Abstract
Electrolytic plasma thermocyclic surface hardening is an attractive solution for both chemical and heat treatment used to improve the properties of the steel surface by structural and phase transformation. Structural and phase transformations occurring during the process of electrolytic plasma thermocyclic hardening are performed repeatedly at varying heating–cooling temperatures, which radically improve the quality of the part and give them properties unattainable by means of one-time processing. The impact of electrolytic plasma thermocyclic hardening modes on the structure and mechanical and tribological properties of 30CrMnSiA steel is investigated. The structural and phase components were examined using optical and scanning electron microscopy, as well as X-ray phase analysis. It is established that the structure of the cross-section is characterized by the following zonality: zone 1—a near-surface hardened zone, which is composed of hardened martensite; zone 2—thermal influence; and zone 3—a matrix consisting of pearlite and ferrite. The microhardness and wear resistance of the hardened surface were evaluated by nanoindentation and “ball on disk” methods, respectively. Nanoindentation analysis demonstrated that the indentation hardening process provides a maximum increase in hardness by three times and an increase in stiffness with a decrease in the elastic modulus by 38% compared to the original steel. The results of tribological studies show that electrolytic plasma thermocyclic hardening increases the resistance of steel to friction by increasing the surface hardness and reduces the area of actual contact during friction. It is established that the microhardness of the cross-section decreases proportionally from the surface to the depth of the layer, which is associated with a decrease in the volume content of martensite.
1. Introduction
The operation of many machine parts occurs under conditions of multifactorial external influence [1,2]. One of the conditions for increasing the operational properties of such parts is the formation of a structure with high-performance characteristics on the surface layer [3]. Therefore, surface hardening remains one of the priority methods for strengthening the surface layers of machine parts. It should be noted that the most common method of surface quenching is represented by quenching with high frequency currents [4,5]. Ensuring the required quality of the hardened surface layer depends, first of all, on the processing modes; however, the high cost of equipment and the low efficiency of the use of material and energy resources limit the utilization of this technology.
Traditionally, only heating and cooling steel has been the most commonly used surface treatment method that existed over time. Conventional heat treatment consists of heating the metal in a furnace and cooling it in air, water, or oil [6]. All these processes require expensive setup and take longer processing times, as well as some of them requiring cumbersome equipment. [7,8]. Electrolytic plasma surface hardening (heating-quenching) is a new method that allows the overcoming of all the aforementioned disadvantages [9,10]. This proves that it can successfully improve the desired physical and mechanical properties in much less time, about a few seconds, when compared to traditional heat treatment processes that require hours and days. Electrolytic plasma hardening (heating-quenching) is a special thermomechanical process in which an electrolyte is used in an aqueous solution under certain conditions, for example, at voltage, current, electrolyte, duration, and rate of heating-quenching [11,12]. In the course of electrolytic plasma hardening, electrical energy is usually transferred from the metal anode to the workpiece itself through the layers of electrolyte and plasma. The plasma layer is formed from the electrolyte material in the gap between the liquid electrode and the conductive surface of the workpiece. Electrolytic plasma hardening is a complex process that combines physical metallurgy and electrochemical processes, such as sample heating in the cathode mode, where phase transformations and deformation occur simultaneously [13,14].
The authors [15] investigated the electrolytic plasma surface hardening of 20X2H4A steel. The application of the electrolytic plasma surface quenching process resulted in a finer microstructure and an increase in hardness due to high heating and cooling rates. Similar results were shown in [16,17], where there was a significant increase in the hardness and wear resistance of tools and highly loaded parts after electrolytic plasma quenching. Aysun Ayday et al. [18] studied the effect of the electrolytic plasma surface hardening on the microstructure and hardness of AISI 1040 steel. The results showed that the microstructure of the hardened zone consisted of phases of martensite. The transition zone consisted of hardened sorbite. It was found that the microhardness was close to 1050 HV—four times higher than that of the base material.
It is known that obtaining the microcrystalline structure of steel with higher values (20%–30% higher) of strength characteristics, including fatigue strength, is obtained by thermocyclic treatment (TCT) [19]. In thermal treatment technology, TDC is usually performed by furnace heating [20]. It is of interest to investigate the possibility of electrolyte plasma thermocyclic treatment of product surface areas [21,22]. The periodic change of electric field intensity between the surfaces of liquid electrode and article changes the power density of surface heating, which in turn provides control of electrolyte-plasma heating and creation of necessary thermal modes for formation of hardening structures [23,24].
The effectiveness of the influence of electrolytic plasma thermocyclic treatment on the structure and properties of steel is largely determined by the mode of its implementation, that is, the temperatures in the cycle, the number of cycles, as well as the rate of heating and cooling [25]. The contradictory understanding of their mutual influence has created prerequisites for the use of a wide range of methods of electrolytic plasma thermocyclic treatment, varying not only in the principle of influencing the structure (with complete phase transformations, with partial phase transformations or without them) but also, most importantly, differing up to 20–50 times in energy consumption to obtain the desired result [26,27]. In this regard, the development and introduction of new, more efficient technologies for strengthening structural steels that improve the quality of the finished part and, ultimately, its operational durability, which provides a significant reduction in energy consumption, are aimed at solving and theoretically substantiating a scientific problem of the national economic importance.
In this regard, the purpose of this work is to study the effect of EPTCH modes on the structural-phase state and mechanical-tribological properties of 30CrMnSiA steel.
2. Materials and Methods
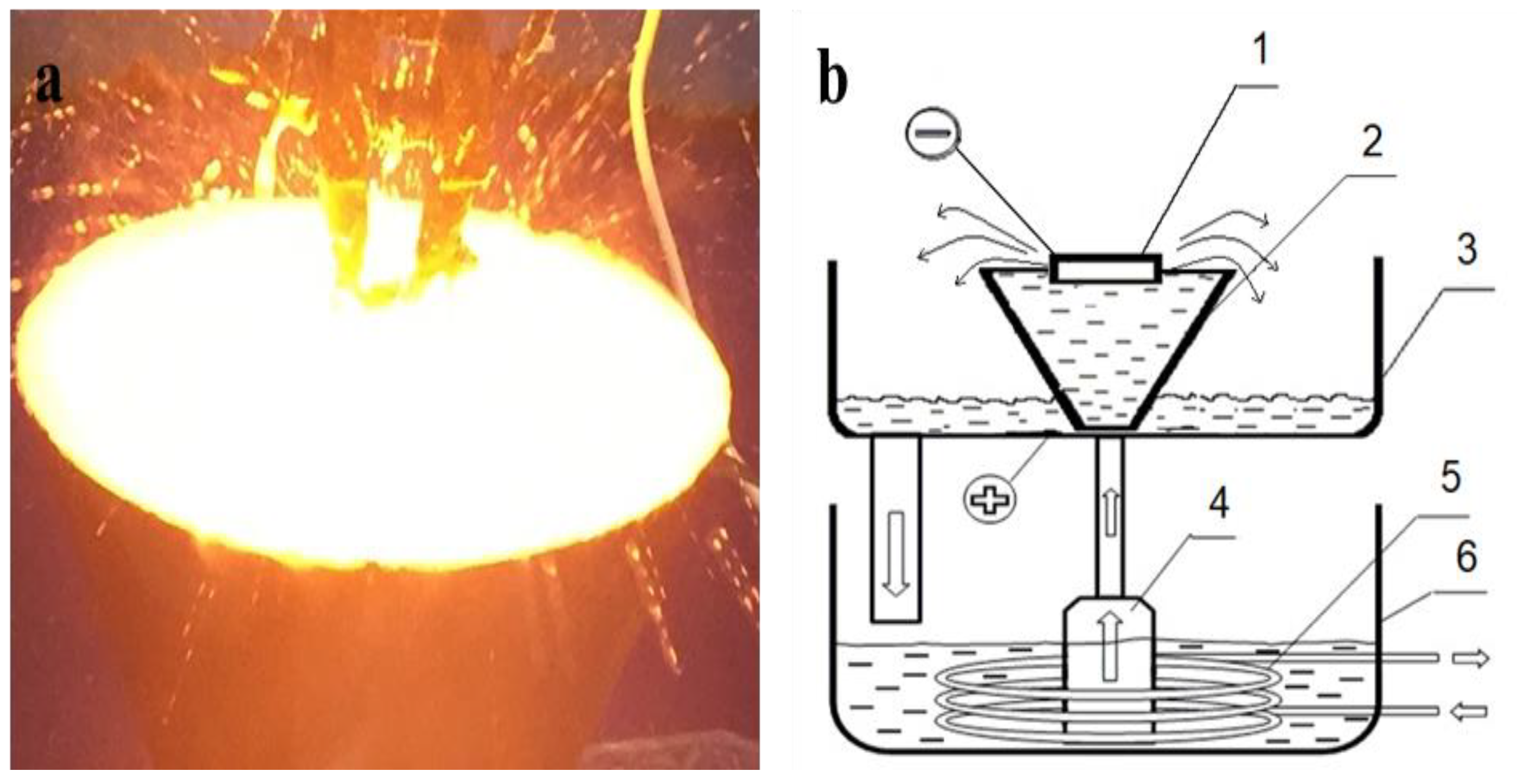
Samples of 30 CrMnSiA steel in delivery condition (quenched at 880 °C and tempered at 540 °C in oil in accordance with GOST 4543-71) were used as a material for the study. The chemical composition of steel: 0.28%–0.35% C; 0.8%–1.1% Cr; 0.8%–1.1% Mn; 0.9%–1% Si; 0.025% P; 0.025% S; and the rest Fe according to GOST 4543-71. Prior to the experimental treatment, the surface of the 2 × 2 × 1 cm3 steel samples was sanded on sanding paper with a grain size from P100 to P2000, then polished on velvet and nylon fabrics with diamond pastes of 0.25–0.5 microns and cleaned with alcohol. The EPTCH of steel was carried out in the cathode mode at the electrolyte-plasma treatment plant [28,29], the scheme of which is shown in Figure 1b. The power source was a powerful rectifier, which gives a maximum output value of 360 V/100 A in the form of direct current [30,31]. A solution of sodium carbonate (Na2CO3) was used as a source of heating and cooling. The composition of the electrolyte is 85% distilled water and 15% sodium carbonate (wt. %). The voltage value (U, V) and processing time (t, s) were different for each sample. The samples were hardened at voltages of 320 V, 250 V, and 50 V sequences. Periodic changes in the electric field strength between the surfaces of the liquid electrode and the product change the power density of surface heating, which provides control over the electrolyte-plasma heating and ensures the necessary thermal conditions are provided for the formation of hardening structures. The modes of EPTCH are given in Table 1.
