Secondary Structures on the Friction Surface of Diamond-like Coating
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rogachev, A.V.; Sidorskiy, S.S. Restoration and Increase of Wear Resistance of Machine Parts; BelTU: Belarus, Gomel, 2005; 343p. [Google Scholar]
- Aisenberg, S.; Chabot, R. Ion-Beam Deposition of Thin Films of Diamondlike Carbon. J. Appl. Phys. 1971, 42, 2953–2958. [Google Scholar] [CrossRef]
- Lettington, A.H. Applications of diamond-like carbon thin films. Carbon 1998, 36, 555–560. [Google Scholar] [CrossRef]
- Memming, R.; Tolle, H.J.; Wierenga, P.E. Properties of polymeric layers of hydrogenated amorphous carbon produced by a plasma-activated chemical vapour deposition process II: Tribological and mechanical properties. Thin Solid Film. 1986, 143, 31–41. [Google Scholar] [CrossRef]
- Grill, A. Tribology of diamondlike carbon and related materials: An updated review. Surf. Coat. Technol. 1997, 94–95, 507–513. [Google Scholar] [CrossRef]
- Tyagi, A.; Walia, R.S.; Murtaza, Q.; Pandey, S.M.; Tyagi, P.K.; Bajaj, B. A critical review of diamond like carbon coating for wear resistance applications. Int. J. Refract. Met. Hard Mater. 2019, 78, 107–122. [Google Scholar] [CrossRef]
- Martins, P.S.; Magalhães, P.A.A.; Carneiro, J.R.G.; Talibouya Ba, E.C.; Vieira, V.F. Study of Diamond-Like Carbon coating application on carbide substrate for cutting tools used in the drilling process of an Al–Si alloy at high cutting speeds. Wear 2022, 498–499, 204326. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef] [Green Version]
