1. Introduction
Tire molds determine the quality and appearance of automobile tires. When tire mold fouling occurs under high temperature, high pressure, or chemical reaction during vulcanization, the quality and appearance of tires are affected and demolding becomes difficult [
1,
2,
3].
Figure 1 shows the structure of tire molds and the surface changes after multiple vulcanizations.
In order to reduce mold contamination and demolding force and extend the service life of molds, anti-sticking coatings must be selected carefully, with consideration for both materials and surface structures. There are many studies on anti-sticking surfaces, but many of them focus on the control of surface wettability [
4,
5]. Micro- and/or nano-surface textures have been obtained to form superhydrophobic surfaces with self-cleaning [
6] or anti-icing [
7] properties. These surfaces are usually exposed to the atmosphere and are viable at normal temperature and pressure. Since tire mold surfaces are subjected to high temperatures (>130 °C) and make contact with rubber tires under high pressures (>10 MPa), surface wettability may not be the right factor to evaluate the anti-sticking property of a given mold.
Some researchers have studied the application performance of anti-sticking coatings on mold surfaces. Novotny [
8] developed a polytetrafluoroethylene (PTFE)-based multilayer micro-coating for aluminum AlMg3 molds and found that it could prevent form contamination during tire production and extend production cycles by 200%–400% between process cleanings. Dong et al. [
9] analyzed the application prospect of a diamond-like carbon (DLC) coating on tire molds and studied its roughness, element composition, and hydrophobicity. Zhang et al. [
10] developed an α-Al
2O
3 multilayer coating with good anti-adhesive property for a precision glass mold. Guan et al. [
11] studied the tribological and anti-adhesive properties of TiAlN, CrN, PTFE, and Ni-P-PTFE coatings on rotary tillage blades and found that the PTFE coating had better wear resistance and anti-adhesive properties. Calderón et al. [
12] investigated the application of anti-adhesive multilayer coatings composed of an organosilane (PFOS) layer and NiO and Ni layers on injection molds and found that the coating displayed improved wear resistance and anti-adhesion properties to polyamide 6 (PA6) compared with the uncoated and TiN-coated mold. Sánchez-Urbano et al. [
13] studied the demolding properties of fluoropolymer, ceramic, and silicone rubber coatings on aluminum molds for the production of polyurethane (PUR) foam. After 1500 cycles of demolding, it was proven that the perfluoroalkoxy (PFA) coating had the best demolding performance. Akinci and Cobanoglu [
14] investigated the adhesion behavior and surface properties of PTFE, fluorinated ethylene propylene (FEP), PFA, and ETFE coatings between molds and PUR and found that PFA had the lowest surface roughness value of and the largest contact angle after a holding period in isocyanate. However, most of these studies focused on the surface hydrophobicity and wear resistance of the coatings and did not investigate the demolding force and its influencing parameters.
Some scholars have studied the factors and mechanisms affecting the demolding performance of injection moldings. These factors include material properties, demolding temperature, polymer pressure history, and mold structures; they affect the chemical, physical, and mechanical interactions between a polymer and the mold during demolding [
15,
16,
17]. Majewski and Hopkinson [
18] reported that the major factors affecting the release force of a polymer surface to a PUR foam part are the surface energy and surface roughness of the substrate and the proportion of isocyanate in the foam. Navabpour et al. [
19] tested the demolding force of low-density polyethylene (LDPE) on eight different coatings and found that the surface composition, surface energy, and roughness affected the adhesion between LDPE and the mold surface, where the surface composition had the greatest influence. Sasaki et al. [
20] found that there is a best core surface roughness for minimizing the demolding force between the resin and the mold: Ra 0.212 μm for polypropylene (PP) and polyethylene terephthalate (PET) and Ra 0.092 μm for methacrylate resin (PMMA). Sorgato et al. [
21,
22] studied the demolding force of two injection molds processed by micro-milling and micro-electro-discharge machining, respectively, and found that the demolding force is not only related to Ra, but also other surface morphology parameters. Correia et al. [
23] reported that the demolding force is related to friction and that there is a physical interlocking between part and mold in injection forming, especially when the roughness is high. These studies mainly focused on the demolding performance of plastic (PUR, PE, PP, PET, PMMA, etc.) injection molding. Research on the demolding force of rubber vulcanization is relatively scant, and rubber has different physical and chemical properties compared to plastic. Moreover, the above studies did not study the anti-fouling performance of the molds.
