2.1. Experimental Materials
The crystal violet lactone (C26H29N3O2, MW: 415.527, CAS: 1552-42-7) was purchased from Nantong Runfeng Petrochemical Co., Ltd., Nantong, China. The bisphenol A (C15H16O2, MW: 228.286, CAS: 80-05-7) was bought from Shanghai HaiHai Chemical Co., Ltd., Shanghai, China. The tetradecanol (C14H30O, MW: 214.387, CAS: 112-72-1) was obtained from Guangzhou Jiangshun Chemical Technology Co., Ltd., Guangzhou, China. Urea (CH4N2O, MW: 60.06, CAS: 57-13-6) was purchased from Guangzhou Suixin Chemical Co., Ltd., Guangzhou, China. The 37% formaldehyde water solution (CH2O, MW: 30.03, CAS: 50-00-0) was purchased from Shandong Xinjiucheng Chemical Technology Co., Ltd., Jinan, China. Triethanolamine (C6H15NO3, MW: 149.19, CAS: 102-71-6) was acquired from Xingtai Xinlanxing Technology Co., Ltd., Xingtai, China. Acetic acid (C2H4O2, MW: 60.052, CAS: 64-19-7), citric acid monohydrate (C6H10O8, MW: 210.139, CAS: 5949-29-1), and hydrochloric acid (HCl, MW: 36.461, CAS: 7647-01-0) were purchased from Jinan Xiaoshi Chemical Co., Ltd., Jinan, China. Absolute ethanol (C2H6O, MW: 46.068, CAS: 64-17-5) was purchased from Guangzhou Chengyi Nuoyi Instrument Co., Ltd., Guangzhou, China. Arabic gum powder (CAS: 9000-01-5) was purchased from Nanjing Jinyou Biotechnology Co., Ltd., Nanjing, China. Acetone (C3H6O, MW: 58.08, CAS: 67-64-1) was purchased from Guangzhou Jiangshun Chemical Technology Co., Ltd., Guangzhou, China. Ethyl acetate (C4H8O2, MW: 88.105, CAS: 141-78-6) was purchased from Jinan Zhengkang Chemical Co., Ltd., Jinan, China. Xylene (C8H10, MW: 106.165, CAS: 1330-20-7) was purchased from Jinan Zhengkang Chemical Co., Ltd., Jinan, China. The main components of commercial photochromic microcapsules include polyformaldehyde melamine (CAS: 9003-08-1, 32–36 wt.%), styrene-maleic anhydride maleic acid monomethyl ester polymer (CAS: 31959-78-1, 6.5–8.0 wt.%), 4-(1-phenylethyl)-o-xylene (CAS: 6196-95-8, 50–60 wt.%), and 1,3,3-trimethylindoline-6’-(1-piperidinyl) spironoxazine (photochromic purple dye, CAS: 114747-45-4, 2.6–4.0 wt.%), and they were purchased from Shenzhen Oriental Rainbow Company, Shenzhen, China. Waterborne acrylic resin coating (mainly composed of waterborne acrylic emulsion, polyurethane emulsion, additives, and water) was purchased from Jiangsu Anyi Chemical Co., Ltd., Nantong, China. Alkyd resin coating was purchased from Nanjing Panfeng Chemical Co., Ltd., Nanjing, China. Aluminum alloy metal plates (50 mm × 50 mm × 0.5 mm) were purchased from Dongguan Guangouli Metal Materials Co., Ltd., Dongguan, China.
2.2. Synthesis of Color-Changing Compound and Thermochromic Microcapsules
- (1)
Synthesis of the color-changing compound
Tetradecanol (30.00 g) was first weighed and placed in a beaker. The beaker was placed in the DF-101 digital display thermostatic water bath (Shenzhen Dingxinyi Experimental Equipment Co., Ltd., Shenzhen, China), where the tetradecanol was heated to a molten state at 50 °C. Then, 1.50 g of bisphenol A and 0.50 g of crystal violet lactone were then added. The rotor was added to stir. The temperature was steadily raised to 90 °C after the mixture was well stirred. After stirring at 400 rpm for 1.5 h, the mixture became clear and transparent. The color-changing compound was a dark blue solid after cooling to room temperature.