- Plotnikov, V.A.; Dem’yanov, B.F.; Yeliseeyev, A.P.; Makarov, S.V.; Zyryanova, A.I. Structural state of diamond-like amorphous carbon films, obtained by laser evaporation of carbon target. Diam. Relat. Mater. 2019, 91, 225–229. [Google Scholar] [CrossRef]
- Meng, W.J.; Meletis, E.I.; Rehn, L.E.; Baldo, P.M. Inductively coupled plasma assisted deposition and mechanical properties of metal-free and Ti-containing hydrocarbon coatings. J. Appl. Phys. 2000, 87, 2840–2848. [Google Scholar] [CrossRef]
- Jacob, W.; Möller, W. On the structure of thin hydrocarbon films. Appl. Phys. Lett. 1993, 63, 1771–1773. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like carbon: State of the art. Diam. Relat. Mater. 1999, 8, 428–434. [Google Scholar] [CrossRef]
- McKenzie, D.R.; Muller, D.; Pailthorpe, B.A. Compressive-Stress-Induced Formation of Thin-Film Tetrahedral Amorphous Carbon. Phys. Rev. Lett. 1991, 67, 773–776. [Google Scholar] [CrossRef] [PubMed]
- Grill, A.; Meyerson, B. Development and Status of Diamondlike Carbon. In Synthetic Diamond: Emerging CVD Science and Technology; Spear, K.E., Dismukes, J.P., Eds.; Wiley: New York, NY, USA, 1994; pp. 91–143. [Google Scholar]
- Donnet, C.; Erdemir, A. Tribology of Diamond-Like Carbon Films: Fundamentals and Applications; Springer: New York, NY, USA, 2008; 673p. [Google Scholar]
- Wang, K.; Zhou, H.; Zhang, K.; Liu, X.; Feng, X.; Zhang, Y.; Chen, G.; Zheng, Y. Effects of Ti interlayer on adhesion property of DLC films: A first principle study. Diam. Relat. Mater. 2021, 111, 108188. [Google Scholar] [CrossRef]
- Xiao, Y.; Shi, W.; Han, Z.; Luo, J.; Xu, L. Residual stress and its effect on failure in a DLC coating on a steel substrate with rough surfaces. Diam. Relat. Mater. 2016, 66, 23–25. [Google Scholar] [CrossRef]