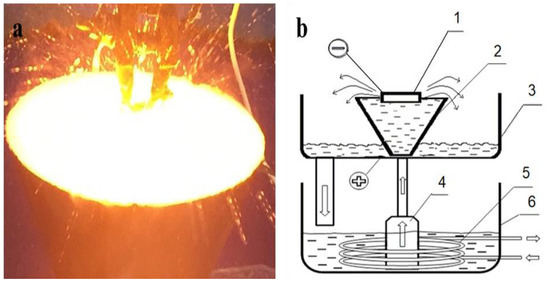
Figure 1.
The process of processing the sample with electrolytic plasma (a) and the functional diagram of the installation (b): 1—the workpiece; 2—a cone-shaped electrolytic cell made of stainless steel; 3—a pallet; 4—a pump; 5—a heat exchanger; 6—a bath with electrolyte.

Table 1.
Technological parameters of the modes of EPTCH steel 30CrMnSiA.
Experimental studies were conducted on the basis of the Scientific Research Center for “Surface Engineering and Tribology” of the S. Amanzholov East Kazakhstan University (Ust-Kamenogorsk, Kazakhstan). The phase composition was determined using an X’pertpro X-ray diffractometer (PANalytical, Amsterdam, Netherlands) with CuKa radiation at 40 kV and 30 mA, the scanning parameters were 35° < 2θ < 85° in 0.02° increments, and the exposure time was 5 s. Data processing and quantitative analysis were carried out using PowderCell 2.4 (Kraus W, Nolze G., 2000, PowderCell for Windows, version 2.4) software. The microstructure of the samples was revealed by chemical etching using a 4% solution of nitric acid (HNO3) in ethyl alcohol. This reagent has been proposed for a long time and remains one of the most widely used reagents in metallographic practice [32,33]. The microstructure was studied using an Altami 5C (Altami Ltd., Saint-Petersburg, Russia) optical metallographic microscope in reflected light at a light field. A scanning electron microscope (SEM) (MIRA3 LMU, TESCAN, Brno, Czech Republic) was used to study the structure at ×4000, ×10000 magnifications. The thickness of the diffusion zone, which had a clear interface with the hardened sublayer, and the thickness of the hardened zone were estimated from cross-sectional microstructure images, as well as analyzing the nature of the microhardness distribution over depth. To determine the hardness by the depth of the samples, a Vickers micro-hardness tester (Metolab 502, Saint-Petersburg, Russia) equipped with a diamond indenter and a load sensor up to 1000 g was used. A nanoindentation tester (Nanoscan 4D compact, Moscow, Russia) equipped with a Berkovich diamond tip, which has a triangular pyramidal shape (nominal angle of 65.3° and the diameter of 200 nm), was used to determine hardness and modulus of elasticity. Five series of tests were carried out for each sample, and the values obtained were averaged to obtain the final value.
One of the main methods of experimental investigation of the mechanical properties of micro- and nanocomposite materials is the indentation method. Thus far, various types of continuous nanoindentation tests are increasingly being used in practice to measure the mechanical properties of materials, due to the high accuracy with which elastic characteristics are measured. The indentation method determines the hardness, Young’s modulus, and elastic recovery of both superhard and soft materials using small loads. Nanoindentation analysis was carried out to evaluate the mechanical properties on a micro-scale of hardened 30CrMnSiA steel samples. The modulus of elasticity is determined by the method of indentation on a complex measuring system according to the Oliver–Farr method [34]. It allows us to distinguish two components from the information contained in the unloading branch of the P−h diagram (force-deformation)—related to the elastic part of the achieved deformation and energy and to the plastic one. The Young’s modulus E is determined by the measured elastic stiffness of the contact S = ∂P/∂h at the unloading stage from the following expression:
here Er = [(1 − ν 2 s)/Es + (1 − ν 2 i)/Ei] − 1 is the reduced Young’s module, taking into account the individual Young modules E and Poisson coefficients v of the contacting bodies, i.e., the sample (with index s) and the indenter (with index i), and Ac is the contact area. Nanohardness H is defined as [35]:
The tribological characteristics of steel were measured in the sliding friction mode according to the “ball-on-disk” scheme on Anton Paar TRB3 tribometer (CSM Instruments, Bern, Switzerland). The sample rotation speed was 2 cm/s, the load was 6 N, and a ball of Si3N4 (Silicon Nitride) with a diameter of 6 mm was used as a counterbody. The total sliding distance reached 60 m, which is enough for the wear testing of the hardened layer. Wear tracks were investigated using a noncontact 3D profilometer MICROMEASURE 3D station (STIL SAS, Aix-en-Provence, France). The surface roughness (Ra) of steel before and after EPTCH was evaluated using a profilometer model 130 (JSC “Plant Proton”, Moscow, Russia).
3. Results and Discussion
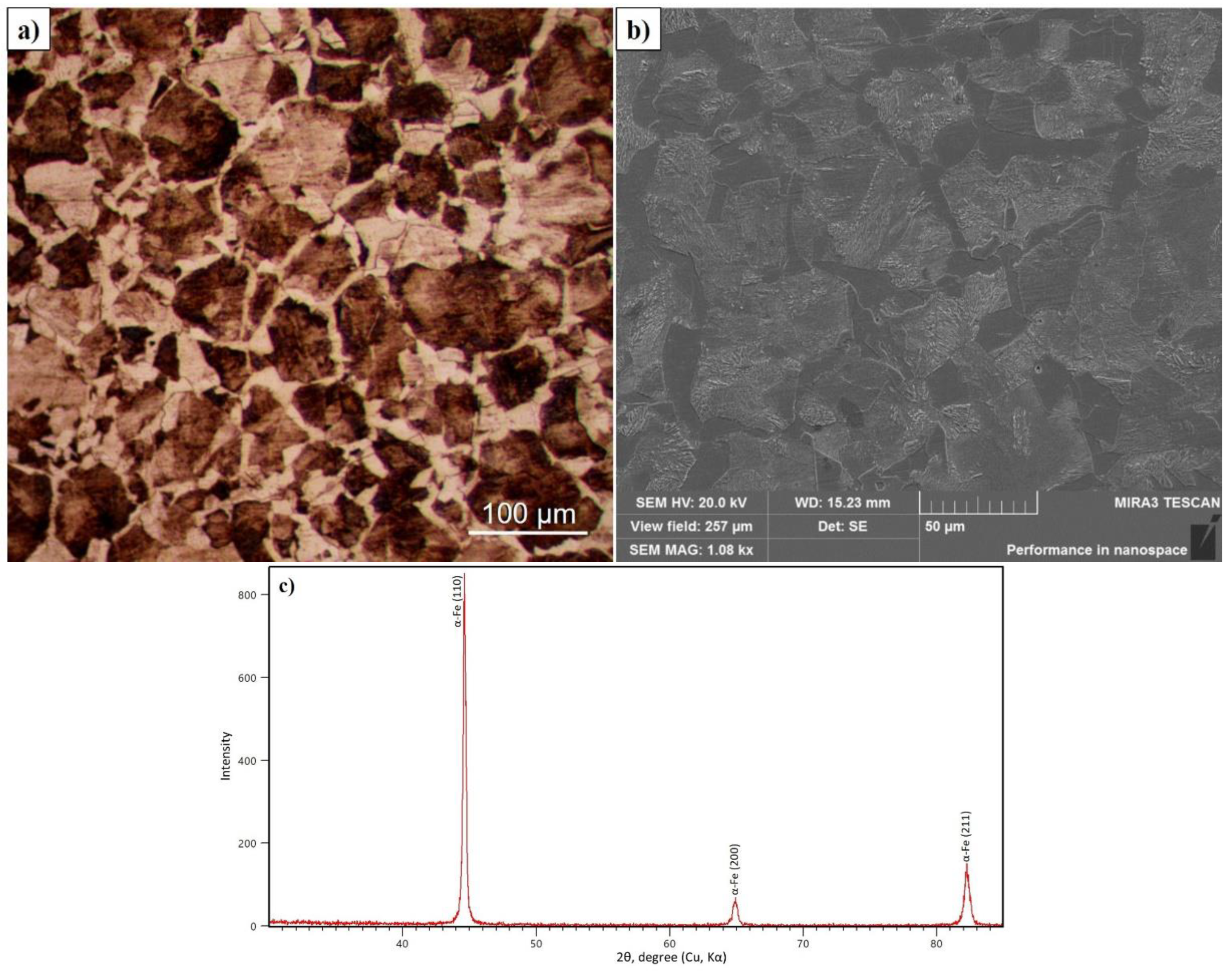
The microstructure of the surface and the diffractogram of 30CrMnSiA steel before EPTCH are shown in Figure 2a–c. As shown in the optical and SEM micrographs, the main phase component of the initial state of 30CrMnSiA steel is ferrite and pearlite [36]. Figure 2 a,b shows that the interplate distance of ferrite (light) and pearlite (dark) looks different depending on the angle to the surface. The thickness of the plates is beyond the resolution of the lenses of an optical microscope, so the grains of pearlite after etching with a 4% solution of nitric acid are colored in dark light [37]. The phase composition of 30CrMnSiA steel samples before EPTCH is investigated. In Figure 2 a diffractogram of 30CrMnSiA steel is presented, and according to X-ray diffraction analysis in the initial state, 30CrMnSiA steel implies an α-Fe phase with a BCC lattice.