- (2)
Preparation of the wall material prepolymer
First, 16.84 g of 37% formaldehyde water solution and 8.00 g of urea were weighed and added to a beaker. When the urea was fully dissolved, the beaker was placed in the water bath and stirred at room temperature. To adjust the pH of the solution to 8.5, a few drops of triethanolamine were added. The temperature was raised to 70 °C. The mixture was stirred at 300 rpm for 1 h.
- (3)
Preparation of core material
First, 5.48 g Arabic gum powder, 104.25 g distilled water, and 8.32 g color-changing compound were weighed and put into a beaker. The beaker was placed into a water bath at 50 °C after a rotor was added, and the mixture was then steadily stirred until the compound was entirely dissolved. The water bath was then heated to 65 °C and stirred for 20 min at 1600 rpm. The mixture was put into the TL-650CT ultrasonic emulsification disperser (Jiangsu Tianling Instrument Co., Ltd., Yancheng, China) for 5 min so that the emulsifier was equally wrapped on the outside surface of the color-changing compound.
- (4)
Encapsulation of microcapsules
The beaker containing the ultrasonic core material emulsion was put into a 35 °C water bath for gradual stirring. Drop by drop, the wall material prepolymer was mixed into the prepared core material solution. Following that, the water bath’s rotational speed was changed to 500 rpm, 1.16 g of silica and 1.16 g of sodium chloride were added, and then 8 wt.% citric acid monohydrate was gradually added. The reaction was continued for 1 h after the pH of the solution was adjusted to about 2.5. To obtain the microcapsule emulsion, the temperature was raised to 68 °C, and the stirring speed was adjusted to 250 rpm to continue the reaction for 30 min. After the microcapsule emulsion was cooled to room temperature, it was filtered by an SHZ-DIII desktop circulating water vacuum pump (Shanghai Yuhua Instrument Equipment Co., Ltd., Shanghai, China) and dried in a 35 °C 202-0AB electric constant temperature blast drying oven. The blue powder obtained was the thermochromic microcapsules.
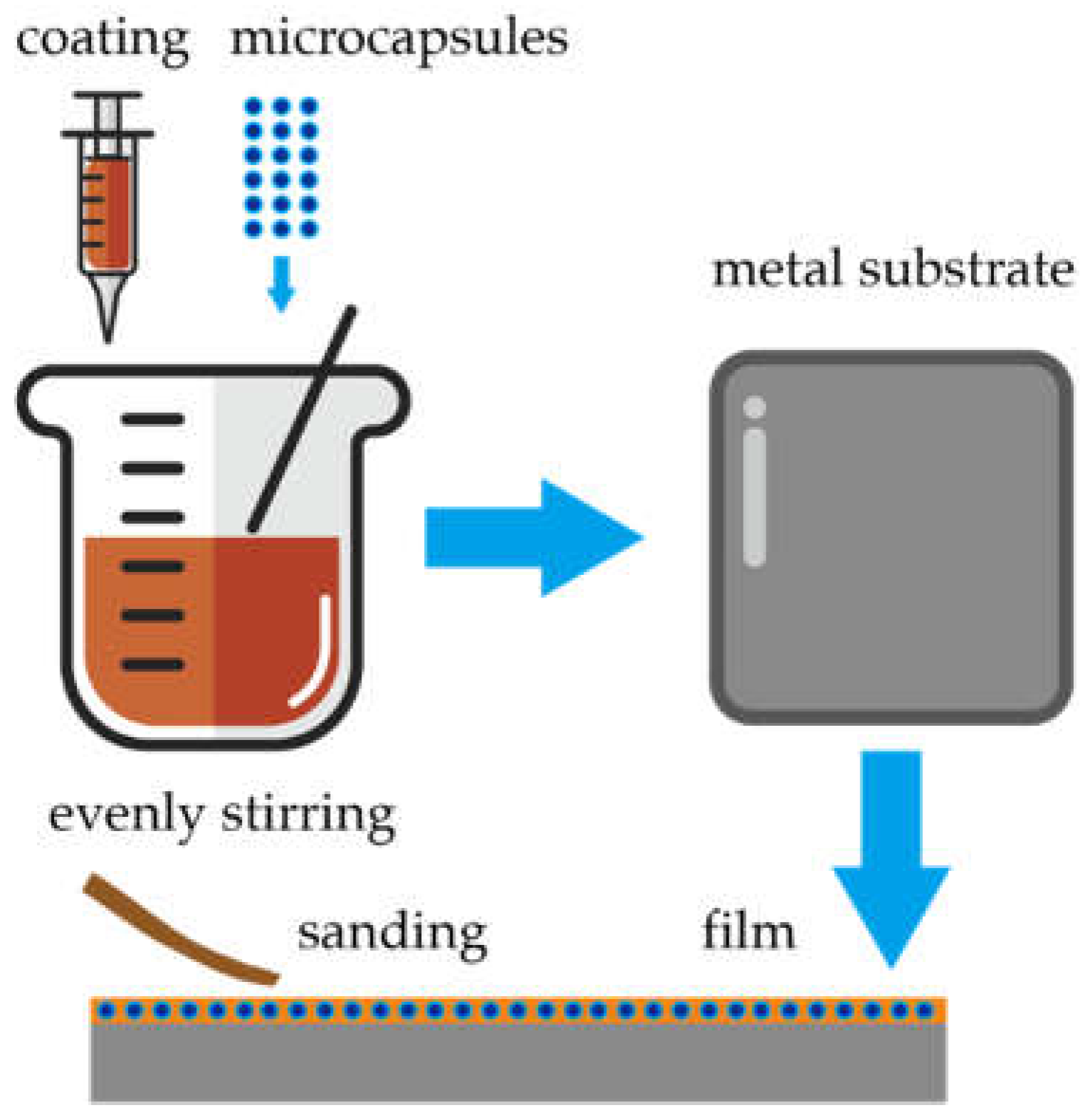
2.3. Finishing Process
A primer layer was obtained by first weighing the microcapsules and coatings of the appropriate quality according to