- Liang, J.H.; Milne, Z.; Rouhani, M.; Lin, Y.-P.; Bernal, R.A.; Sato, T.; Carpick, R.W.; Jeng, Y.R. Stress-dependent adhesion and sliding-induced nanoscale wear of diamond-like carbon studied using in situ TEM nanoindentation. Carbon 2022, 193, 230–241. [Google Scholar] [CrossRef]
- Dimigen, H.; Hübsch, H.; Memming, R. Tribological and electrical properties of metal-containing hydrogenated carbon films. Appl. Phys. Lett. 1987, 50, 1056–1058. [Google Scholar] [CrossRef]
- Qiang, L.; Zhang, B.; Zhou, Y.; Zhang, J. Improving the internal stress and wear resistance of DLC film by low content Ti doping. Solid State Sci. 2013, 20, 17–22. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, D.; Wu, W. Catalysis effect of metal doping on wear properties of diamond-like carbon films deposited by a cathodic-arc activated deposition process. Thin Solid Film. 2002, 420–421, 241–247. [Google Scholar] [CrossRef]
- Gayathri, S.; Kumar, N.; Krishnan, R.; Ravindran, T.R.; Amirthapandian, S.; Dash, S.; Tyagi, A.K.; Sridharan, M. Influence of transition metal doping on the tribological properties of pulsed laser deposited DLC films. Ceram. Int. 2015, 41, 1797–1805. [Google Scholar] [CrossRef]
- Orrit-Prat, J.; Bonet, R.; Ruperez, E.; Punset, M.; Ortiz-Hernandez, M.; Guillem-Marti, J.; Lousa, A.; Cano, D.; Diaz, C.; Garci Fuentes, G.; et al. Bactericidal silver-doped DLC coatings obtained by pulsed filtered cathodic arc co-deposition. Surf. Coat. Technol. 2021, 411, 126977. [Google Scholar] [CrossRef]