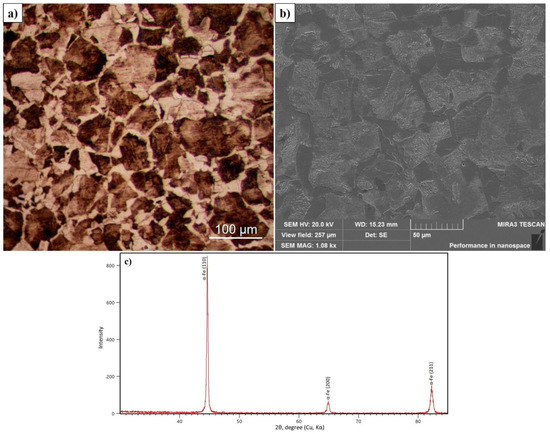
Figure 2.
Micrographs of the initial state of 30CrMnSiA steel obtained by optical (a) and scanning (b) microscopes and its diffractogram (c).
As is known, the results of the microstructure assessment significantly affect the determination of the nature of changes in the properties of the material in the process of temperature exposure to the material.
One of the essential parameters determining the nature of structural changes during electrolytic plasma thermocyclic heating is a periodic temperature increase at which the metal is heated above the temperature of the phase transition α→γ, which is known as the austenitization process [38]. Ferrite and cementite are unstable at such high temperatures and dissolve when heated. The resulting austenite provides enough space between the iron atoms for the carbon atoms of the substance. It dissolves in a lattice of iron [39]. According to the Fe-C phase diagram, it is known that after EPTCH, the steel cools from the AC1 point to room temperature, the arrangement of the iron atoms will change to each other again. The transformation of the γ-phase into the α-phase is explained by the high rate of electrolyte cooling [40]. The high cooling rate of the electrolyte does not allow carbon to be properly distributed to obtain the phases of pearlite or bainite, which leads to the formation of martensite. This tension makes the steel rigid. As we know, martensite is the main structural component of hardened steel, which is an ordered supersaturated solid solution of carbon in α-iron [41].
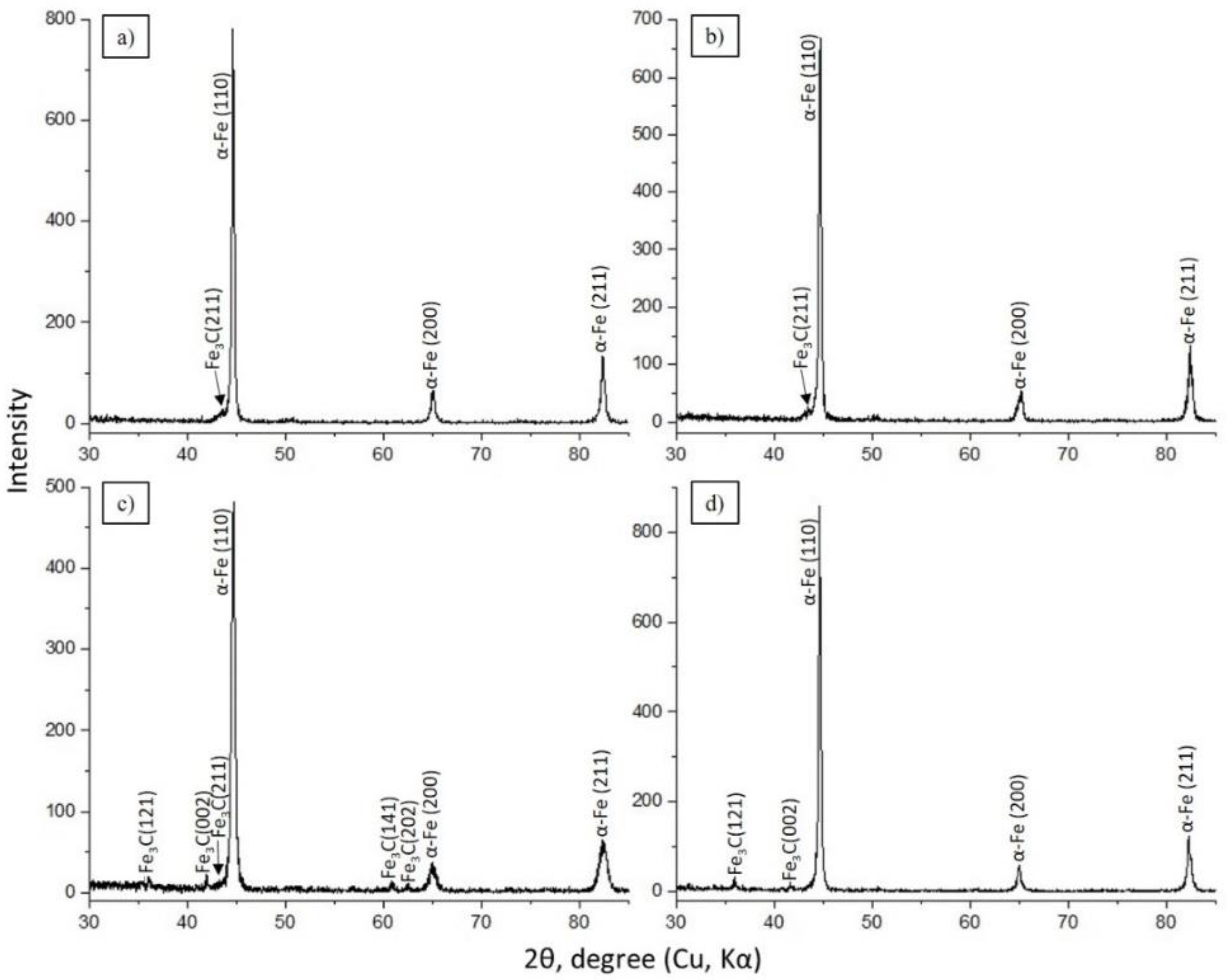
The determination of morphological phase components of structures in the material is undoubtedly an important task for establishing the optimal mode of hardening of the EPTCH, which can be implemented using X-ray diffraction analysis methods on an X-ray diffractometer. Figure 3 illustrates the results of the obtained diffractograms of 30CrMnSiA steel samples treated with EPTCH according to the modes No. 1, No. 2, No. 3, and No. 4. As shown in Figure 3a–d, the phase structure of 30CrMnSiA steel mainly implies of α’–Fe and Fe3C grains, which are α’-martensite and Fe3C–cementite (iron carbide), as well as the broadening of interference lines from the crystallographic plane (110). The broadening of the interference lines of the plane (110) and the increase in their relative intensity are presumably associated with an increase in the density of dislocations during the formation of martensite and is determined mainly by the tetragonality of martensite, which is consistent with the results reported by other authors [42,43,44]. However, it is known that with the help of X-ray diffraction analysis, it is possible to detect the presence of any phase with its content in the amount of at least 2%, and to identify phases in smaller quantities, it is necessary to use other methods of analysis.
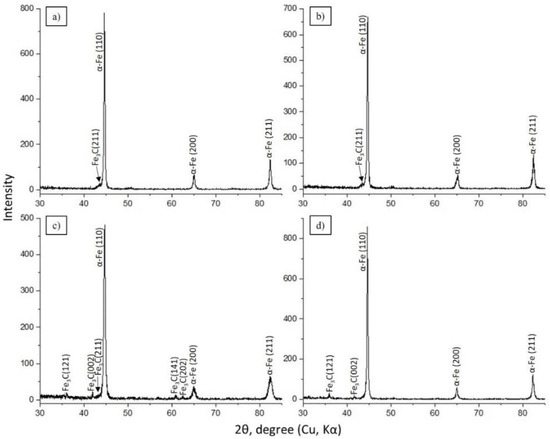
Figure 3.
Samples processed according to the modes: (a) No. 1; (b) No. 2; (c) No. 3; and (d) No. 4 (Table 1). Diffractogram of 30CrMnSiA steel after EPTCH.
It should be noted that the martensitic transformation during cooling does not occur at a constant temperature but in a certain temperature range, while the transformation does not begin at the temperature of austenite decay under equilibrium conditions but several hundred degrees below. Phase transformations end at a temperature that is well below room temperature. As a result, phase and structural changes occur in the heating zone: the formation of austenite, the dissolution of the carbide phase during heating, and the transformation of austenite into martensite during cooling.