Table 1, stirring them evenly, and then painting them onto the surface of metal boards with a BEVS1803 coating preparation machine (Guangzhou Keyu New Material Technology Co., Ltd., Guangzhou, China). After they had been dried at room temperature for 1 h, they were finely sanded with 800 mesh sandpaper [
32]. In
Figure 1, the experimental flow is depicted. The coating was applied in a coating amount of 15–20 g/m
2, and it was then dried for 1 day in the oven at 30 °C. After drying, the thickness of the primer layer was about 60 μm [
33]. The same procedure was used to apply the topcoat. Finally, a double-layer coating on the metal substrate was successfully obtained.
In different finishing sequences, the photochromic and thermochromic microcapsules were added to the coating and coated on the metal substrate, respectively.
Table 1 displays the multi-functional coating’s finishing sequences. Alkyd resin was the foundation of the coating containing thermochromic microcapsules, while the waterborne acrylic resin was the base of the coating with photochromic microcapsules. The previous experiments showed that when our prepared thermochromic microcapsules were added to the waterborne acrylic resin coating, the discoloration performance of the microcapsules was reduced, the microcapsules in the paint film would be severely reunited, and the surface of the paint film would be uneven [
34]. Therefore, the alkyd resin coating served as the base for the combined usage of thermochromic and photochromic microcapsules.
2.4. Testing and Characterization
- (1)
Micromorphology characterization and chemical composition testing
The software “Nano measurer”, with a measurement capacity of 100, was used to gauge the particle size distribution of microcapsules [
35]. To describe the micromorphology of the prepared coating, a VERITAS scanning electron microscope (SEM, Shanghai Junzhun Instrument Equipment Manufacturer, Shanghai, China) was chosen. The ATR tablet pressing method was used to manufacture the coating, and a BOEN-85697F Fourier transform infrared spectrometer (FTIR, Fribourne Industrial Development Co., Ltd., Shanghai, China) was chosen to study the coating’s chemical composition.