- Murata, Y.; Choo, C.-K.; Ono, H.; Nagai, Y.; Tanaka, K. Characterization of N-doped DLC Thin Films Prepared by Hydrocarbons Pyrolysis Method. Mater. Today Proc. 2016, 3, 197–202. [Google Scholar] [CrossRef]
- Xiao, Y.; Sun, W.; Ma, M.; Jia, Y.-P.; Liu, J.; Zhang, T. Microstructure, wear and corrosion behavior of nano-CeO2 doped diamond-like carbon (DLC) composite films. Diam. Relat. Mater. 2022, 126, 109087. [Google Scholar] [CrossRef]
- Hofmann, D.; Kunkel, S.; Bewilogua, K.; Wittorf, R. From DLC to Si-DLC based layer systems with optimized properties for tribological applications. Surf. Coat. Technol. 2013, 215, 357–363. [Google Scholar] [CrossRef]
- Baia Neto, A.L.; Santos, R.A.; Freire, F.L., Jr.; Camargo, S.S., Jr.; Carius, R.; Finger, F.; Beyer, W. Relation between mechanical and structural properties of silicon-incorporated hard a-C:H films. Thin Solid Film. 1997, 293, 206–211. [Google Scholar] [CrossRef]
- Gershman, I.; Mironov, A.; Mezrin, A.; Torskaya, E.; Kuznetsova, T.; Lapitskaya, V.; Rogachev, A. Effect of sp3–sp2 Transformation on the Wear Rate of the DLC Coating. Lubricants 2022, 10, 85. [Google Scholar] [CrossRef]
- Weicheng, K.; Zhou, Y.; Jun, H. Effect of carburizing treatment on microstructural, mechanical and tribological performances of Cr doped DLC coating deposited on Ti6Al4V alloy. Ceram. Int. 2021, 47, 34425–34436. [Google Scholar] [CrossRef]
- Li, A.; Chen, Q.; Wu, G.; Wang, Y.; Lu, Z.; Zhang, G. Probing the lubrication mechanism of multilayered Si-DLC coatings in water and air environments. Diam. Relat. Mater. 2020, 105, 107772. [Google Scholar] [CrossRef]