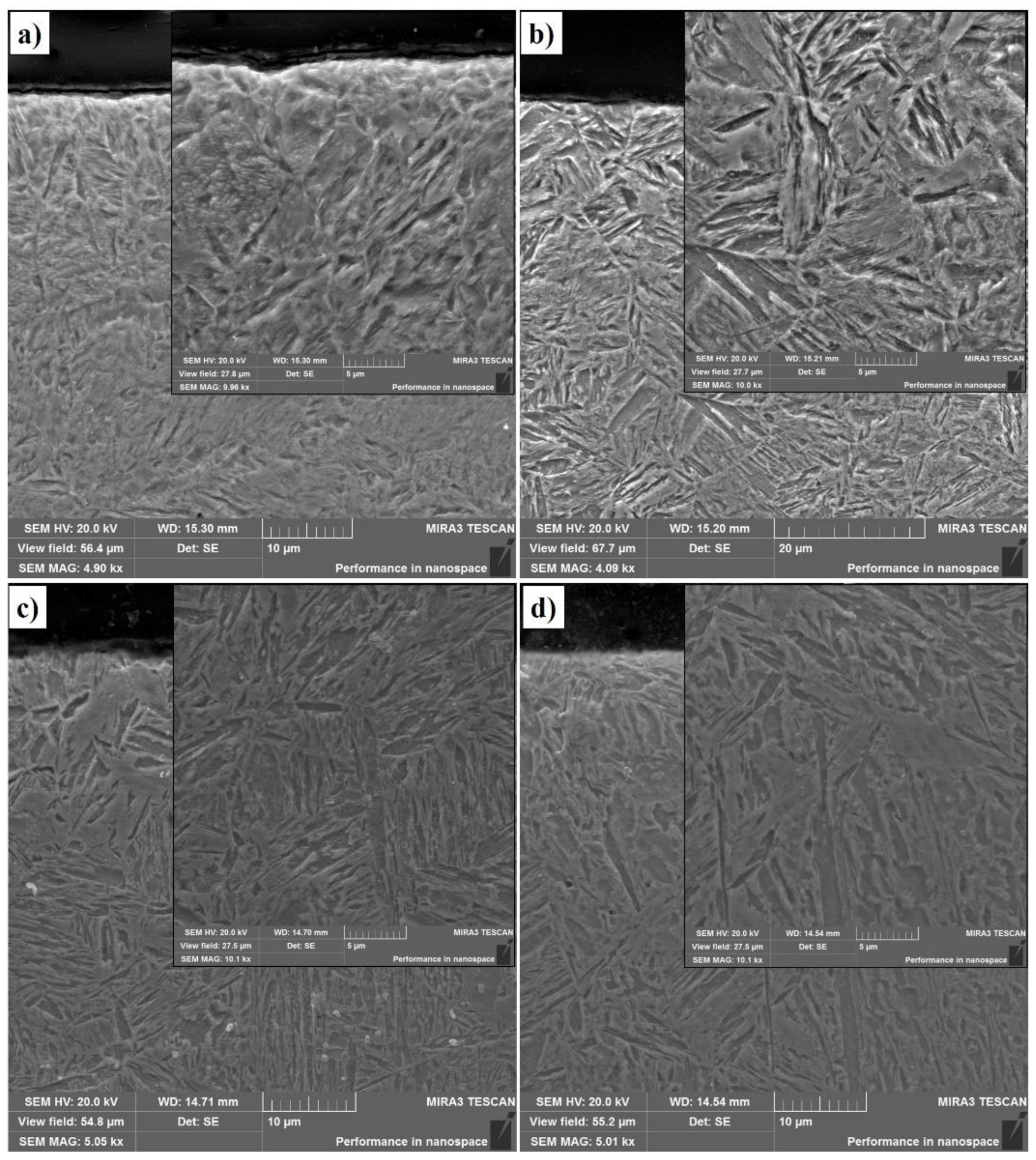
According to the results obtained, it was found that the process of EPTCH has a significant effect on the microstructure and structural-phase state of 30CrMnSiA steel. SEM images of a hardened cross-sectional layer obtained by scanning electron microscopy are shown in Figure 4a–d. Surface hardening due to EPTCH creates a unique microstructure that differs from the base material. The resulting micrograph shows the formation of martensitic structures. Martensitic steels in the hardened state have high strength, along with good ductility [45].
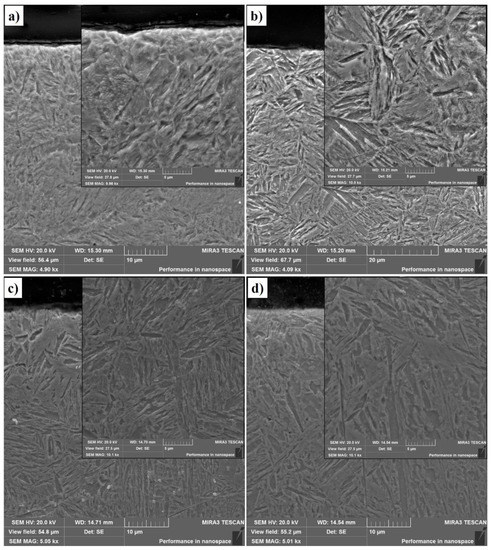
Figure 4.
Microstructure of the 30CrMnSiA steel after EPTCH: (a) No. 1; (b) No. 2; (c) No. 3; (d) No. 4.
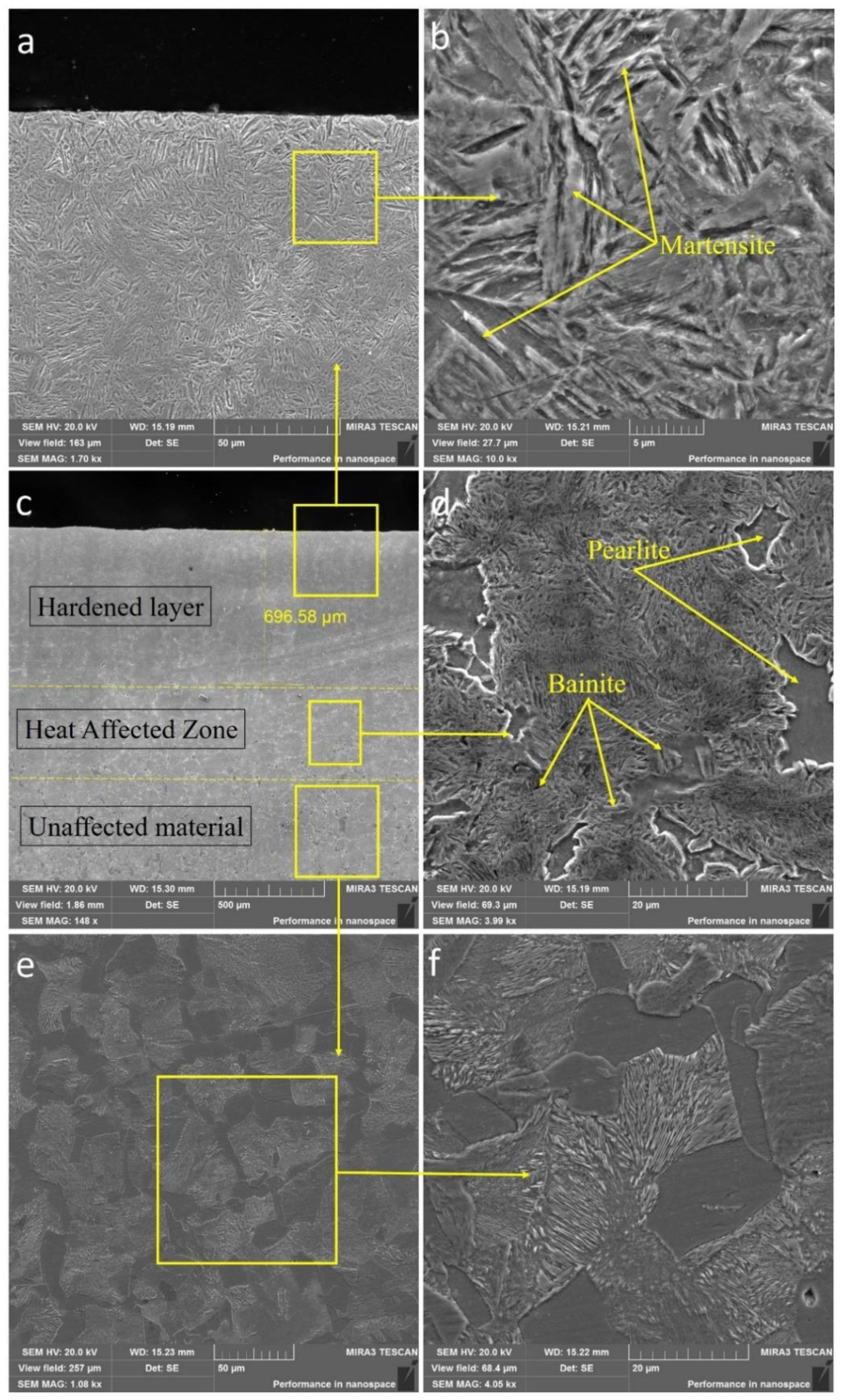
From Figure 4 and Figure 5, obtained using a scanning electron microscope, it can be seen that the structure of the cross-sections of steels as they move away from the surface into the depth of the material has a zoning; thus, a zone of a hardened layer is observed on the surface, followed by a heat-affected zone, which together form a modified layer, and finally a zone of the source material.
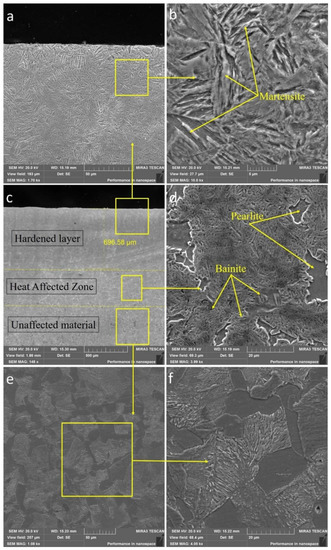
Figure 5.
SEM images of the cross-section of sample No. 2. (a) hardened layer at ×1700 and (b) ×10,000, (c) micrography at ×150, (d) heat affected zone at ×4000, (e) unaffected material at ×1000 and (f) ×4000.
For the purpose of conducting a detailed study of the microstructure, a SEM analysis of the zones (Figure 5a–f) of the cross-section of 30CrMnSiA steel treated according to mode No. 2 with a large magnification was carried out. As shown in Figure 5e–f, the microstructure of the initial state of 30CrMnSiA steel consists of a ferrite-pearlite phase. A characteristic feature of the used EPTCH hardening technology is the zonality of the formed structures as the layer had a different microstructure in depth. They could be broadly divided into a hardening zone and a zone of thermal action from the surface in the direction of depth. The microstructure of the hardened layer consisted of a needle-like martensitic structure (Figure 5a–b), which is similar to a typical hardening microstructure [46]. Due to the high temperature reached during heating, the atoms in this region can be easily redistributed, which, upon subsequent cooling, forms a finely dispersed lamellar martensite. The depth of the hardened layer of sample No. 2 was measured and was approximately 700 microns, respectively. The heat-affected zone contains bainite, ferrite, and pearlite.