- (2)
Chromatic difference testing
After a SEGT-J chromatic difference meter (Beijing Shidai Shanfeng Technology Co., Ltd., Beijing, China) was calibrated, the test hole with the sample was aligned to test and record the values of
L,
a, and
b. On one test item, a total of 4 tests were performed. The
L value denotes lightness. The color becomes brighter with the higher
L value. The red–green color is represented by the value
a. The color is red if the
a is positive. The color is green if the
a is negative. Yellow–blue color is represented by the
b value. The color is yellow when the
b is a positive value. The color is blue when the
b is negative.
L1,
a1, and
b1 represent the data of the coating before the treatment, and
L2,
a2, and
b2 represent the data of the coating after the treatment. The chromatic difference in the coating before and after treatment (Δ
E) is calculated [
36] according to Formula (1):
- (3)
Gloss testing
The gloss tests were carried out with a DT60 gloss meter (Changzhou Dude Precision Instrument, Changzhou, China) according to GB/T4893.6-2013 [
37]. After the calibration by pressing the test key, the lens cover of the gloss meter was taken off. The test sample was aligned with the test hole to record the coating gloss at 20°, 60°, and 85°. With the gloss data at a 60° incidence angle as a reference, the light loss rate of the film before and after adding microcapsules was calculated according to Formula (2).
GL stands for the light loss rate,
G0 for the gloss of the film without microcapsules, and
G1 for the gloss of the film with microcapsules.
- (4)
Mechanical properties and roughness testing
According to GB/T 6739-2006, a QHQ-A portable paint film hardness tester (6H–6B pencils, Dongguan Huaguo Precision Instrument Co., Ltd., Dongguan, China) was used to gauge the hardness of the film on the metal substrate [
38]. The values 6B–6H were from softest to hardest. The maximum hardness of the pencil was recorded as the coating’s hardness when there is no indentation on the coated surface. The adhesion grade of the coating was examined using the QFH-HG600 film scribing device from Shanghai Le’ao Test Instrument Co., Ltd., Shanghai, China. There were 6 degrees, with grade 0 denoting the coating’s best adhesion and grade 5 denoting the coating’s poorest adhesion. The impact strength of the coating was evaluated using a BEVS1601 paint film impactor tester (Guangzhou Xinyi Laboratory Equipment Co., Ltd., Guangzhou, China) in accordance with GB/T 1731-1993 [
39]. The larger the number, the better the impact resistance. An SJ-210 precise roughness tester, which is offered by Shenzhen Fengteng Precision Instrument Co., Ltd., Shenzhen, China, was used to gauge the coating’s roughness.
- (5)
Cold liquid resistance testing
Acetic acid, ethanol, coffee, and 15 wt.% NaCl solution were chosen as the cold liquid resistance testing agents of the coating to evaluate the coating’s cold liquid resistance in accordance with GB/T4893.1-2005. The coating’s center was chosen as the test area for its resistance to cold liquids. With tweezers, the filter paper from different testing agents was removed after being soaked for 5 s. It was placed on the coating surface, and then a glass cover was placed on the testing sample surface for 24 h. After removing the glass cover and the filter paper, the remaining liquid was wiped off. After the coating was completely dry, the coating surface was observed, and the chromatic difference and gloss of the testing area were tested to determine the coating’s level of cold liquid resistance. Grade 1 indicates that the testing area is not distinguished from other areas on the sample [
40].
- (6)
Ultraviolet (UV) photooxidation aging resistance testing
According to GB/T 1865-2009 [
41], the artificially accelerated aging test (UV photooxidation) was performed in a UV weather resistance test chamber (Nanjing Environmental Testing Equipment Co., Ltd., Nanjing, China). The irradiance of the xenon light is 50 W/m
2. The film based on the metal substrate was placed in the UV test chamber. Every 24 h until the coating had no discoloration performance, the chromaticity value of the coated surface was checked.