- Kim, J.-I.; Lee, W.-Y.; Tokoroyama, T.; Murashima, M.; Umehara, N. Tribo-chemical wear of various 3d-transition metals against DLC: Influence of tribo-oxidation and low-shear transferred layer. Tribol. Int. 2023, 177, 107938. [Google Scholar] [CrossRef]
- Kuznetsova, T.; Lapitskaya, V.; Khabarava, A.; Trukhan, R.; Chizhik, S.; Torskaya, E.; Mezrin, A.; Fedorov, S.; Rogachev, A.; Warcholinski, B. Silicon addition as a way to control properties of tribofilms and friction of DLC coatings. Appl. Surf. Sci. 2023, 608, 155115. [Google Scholar] [CrossRef]
- Arief, I.; Mukhopadhyay, P.K. Yielding behavior and temperature-induced on-field oscillatory rheological studies in a novel MR suspension containing polymer-capped Fe3Ni alloy microspheres. J. Magn. Magn. Mater. 2017, 429, 236–240. [Google Scholar] [CrossRef]
- Guo, T.; Chen, Y.; Cao, R.; Pang, X.; He, J.; Qiao, L. Cleavage cracking of ductile-metal substrates induced by brittle coating fracture. Acta Mater. 2018, 152, 77–85. [Google Scholar] [CrossRef]
- Guo, T.; Qiao, L.; Pang, X.; Volinsky, A.A. Brittle film-induced cracking of ductile substrates. Acta Mater. 2015, 99, 273–280. [Google Scholar] [CrossRef]
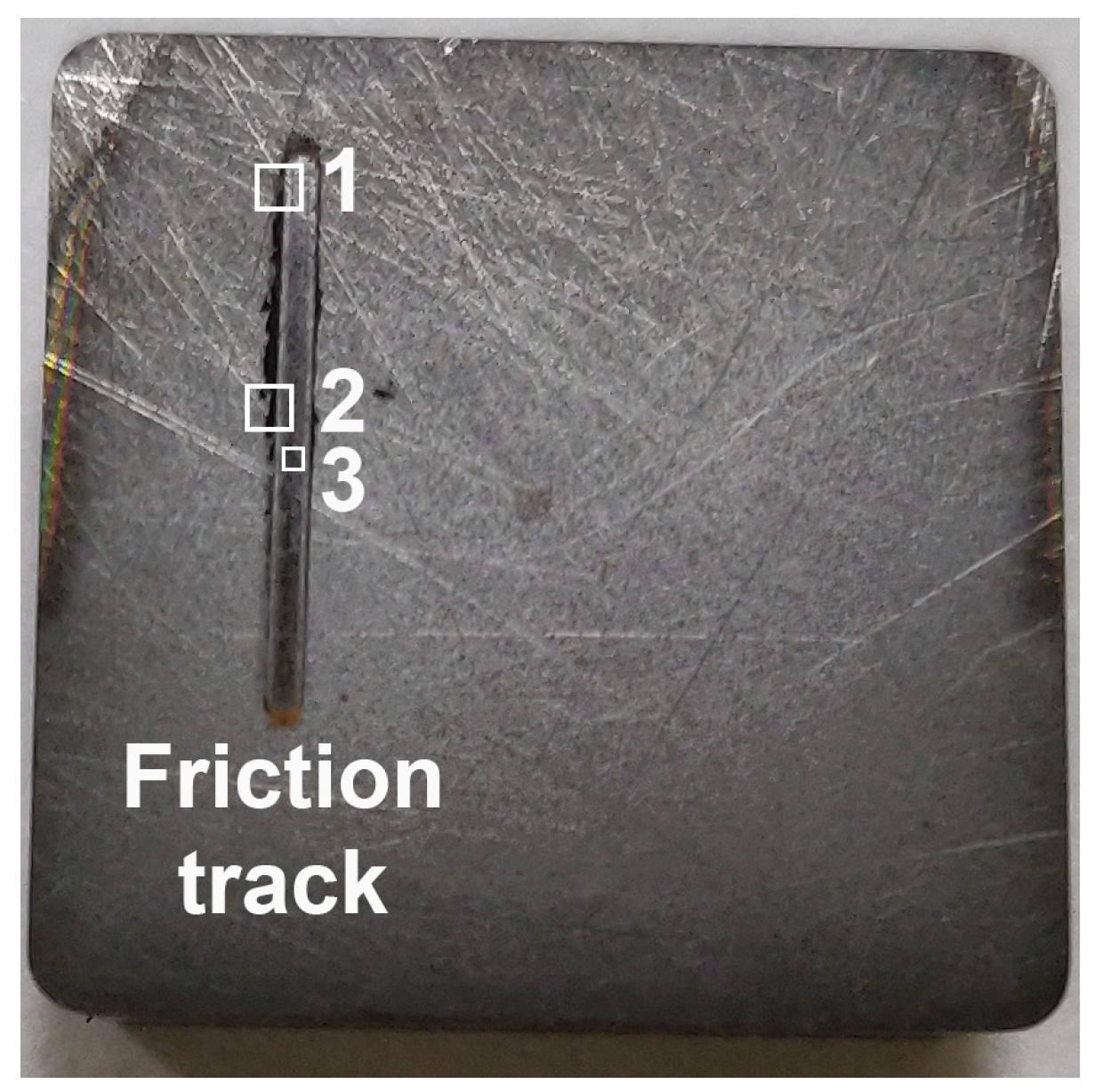
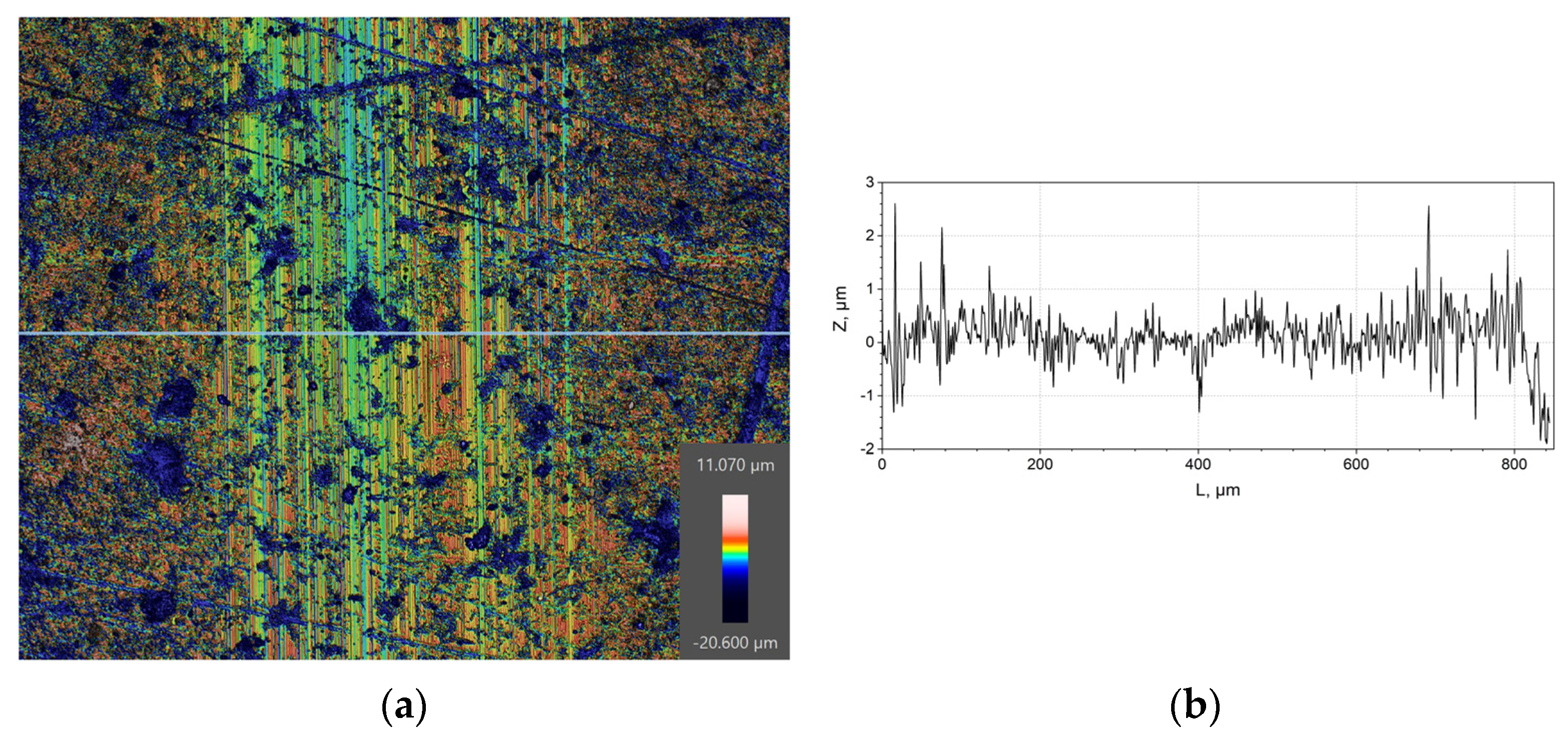

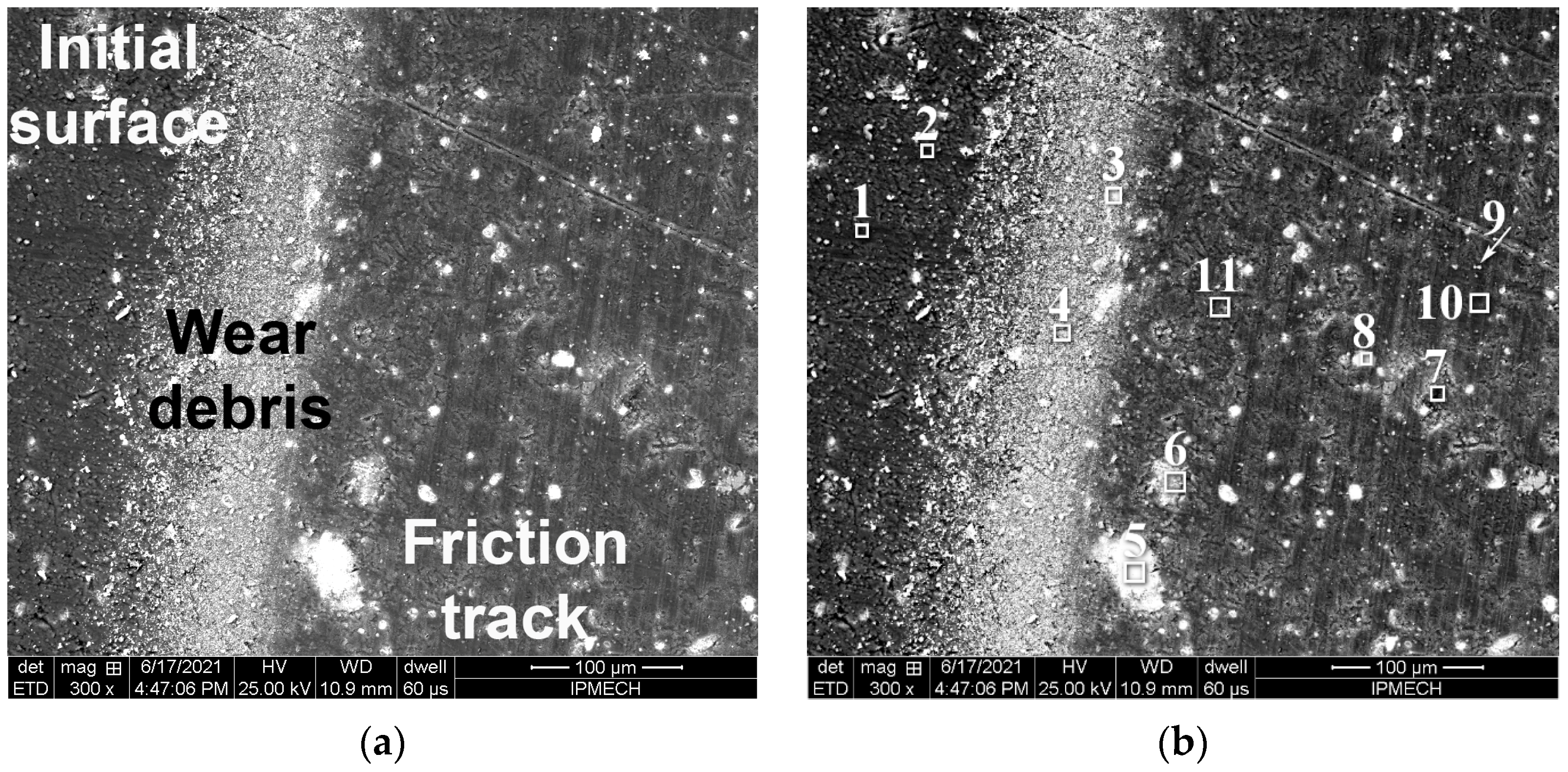

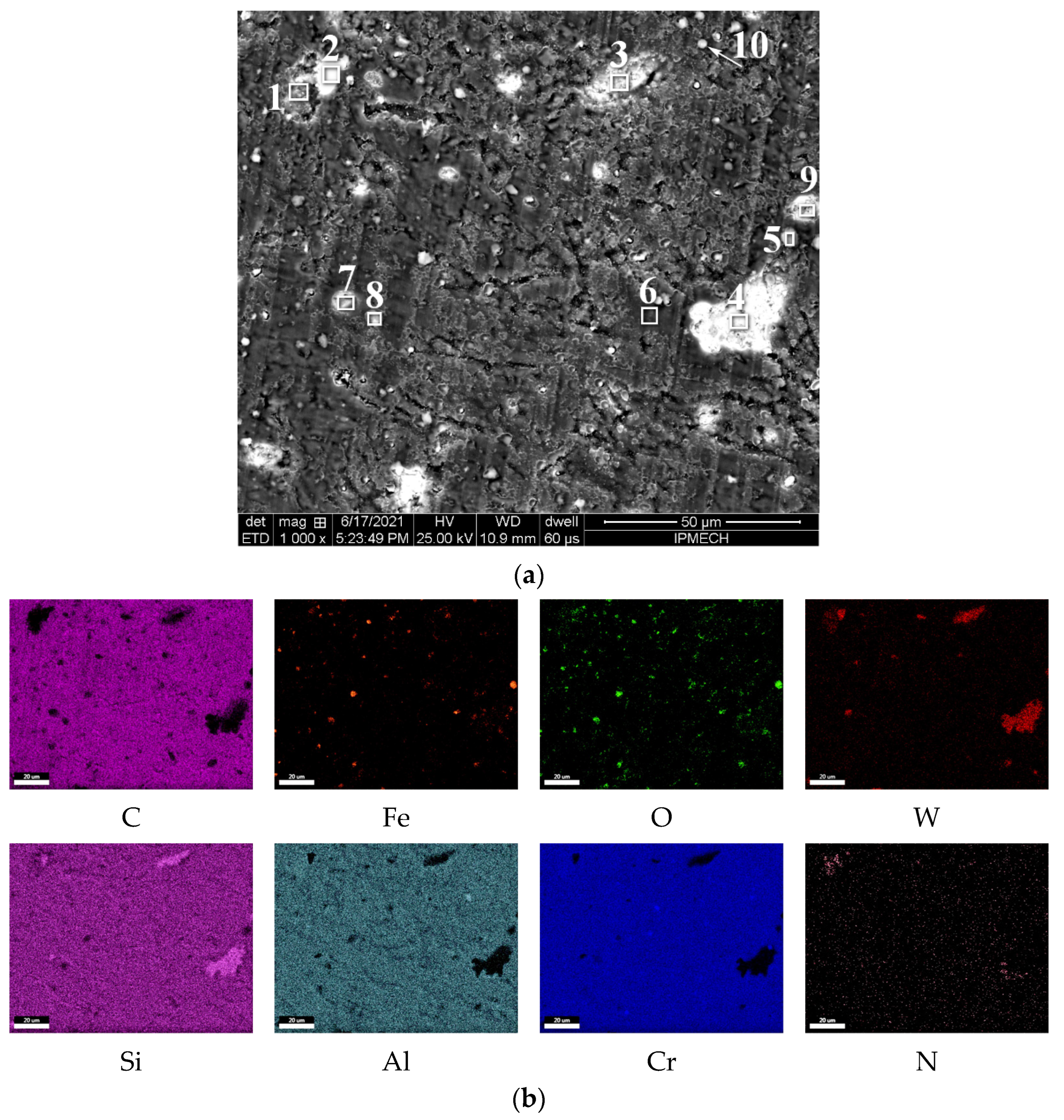
| C | Si | Mn | Ni | S | P | Cr | Cu | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.95–1.05 | 0.17–0.37 | 0.2–0.4 | up to 0.3 | up to 0.02 | up to 0.027 | 1.3–1.65 | up to 0.25 | bal. |
| Zone | Content of Element, wt.% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | O | Al | Si | Cr | Mn | Fe | Ni | Cu | Co | W | C | Other | |