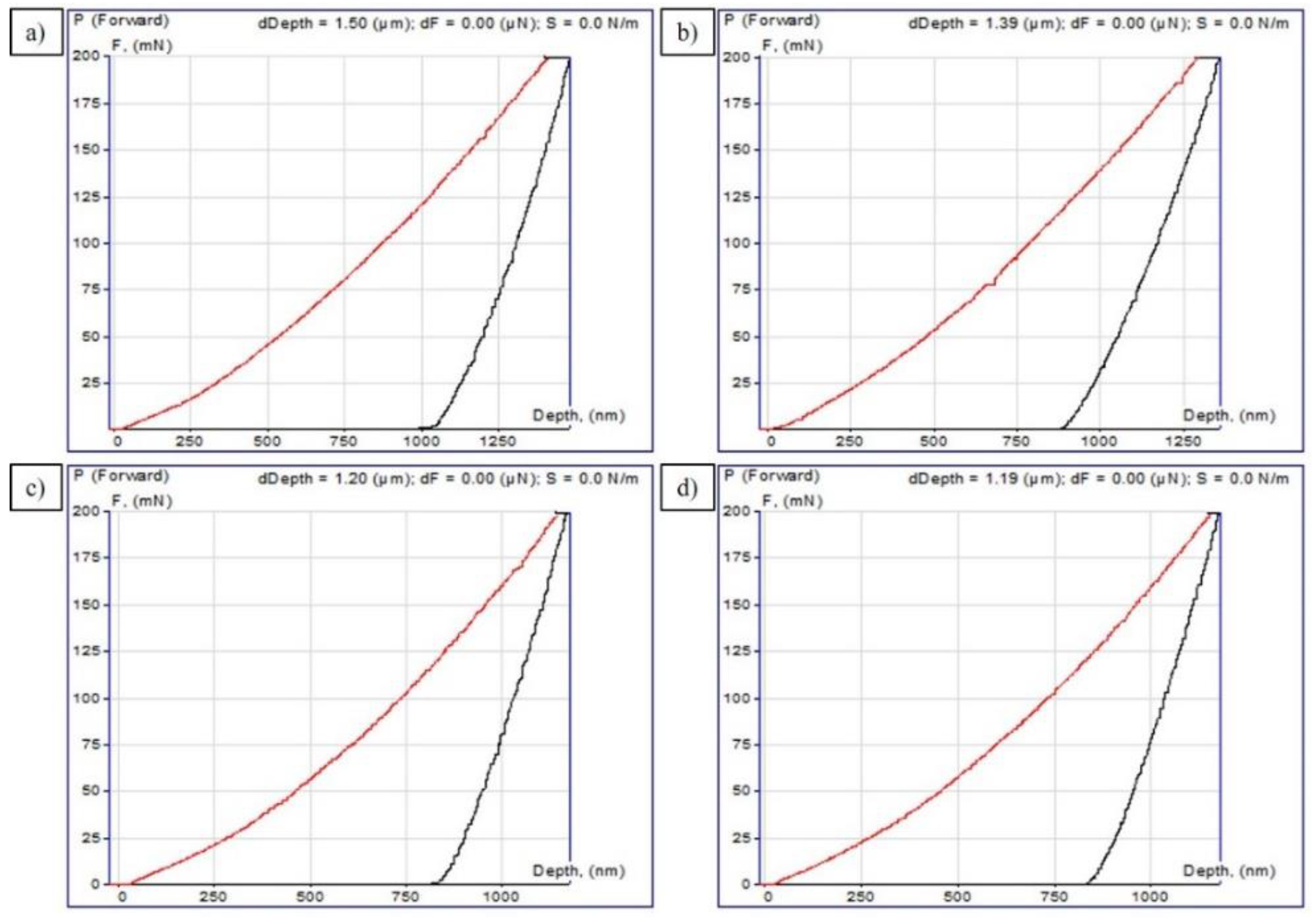
The EPTCH process (heating-quenching) transformed the α’-phase and iron carbide into Fe3C, respectively. Table 2 shows the values of hardness (H) and modulus of elasticity (Er) obtained as a result of nanoindentation tests. The formation of the α’ phase and Fe3C resulted in a 66% improvement in H, a threefold improvement in HV, and a 38% decrease in Er compared to the original steel. These results demonstrate that EPTCH treatment increases the microhardness and reduces the modulus of elasticity of the samples. Moreover, higher microhardness and lower modulus of elasticity will lead to higher rigidity, which can further increase wear resistance.

Table 2.
Results of nanoindentation of 30CrMnSiA EPTCH steel.
This technique implies selecting the parameters of a power function describing the experimental dependence of the depth of immersion of the indenter and the contact area on the applied force, as well as calculating the hardness and modulus of elasticity according to these data. The curves of the dependence of the normal force on the penetration depth are shown in Figure 6a–d, and the corresponding measured values, i.e., indentation hardness (H) and the reduced modulus of elasticity (Er), are given in Table 2.
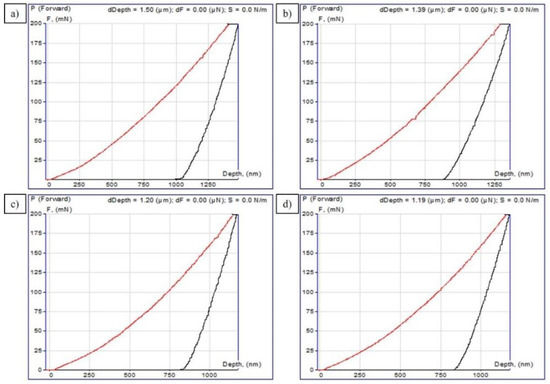
Figure 6.
P−h-diagrams curves of 30CrMnSiA steel after EPTCH: (a) No. 1; (b) No. 2; (c) No. 3; and (d) No. 4.
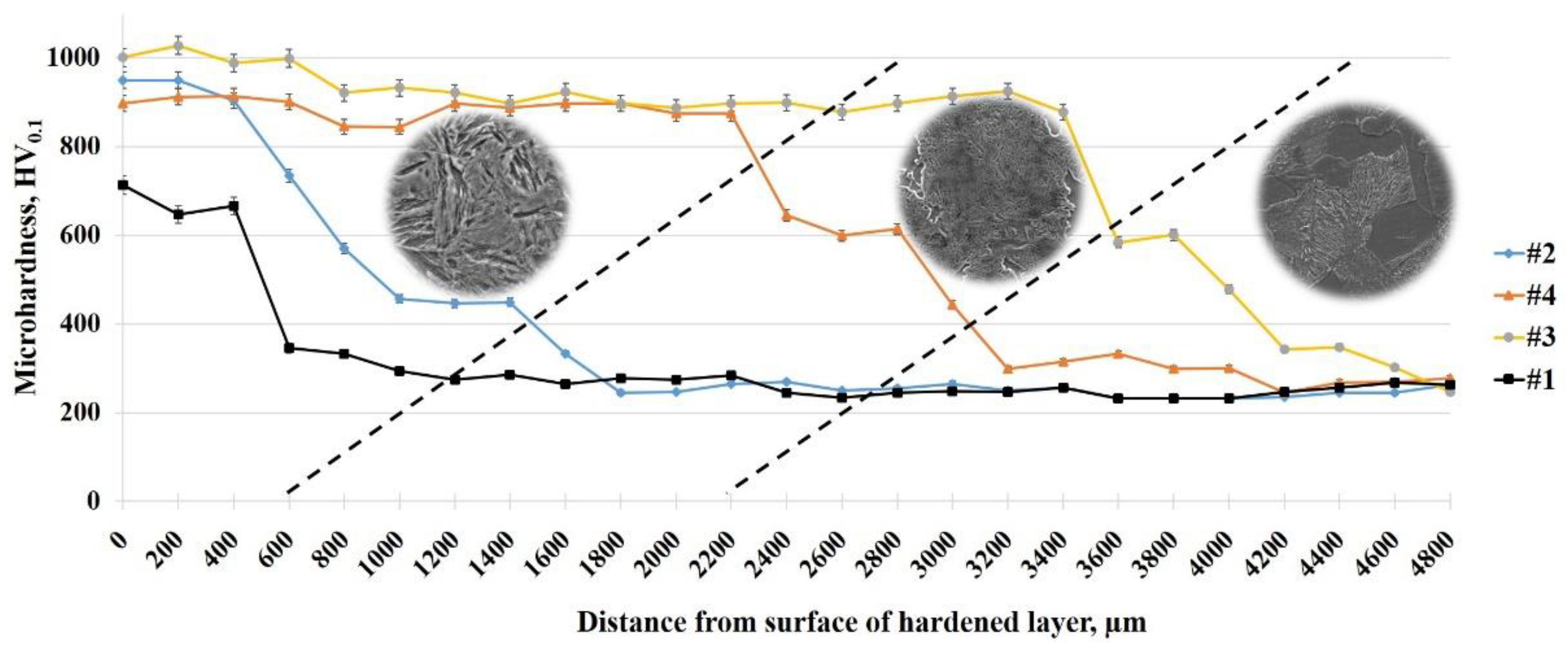
At the periodical switching of high voltage (320 V), medium voltage (200 V), and low voltage (50 V) the heating rate is periodically increased and decreased, which allows an increase in time and obtaining a thicker heated layer. The periodic variation of electric field strength between the surfaces of the liquid electrode and the product changes the power density of the surface heating, which controls the electrolyte-plasma heating and creates the necessary thermal conditions for the formation of hardening structures. By periodically changing the power density of heating, it is possible to obtain hardened layers with thickness of 0.6, 2.2, and 3.4 mm. The hardness of the hardened layer was measured using a hardness tester. The microhardness of the cross section has a maximum value at the edge of the surface in the hardened layer and then decreases into the depth of the sample (Figure 7). The highest value of the microhardness of the steel surface of 30CrMnSiA in the zone of the hardened layer with a thickness of about 3 mm is achieved after EPTCH according to mode No. 3 and is ~1000 HV with a martensitic structure, and a lower value is observed after EPTCH according to mode No. 1, where the thickness of the hardened layer is approximately 0.6 mm. Further into the depth of the material along the cross section, these indicators decrease, in the heat-affected zone with a martensitic-pearlite structure, the value of microhardness gradually decreases to the zone of the matrix with a ferrite–pearlite structure, where microhardness has the initial value. The increase in hardness is due to the presence of solid Fe3C and martensite, as confirmed by X-ray diffraction. According to the changes in microstructure and microhardness, it is clear that electrolytic plasma heating affects the structure and properties of the material, so the modified layer can be clearly distinguished from the main microstructure using simple optical microscopy to assess the effectiveness of the material hardening [47].
