26, 101389. [Google Scholar] [CrossRef]
- Tavakol, S.; Azami, M.; Khoshzaban, A.; Ragerdi Kashani, I.; Tavakol, B.; Hoveizi, E.; Rezayat Sorkhabadi, S.M. Effect of laminated hydroxyapatite/gelatin nanocomposite scaffold structure on osteogenesis using unrestricted somatic stem cells in rat. Cell Biol. Int. 2013, 37, 1181–1189. [Google Scholar] [CrossRef]
- Kim, H.W.; Kim, H.E.; Salih, V. Stimulation of Osteoblast Responses to Biomimetic Nanocomposites of Gelatin-Hydroxyapatite for Tissue Engineering Scaffolds. Biomaterials 2005, 26, 5221–5230. [Google Scholar] [CrossRef]
- Sadeghi-Avalshahr, A.; Khorsand-Ghayeni, M.; Nokhasteh, S.; Mahdavi Shahri, M.; Molavi, A.M.; Sadeghi-Avalshahr, M. Effects of hydroxyapatite (HA) particles on the PLLA polymeric matrix for fabrication of absorbable interference screws. Polym. Bull. 2018, 75, 2559–2574. [Google Scholar] [CrossRef]
- Chen, J.; Que, W.; He, Z.; Zhang, X. PDMS-modified CaO-SiO2 hybrids derived by a sol–gel process for biomedical applications. Polym. Compos. 2014, 35, 1193–1197. [Google Scholar] [CrossRef]
- Hutchens, S.A.; Benson, R.S.; Evans, B.R.; O’Neill, H.M.; Rawn, C.J. Biomimetic synthesis of calcium-deficient hydroxyapatite in a natural hydrogel. Biomaterials 2006, 27, 4661–4670. [Google Scholar] [CrossRef]
- Zhang, Y.; Reddy, V.J.; Wong, S.Y.; Li, X.; Su, B.; Ramakrishna, S.; Lim, C.T. Enhanced biomineralization in osteoblasts on a novel electrospun biocomposite nanofibrous substrate of hydroxyapatite/collagen/chitosan. Tissue Eng. Part A 2010, 16, 1949–1960. [Google Scholar] [CrossRef]
- Altamura, D.; Pastore, S.G.; Raucci, M.G.; Siliqi, D.; De Pascalis, F.; Nacucchi, M.; Ambrosio, L.; Giannini, C. Scanning Small- and Wide-Angle X-ray Scattering Microscopy Selectively Probes HA Content in Gelatin/Hydroxyapatite Scaffolds for Osteochondral Defect Repair. ACS Appl. Mater. Interfaces 2016, 8, 8728–8736. [Google Scholar] [CrossRef]
- Li, M.; Liu, W.; Sun, J.; Xianyu, Y.; Wang, J.; Zhang, W.; Zheng, W.; Huang, D.; Di, S.; Long, Y.-Z. Jiang, X. Culturing Primary Human Osteoblasts on Electrospun Poly (lactic-co-glycolic acid) and Poly (lactic-co-glycolic acid)/Nanohydroxy apatite Scaffolds for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2013, 5, 5921–5926. [Google Scholar] [CrossRef] [PubMed]
- Perssona, M.; Loritea, G.S.; Kokkonena, H.E.; Choc, S.W.; Lehenkari, P.P.; Skrifvars, M.; Tuukkanena, J. Effect of bioactive extruded PLA/HA composite films on focal adhesion formation of preosteoblastic cells. Colloids Surf. B 2014, 121, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Nihonmatsu, S.; Okawara, T.; Onuki, H.; Sakagami, H.; Nakajima, H.; Takeishi, H.; Shimada, J. Adhesion and Proliferation of Osteoblastic Cells on Hydroxyapatite-dispersed Ti-based Composite Plate. In Vivo 2019, 33, 1067–1079. [Google Scholar] [CrossRef] [Green Version]
- Monmaturapoj, N.; Srion, A.; Chalermkarnon, P.; Buchatip, S.; Petchsuk, A.; Noppakunmongkolchai, W.; Mai-Ngam, K. Properties of poly (lactic acid)/hydroxyapatite composite through the use of epoxy functional compatibilizers for biomedical application. J. Biomater. Appl. 2017, 32, 088532821771578. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Que, W.; Lei, B.; Li, B. Highly Bioactive Elastomeric Hybrid Nanoceramics for Guiding Bone Tissue Regeneration. Coatings 2022, 12, 1633. https://doi.org/10.3390/coatings12111633
Chen J, Que W, Lei B, Li B. Highly Bioactive Elastomeric Hybrid Nanoceramics for Guiding Bone Tissue Regeneration. Coatings. 2022; 12(11):1633. https://doi.org/10.3390/coatings12111633
Chicago/Turabian StyleChen, Jing, Wenxiu Que, Bo Lei, and Beibei Li. 2022. "Highly Bioactive Elastomeric Hybrid Nanoceramics for Guiding Bone Tissue Regeneration" Coatings 12, no. 11: 1633. https://doi.org/10.3390/coatings12111633
APA StyleChen, J., Que, W., Lei, B., & Li, B. (2022). Highly Bioactive Elastomeric Hybrid Nanoceramics for Guiding Bone Tissue Regeneration. Coatings, 12(11), 1633. https://doi.org/10.3390/coatings12111633