| 1–1 | 1.77 | 12.29 | 0.45 | 0.23 | 5.04 | 0.69 | 65.80 | 0.23 | 0.04 | 4.53 | 0.72 | 8.16 | bal. |
| 1–2 | 1.03 | 8.45 | 0.22 | 0.51 | 4.74 | 0.65 | 71.06 | 0.23 | 0.04 | 4.79 | 0.71 | 7.52 | bal. |
| 1–3 | 6.04 | 0.43 | 3.45 | 3.82 | 17.60 | 1.02 | 0.05 | 0.02 | 0.01 | 0.65 | 0.87 | 65.78 | bal. |
| 1–4 | 6.77 | 0.40 | 3.24 | 3.30 | 15.90 | 0.97 | 0.04 | 0.02 | 0.01 | 0.57 | 3.67 | 64.91 | bal. |
| 1–5 | 13.89 | 1.11 | 2.02 | 1.96 | 11.88 | 0.76 | 0.06 | 0.02 | 0.02 | 0.22 | 0.70 | 67.29 | bal. |
| 1–6 | 16.73 | 1.91 | 10.40 | 5.26 | 32.76 | 2.23 | 0.26 | 0.21 | 0.06 | 3.78 | 17.95 | 7.44 | bal. |
| 1–7 | 11.44 | 1.56 | 8.97 | 5.77 | 31.96 | 1.71 | 0.05 | 0.06 | 0.00 | 3.10 | 20.80 | 13.73 | bal. |
| 1–8 | 4.84 | 1.24 | 3.02 | 3.75 | 17.43 | 1.18 | 0.26 | 0.11 | 0.08 | 1.15 | 4.76 | 61.87 | bal. |
| 1–9 | 0.03 | 1.99 | 0.37 | 3.66 | 4.48 | 0.71 | 1.36 | 0.64 | 0.37 | 8.00 | 30.25 | 46.07 | bal. |
| 1–10 | 4.67 | 1.21 | 3.70 | 3.83 | 17.77 | 1.10 | 0.19 | 0.05 | 0.02 | 0.82 | 4.84 | 61.52 | bal. |
| Zone | Content of Element, wt.% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | O | Al | Si | Cr | Mn | Fe | Ni | Cu | Co | W | C | Others | |
| 2–1 | 9.43 | 1.07 | 2.55 | 2.95 | 12.27 | 0.73 | 0.05 | 0.02 | 0.02 | 0.19 | 1.69 | 68.93 | bal. |
| 2–2 | 4.69 | 0.28 | 3.38 | 3.71 | 16.66 | 1.07 | 0.08 | 0.05 | 0.03 | 0.72 | 4.42 | 64.68 | bal. |
| 2–3 | 14.85 | 18.27 | 4.52 | 2.16 | 12.59 | 1.05 | 23.66 | 0.03 | 0.02 | 1.42 | 0.95 | 20.39 | bal. |
| 2–4 | 3.88 | 2.39 | 3.20 | 4.06 | 18.34 | 1.26 | 1.36 | 0.06 | 0.03 | 0.82 | 4.90 | 59.49 | bal. |
| 2–5 | 0.31 | 2.34 | 0.56 | 5.20 | 3.14 | 0.35 | 1.35 | 0.35 | 0.07 | 6.69 | 33.24 | 44.92 | bal. |