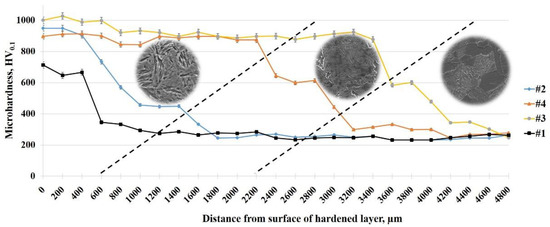
Figure 7.
The distribution of microhardness over the depth of the cross-section of steel 30CrMnSiA under different modes of thermal cycling.
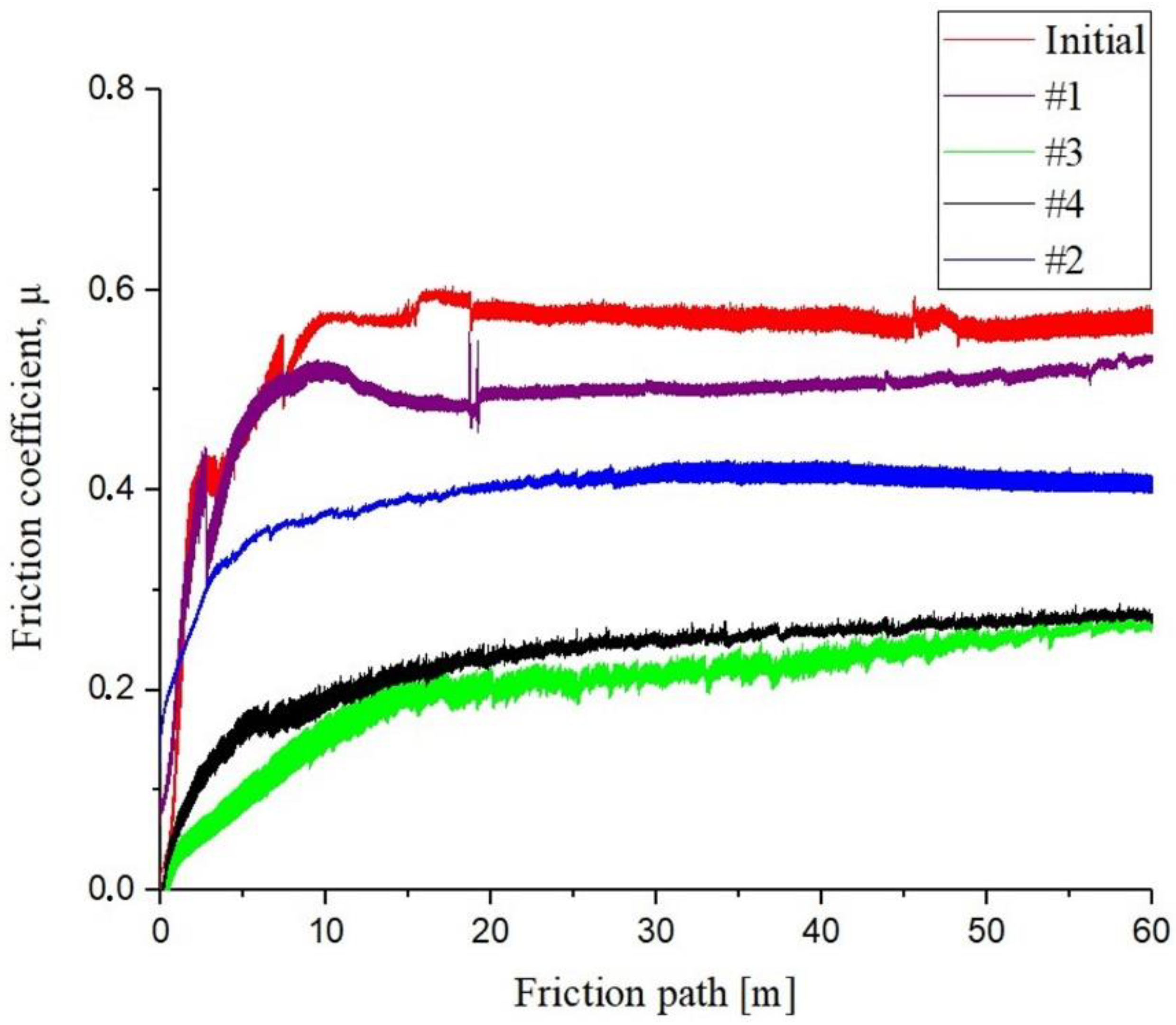
The number of EPTCH cycles has a significant impact on tribological characteristics. To determine the tribological parameters of the surface, tribological tests were performed on the sliding friction of surfaces according to the “ball-on-disk” scheme using an automated friction machine Tribometer TRB3 [48]. The graph of the distribution of the sliding friction coefficient of steel 30CrMnSiA under different thermal cycling modes is shown in Figure 8. It was determined that the value of the sliding friction coefficient µ of the initial state of the 30CrMnSiA steel sample is ~0.55, and after the EPTCH, the value of the average sliding friction coefficient decreases by 2.4 times compared to the initial state. An increase in wear resistance and a decrease in the coefficient of friction is a result of the presence of solid Fe3C and martensite. It was determined that the sample with the lowest coefficient of friction (No. 3) has the largest amount of cementite in the phase composition.
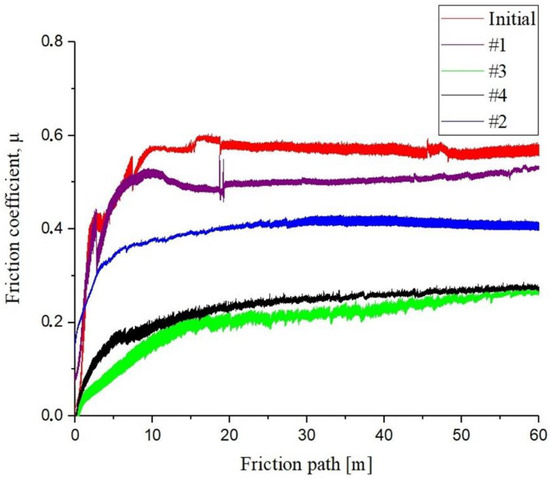
Figure 8.
Graph of the dependence of the sliding friction coefficient on the path 30CrMnSiA steel at different modes of thermocyclic hardening.
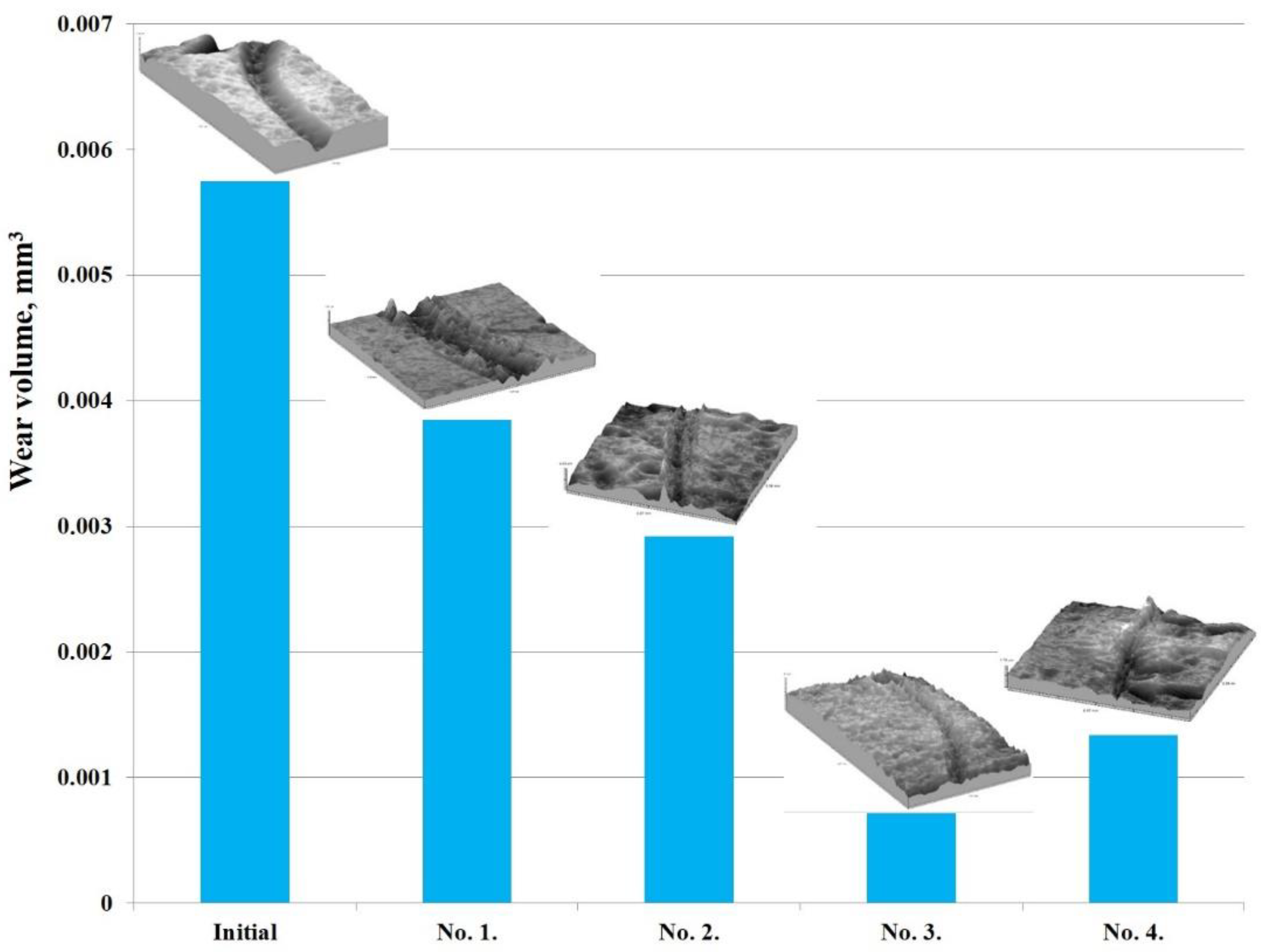
According to the results obtained, it can be argued that the hardening of EPTCH can lead to a decrease in the friction coefficient of the material surface and thereby increase its wear resistance. The friction coefficient determines the friction strength of the rubbing materials and the abrasion resistance according to the wear volume, that is, the less is the wear, the higher is the abrasion resistance [49]. The wear volume of 30CrMnSiA steel at different modes of thermal cycling was calculated by the area of transverse profile of wear track (Figure 9). Table 3 shows the results of tribological tests and surface roughness of 30CrMnSiA steel before and after EPTCH.