| 2–6 | 12.97 | 1.61 | 9.60 | 5.48 | 32.31 | 2.26 | 0.42 | 0.26 | 0.12 | 3.43 | 20.94 | 9.32 | bal. |
| 2–7 | 0.12 | 2.58 | 4.51 | 4.26 | 22.76 | 1.79 | 1.01 | 0.26 | 0.20 | 3.60 | 19.90 | 37.84 | bal. |
| 2–8 | 0.03 | 2.14 | 0.00 | 5.65 | 1.53 | 0.15 | 0.66 | 0.15 | 0.01 | 3.99 | 66.82 | 16.26 | bal. |
| 2–9 | 3.19 | 17.18 | 2.47 | 2.32 | 16.90 | 1.61 | 20.22 | 0.07 | 0.02 | 1.65 | 1.82 | 32.43 | bal. |
| 2–10 | 3.62 | 0.60 | 4.05 | 3.61 | 18.34 | 1.25 | 0.20 | 0.13 | 0.10 | 1.76 | 6.76 | 59.13 | bal. |
| 2–11 | 5.58 | 0.48 | 3.40 | 3.02 | 16.18 | 1.04 | 0.10 | 0.08 | 0.03 | 1.12 | 5.03 | 63.68 | bal. |
| Zone | Content of Element, wt.% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | O | Al | Si | Cr | Mn | Fe | Ni | Cu | Co | W | C | Others | |
| 3–1 | 15.93 | 1.81 | 9.36 | 6.05 | 27.94 | 1.83 | 0.21 | 0.24 | 0.04 | 4.40 | 21.71 | 9.59 | bal. |
| 3–2 | 0.97 | 10.87 | 0.36 | 4.52 | 4.52 | 0.74 | 5.62 | 0.33 | 0.28 | 4.71 | 49.68 | 14.84 | bal. |
| 3–3 | 0.00 | 1.49 | 0.32 | 5.55 | 2.22 | 0.34 | 0.78 | 0.27 | 0.12 | 4.16 | 50.07 | 32.54 | bal. |
| 3–4 | 0.02 | 1.78 | 0.03 | 5.86 | 1.50 | 0.20 | 0.74 | 0.25 | 0.05 | 4.55 | 59.81 | 22.91 | bal. |
| 3–5 | 1.56 | 25.18 | 1.55 | 2.71 | 6.25 | 0.76 | 29.20 | 0.15 | 0.05 | 3.05 | 7.98 | 21.15 | bal. |
| 3–6 | 3.19 | 0.25 | 3.83 | 3.82 | 18.29 | 1.26 | 0.20 | 0.14 | 0.13 | 1.33 | 7.44 | 59.57 | bal. |
| 3–7 | 0.58 | 0.98 | 0.42 | 5.30 | 4.88 | 0.61 | 0.60 | 0.42 | 0.17 | 7.45 | 50.88 | 25.24 | bal. |
| 3–8 | 3.39 | 18.22 | 3.77 | 3.44 | 14.47 | 1.25 | 12.04 | 0.08 | 0.03 | 1.62 | 4.92 | 36.53 | bal. |
| 3–9 | 5.55 | 2.58 | 1.96 | 4.88 | 5.96 | 0.72 | 0.52 | 0.38 | 0.20 | 4.86 | 42.14 | 28.03 | bal. |
| 3–10 | 3.51 | 19.40 | 2.83 | 2.94 | 14.73 | 1.29 | 11.93 | 0.05 | 0.03 | 1.19 | 2.80 | 39.15 | bal. |
| Zone | Content of Element, wt.% | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | O | Al | Si | Cr | Mn | Fe | Ni | Cu | Co | W | Others | C | |
| Average composition of the initial surface | 8.7 | 0.72 | 2.8 | 2.98 | 14.18 | 0.88 | 0.06 | 0.03 | 0.02 | 0.43 | 2.62 | 0.15 | 66.45 |
| Secondary structures with wear near 10% | 3.19 | 0.25 | 3.83 | 3.82 | 18.29 | 1.26 | 0.20 | 0.14 | 0.13 | 1.33 | 7.44 | 0.55 | 59.57 |
| Secondary structures with wear near 85% | 15.93 | 1.81 | 9.36 | 6.05 | 27.94 | 1.83 | 0.21 | 0.24 | 0.04 | 4.4 | 21.71 | 0.89 | 9.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gershman, I.; Mironov, A.; Fox Rabinovich, G.; Muravyeva, T.; Shkalei, I.; Shcherbakova, O.; Torskaya, E.; Fedorov, S.; Endrino, J.L. Secondary Structures on the Friction Surface of Diamond-like Coating. Coatings 2022, 12, 1685. https://doi.org/10.3390/coatings12111685
Gershman I, Mironov A, Fox Rabinovich G, Muravyeva T, Shkalei I, Shcherbakova O, Torskaya E, Fedorov S, Endrino JL. Secondary Structures on the Friction Surface of Diamond-like Coating. Coatings. 2022; 12(11):1685. https://doi.org/10.3390/coatings12111685
Chicago/Turabian StyleGershman, Iosif, Alexander Mironov, German Fox Rabinovich, Tamara Muravyeva, Ivan Shkalei, Olga Shcherbakova, Elena Torskaya, Sergey Fedorov, and Jose Luis Endrino. 2022. "Secondary Structures on the Friction Surface of Diamond-like Coating" Coatings 12, no. 11: 1685. https://doi.org/10.3390/coatings12111685