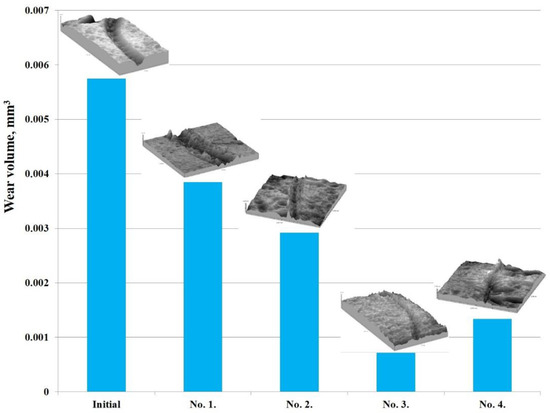
Figure 9.
Graph of the sliding friction coefficient dependence on the wear path of 30CrMnSiA steel under different modes of thermocyclic hardening.

Table 3.
Tribological tests results and roughness of 30CrMnSiA steel before and after EPTCH.
It should be noted that after EPTCH, no significant melting of 30CrMnSiA steel surface was observed. Sample No. 1 showed the relatively maximum values of surface roughness of 0.157 ± 0.014 μm. The subsequent repetition of the cycle of heating and cooling led to a decrease in surface roughness, and the number of microroughness decreased due to their microfusion. The decrease in surface roughness has a favorable effect on the tribological properties of 30CrMnSiA steel. Thus, thermal cyclic treatment allows to increase the time and to obtain a thicker heated layer without significant melting of the surface. Additionally, on the basis of previously conducted metallographic and X-ray phase investigations of 30XГCA steel samples after EPTCH it is possible to assume that the increase in wear resistance of surface is mainly connected with formation of martensite and cementite.
4. Conclusions
In this paper, the results of determining the microstructure, structural-phase state, and tribological properties of 30CrMnSiA steel with EPTCH were investigated and systematically studied. According to the results obtained, the following conclusions can be drawn:
- –
- It was established that as the number of cycles of electrolytic plasma hardening increases, the transformation of large-needle martensite into needle martensite and the formation of iron carbides occurs, and the thickness of the reinforced layer increases significantly.
- –
- It was determined that the microstructure of 30CrMnSiA steel after electrolytic plasma thermocyclic hardening has a characteristic zonal structure, which is conditionally divided into three zones: zone 1 hardens with a martensitic morphological component with a thickness of up to 3 mm; zone 2 is thermal influence, where the structure consists of martensite and pearlite; and zone 3 is a matrix with a ferrite–pearlite structural component.
- –
- It was established that surface hardening creates a gradient microstructure in the plasma hardening region with a predominantly martensitic microstructure. In the hardened zone, a maximum hardness of approximately 9.1 GPa was obtained, which is three times higher than that in the main material.
- –
- It was found that the electrolytic plasma thermocyclic hardening of 30CrMnSiA steel has a positive effect on tribological parameters, so the value of the sliding friction coefficient decreased by 2.4 times compared to the original sample.
Thus, the conducted studies have demonstrated the prospects and feasibility of using the method of electrolytic plasma thermocyclic hardening to improve the performance properties of parts made of 30CrMnSiA structural steel operating under conditions of friction and wear.
5. Patents
Rakhadilov, B.K.; Stepanova, O.A.; Sagdoldina, Z.B.; Satbayeva, Z.A. Method for hardening steel products. KZ Utility model patent No. 4891; Application No. 18.09.18, publ. 28.04.20.
Author Contributions
Conceptualization, B.R.; methodology, B.R., D.B. and Y.T.; investigation, L.Z. and Z.S.; writing—original draft preparation, R.K.; visualization, M.A., L.Z. and Y.T.; writing—review and editing, B.R. and L.Z.; All authors have read and agreed to the published version of the manuscript.
Funding
This research has been funded by the Science Committee of the Ministry of Education and Science of the Republic of Kazakhstan (Grant No. AP09261164).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Belkin, P.N.; Kusmanov, S.A. Plasma electrolytic hardening of steels: Review. Surf. Eng. Appl. Electrochem. 2016, 52, 531–546. [Google Scholar] [CrossRef]
- Pantelis, D.I.; Bouyiouri, E.; Kouloumbi, N.; Vassiliou, P.; Koutsomichalis, A. Wear and corrosion resistance of laser surface hardened structural steel. Surf. Coat. Technol. 2002, 161, 125–134. [Google Scholar] [CrossRef]
- Kulka, M.; Pertek, A. Laser surface modification of carburized and borocarburized 15CrNi6 steel. Mater. Charact. 2007, 58, 461–470. [Google Scholar] [CrossRef]
- Skeeba, V.Y.; Ivancivsky, V.V.; Martyushev, N.V. Peculiarities of High-Energy Induction Heating during Surface Hardening in Hybrid Processing Conditions. Metals 2021, 11, 1354. [Google Scholar] [CrossRef]
- Iliescu, M.; Roşu, M.-M.; Căpăţină, D. Optimization of the Induction Process on Light Gauge Steel Profiles Used in Metallic Framed Sustainable Eco-Constructions. Sustainability 2019, 11, 6686. [Google Scholar] [CrossRef]
- Popova, N.A.; Zhurerova, L.G.; Nikonenko, E.L.; Skakov, M.K. Effect of plasma electrolytic nitrocarburizing on phase composition of 0.3C-1Mn-1Si-fe steel. Inorg. Mater. Appl. Res. 2017, 8, 130–135. [Google Scholar] [CrossRef]
- Zhu, X. Effect of fast cooling on mechanical properties of continuous annealed cold rolled ultra high strength steel. Cailiao Rechuli Xuebao Trans. 2006, 27, 49–52. [Google Scholar]
- Hurtado-Delgado, E.; Huerta-Larumbe, L.; Miranda-Pérez, A.; Aguirre-Sánchez, Á. Microcracks reduction in laser hardened layers of ductile iron. Coatings 2021, 11, 368. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Buranich, V.V.; Satbayeva, Z.A.; Sagdoldina, Z.B.; Kozhanova, R.S.; Pogrebnjak, A.D. The cathodic electrolytic plasma hardening of the 20Cr2Ni4A chromium-nickel steel. J. Mater. Res. Technol. 2020, 9, 6969–6976. [Google Scholar] [CrossRef]
- Tyurin, Y.N.; Pogrebnjak, A.D. Electric heating using a liquid electrode. Surf. Coat. Technol. 2001, 142–144, 293–299. [Google Scholar] [CrossRef]
- Zhurerova, L.G.; Rakhadilov, B.K.; Popova, N.A.; Kylyshkanov, M.K.; Buranich, V.V.; Pogrebnjak, A.D. Effect of the PEN/C surface layer modification on the microstructure, mechanical and tribological properties of the 30CrMnSiA mild-carbon steel. J. Mater. Res. Technol. 2020, 9, 291–300. [Google Scholar] [CrossRef]
- Ayday, A.; Durman, M. Effect of different surface-heat-treatment methods on the surface properties of AISI 4140 steel. Mater. Tehnol. 2014, 48, 787–790. [Google Scholar]
- Rakhadilov, B.; Seitkhanova, A.; Satbayeva, Z.; Yerbolatova, G.; Icheva, Y.; Sagdoldina, Z. Investigation of the structural, mechanical and tribological properties of plasma electrolytic hardened chromium-nickel steel. Lubricants 2021, 9, 108. [Google Scholar] [CrossRef]
- Tabiyeva, Y.Y.; Rakhadilov, B.K.; Uazyrkhanova, G.K.; Zhurerova, L.G.; Maulit, A.; Baizhan, D. Surface modification of steel mark 2 electrolytic-plasma exposure. Eurasian J. Phys. Funct. Mater. 2019, 3, 355–362. [Google Scholar] [CrossRef]
- Kozlov, E.V.; Malinovskaya, V.A.; Popova, N.L.; Sizonenko, N.R.; Gromov, V.E. Formation of structure-phase states in gradient layers of 20X2H4A steel after nitrocementation. Steel Transl. 2007, 37, 986–988. [Google Scholar] [CrossRef]
- Rahadilov, B.K.; Zhurerova, L.G.; Sagdoldina, Z.B.; Kenesbekov, A.B.; Bayatanova, L.B. Morphological changes in the dislocation structure of structural steel 20GL after electrolytic-plasma hardening of the surface. J. Surf. Investig. 2021, 15, 408–413. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Satbayeva, Z.A.; Wieleba, W.; Kylyshkanov, M.K.; Baizhan, D.R. Changes in structure and properties of structural chromonickel steels after plasma electrolyte hardening. News Natl. Acad. Sci. Repub. Kazakhstan 2021, 4, 76–82. [Google Scholar] [CrossRef]
- Ayday, A.; Kırsever, D.; Demirkıran, A.Ş. The effects of overlapping in electrolytic plasma hardening on wear behavior of carbon steel. Trans. Indian Inst. Met. 2022, 75, 27–33. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Satbayeva, Z.; Baizhan, D. Effect of electrolytic-plasma surface strengthening on the structure and properties of steel 40kHN. In Proceedings of the 28th International Conference on Metallurgy and Materials (METAL), Brno, Czech Republic, 22–24 May 2019; pp. 950–955. [Google Scholar]
- Kumruoğlu, L.C.; Özel, A. Surface Modification of AISI 4140 Steel Using Electrolytic Plasma Thermocyclic Treatment. Mater. Manuf. Process. 2010, 25, 923–931. [Google Scholar] [CrossRef]
- Shi, L.; Cui, X.; Li, J.; Jin, G.; Liu, J.; Tian, H. Improving the wear resistance of heavy-duty gear steels by cyclic carburizing. Tribol. Int. 2022, 171, 107576. [Google Scholar] [CrossRef]
- Di Schino, A.; Testani, C. Heat Treatment of Steels. Metals 2021, 11, 1168. [Google Scholar] [CrossRef]
- Doudkin, M.; Kim, A.; Savelyev, A.; Zhileikin, M.; Gribb, V.; Mikhailovskaya, V. Modernization of the Metal Structure of the Grader Working Equipment. Int. Rev. Mech. Eng. 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Dyakov, I.G.; Burov, S.V.; Belkin, P.N.; Rozanov, E.V.; Zhukov, S.A. Increasing wear and corrosion resistance of tool steel by anodic plasma electrolytic nitriding. Surf. Coat. Technol. 2019, 362, 124–131. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Kozhanova, R.S.; Baizhan, D.; Zhurerova, L.G.; Yerbolatova, G.U.; Kalitova, A.A.; Zhanuzakova, L.N. Influence of plasma electrolytic hardening modes on the structure and properties of 65G steel. Eurasian J. Phys. Funct. Mater. 2021, 5, 209–221. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Tabiyeva, Y.Y.; Uazyrkhanova, G.K.; Zhurerova, L.G.; Baizhan, D. Influence of electrolytic-plasma surface quenching on the structure and strength properties of ferritic-pearlite class wheel steel. Eurasian J. Phys. Funct. Mater. 2020, 4, 167–173. [Google Scholar] [CrossRef]
- Dayança, A.; Karaca, B.; Kumruoǧlu, L.C. The cathodic electrolytic plasma hardening of steel and cast iron based automotive camshafts. Acta Phys. Pol. A 2017, 131, 374–378. [Google Scholar] [CrossRef]
- Kusmanov, S.A.; Silkin, S.A.; Smirnov, A.A.; Belkin, P.N. Possibilities of increasing wear resistance of steel surface by plasma electrolytic treatment. Wear 2017, 386–387, 239–246. [Google Scholar] [CrossRef]
- Kozlov, E.; Popova, N.; Zhurerova, L.; Nikonenko, E.; Kalashnikov, M.; Skakov, M. Structural and phase transformations in 0.3C-1Cr-1Mn-1Si-fe steel after electrolytic plasma treatment. AIP Conf. Proc. 2016, 1783, 020112. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Satbayeva, Z.; Ramankulov, S.; Shektibayev, N.; Zhurerova, L.; Popova, N.; Uazyrkhanova, G.; Sagdoldina, Z. Change of 0.34Cr-1Ni-Mo-Fe steel dislocation structure in plasma electrolyte hardening. Materials 2021, 14, 1928. [Google Scholar] [CrossRef] [PubMed]
- Rakhadilov, B.; Baizhan, D. Creation of bioceramic coatings on the surface of ti–6al–4v alloy by plasma electrolytic oxidation followed by gas detonation spraying. Coatings 2021, 11, 1433. [Google Scholar] [CrossRef]
- Kumruoglu, L.C.; Becerik, D.A.; Ozel, A.; Mimaroglu, A. Surface modification of medium carbon steel by using electrolytic plasma thermocyclic treatment. Mater. Manuf. Process. 2009, 24, 781–785. [Google Scholar] [CrossRef]
- Bayati, M.R.; Molaei, R.; Janghorban, K. Surface alloying of carbon steels from electrolytic plasma. Met. Sci. Heat Treat. 2011, 53, 91–94. [Google Scholar] [CrossRef]
- Oliver, W.; Pharr, G. An Improved Technique for Detemining Hardness and Elastic Modulus Using Load and Displacement Sensing Indentation Experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- ArslanHafeez, M.; Usman, M.; Arshad, M.A.; AdeelUmer, M. Nanoindentation-Based Micro-Mechanical and Electrochemical Properties of Quench-Hardened, Tempered Low-Carbon Steel. Crystals 2020, 10, 508. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Taheri, P.; Rouhaghdam, A.S.; Dehghanian, C. Systematic study of nanocrystalline plasma electrolytic nitrocarburising of 316L austenitic stainless steel for corrosion protection. J. Mater. Sci. Technol. 2007, 23, 665–671. [Google Scholar]
- Chen, J.; Zuo, Z.; Zhou, S.; Wang, X.; Chen, Y.; Ling, G. Study on the Compressive Stress Retention in Quenched Cam of 100Cr6 Steel Based on Coupled Thermomechanical and Metallurgical Modeling. Materials 2021, 14, 5912. [Google Scholar] [CrossRef] [PubMed]
- Merah, N.; Abdul Azeem, M.; Abubaker, H.M.; Al-Badour, F.; Albinmousa, J.; Sorour, A.A. Friction Stir Processing Influence on Microstructure, Mechanical, and Corrosion Behavior of Steels: A Review. Materials 2021, 14, 5023. [Google Scholar] [CrossRef]
- Eres-Castellanos, A.; Garcia-Mateo, C.; Caballero, F.G. Future Trends on Displacive Stress and Strain Induced Transformations in Steels. Metals 2021, 11, 299. [Google Scholar] [CrossRef]
- Litovchenko, I.; Akkuzin, S.; Polekhina, N.; Almaeva, K.; Moskvichev, E. Structural Transformations and Mechanical Properties of Metastable Austenitic Steel under High Temperature Thermomechanical Treatment. Metals 2021, 11, 645. [Google Scholar] [CrossRef]
- Li, J.; Yan, H.; Li, S.; Zhang, Y.; Wu, S. Sliding Friction and Wear Properties of 40CrNiMo Steel after Laser Hardening against GCr15 Steel under Oil Lubrication. Coatings 2022, 12, 604. [Google Scholar] [CrossRef]
- Berladir, K.; Hatala, M.; Hovorun, T.; Pavlenko, I.; Ivanov, V.; Botko, F.; Gusak, O. Impact of Nitrocarburizing on Hardening of Reciprocating Compressor’s Valves. Coatings 2022, 12, 574. [Google Scholar] [CrossRef]
- Fricke, L.V.; Gerstein, G.; Kotzbauer, A.; Breidenstein, B.; Barton, S.; Maier, H.J. High Strain Rate and Stress-State-Dependent Martensite Transformation in AISI 304 at Low Temperatures. Metals 2022, 12, 747. [Google Scholar] [CrossRef]
- Savrai, R.A.; Skorynina, P.A. Structural-phase transformations and changes in the properties of AISI 321 stainless steel induced by liquid carburizing at low temperature. Surf. Coat. Technol. 2022, 443, 128613. [Google Scholar] [CrossRef]
- Kim, A.; Doudkin, M.; Vavilov, A.; Guryanov, G. New vibroscreen with additional feed elements. Arch. Civ. Mech. Eng. 2017, 17, 786–794. [Google Scholar] [CrossRef]
- Tarakci, M.; Korkmaz, K.; Gencer, Y.; Usta, M. Plasma electrolytic surface carburizing and hardening of pure iron. Surf. Coat. Technol. 2005, 199, 205–212. [Google Scholar] [CrossRef]
- Gharbi, A.; Bouhamla, K.; Ghelloudj, O.; Ramoul, C.E.; Berdjane, D.; Chettouh, S.; Remili, S. Heat Treatment Effect on the Microstructural, Hardness and Tribological Behavior of A105 Medium Carbon Steel. Defect Diffus. Forum 2021, 406, 419–429. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Baizhan, D.R.; Sagdoldina, Z.B.; Buitkenov, D.B.; Maulet, M. Phase composition and structure of composite Ti/HA coatings synthesized by detonation spraying. AIP Conf. Proc. 2020, 2297, 020022. [Google Scholar] [CrossRef]
- Rakhadilov, B.K.; Stepanova, O.A.; Sagdoldina, Z.B.; Satbayeva, Z.A. Method for Hardening Steel Products. KZ Utility Model Patent No. 4891; Application No. 18.09.2018, 28 April 2020. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).