This study aimed to identify coatings with lower demolding force and better anti-fouling performance for tire molds and find the influencing mechanism of coating composition, surface wettability, and surface micro-morphology on the anti-sticking properties. Six types of coatings, including a nano-ceramic coating, two commercial fluororesin coatings, and three Teflon coatings, were selected to modify type-45 steel specimens by air spraying. The literature on the anti-sticking performance of these coatings for tire molds is still limited. The water contact angle, bonding ISO grade with substrate, and surface roughness of the coatings were tested. The surface micro-morphology was observed by scanning electron microscopy (SEM). The demolding forces of 10 rubber vulcanization tests were recorded for each coating to study their demolding performance, and surface chemical compositions before and after rubber vulcanization tests were investigated using energy dispersive X-ray spectroscopy (EDS) to evaluate their anti-fouling performance. To explain the performance differences of the three Teflon coatings, their molecular structures were studied by Fourier transform infrared spectrometry (FTIR). The elemental composition and chemical states of contaminants were analyzed by X-ray photoelectron spectrometry (XPS).
3. Results and Discussion
Table 1 shows the basic information of the specimens for the vulcanization test. The water contact angle of the uncoated specimen was 42°, which means that the surface was hydrophilic. The water contact angles of the six coatings were all larger than 90°, indicating hydrophobicity. The water contact angle of the bilayer waterborne PTFE coating was the largest, at 133.1°. The adhesion ISO grades of the three commercial coatings were all grade 0, meaning that the best grade of adhesion and the adhesion strengths of the three fluororesin coatings were relatively low.
Figure 5 shows the SEM micrographs of the coatings on top-view. The microstructure may be used to analyze the demolding forces of the specimens. The nano-ceramic coating exhibited micro-nanostructures with visible nanoparticles (250–400 nm) and a porous structure, which is the reason for its good hydrophobicity (126.6°). The bilayer waterborne PTFE coating also had micro-nanostructures due to the large amount of inorganic fillers, and the PTFE resin did not form a continuous film. There were fibrous structures on the surface of the PTFE coating, and the fiber diameter was about 400~500 nm. This fibrous structure contained many micro-gaps, which may increase the penetration of hot rubber, leading to more residues. The surfaces of the FEP-based Whitford Xylan 8840 coating, the PFA coating, and the FEP coating were flat and smooth, forming a continuous film as shown in
Figure 5c,e,f.
Figure 6 shows the demolding force variation of the seven specimens for ten rubber vulcanization tests. The demolding force of the uncoated specimen was the largest with a maximum value of 154 N, while the maximum demolding force of the coating specimens was 72 N. This indicates that all six coatings could reduce the demolding force by at least 24%. The demolding force of the nano-ceramic coating ranged from 29 N to 72 N, and in general, it increased with increasing amount of rubber vulcanization. A similar trend was found in the case of PUR foam demolding on the aluminum mold with the sol-gel ceramic coating [
13]. This might be because the rubber contaminant adhered to the coating surface and increased the further adhesion of rubber. The demolding force of the bilayer waterborne PTFE coating was lower than that of the nano-ceramic coating and was 30~40 N after four vulcanization tests. The demolding force of the PTFE coating was 14~22 N. The Whitford Xylan 8840 coating, the PFA, and the FEP coatings showed good demolding performance with zero demolding force. The PFA and FEP coatings also showed low release force (5~20 N from specimens of 45 × 45 mm
2) for PUR foam, as in [
13]. These results indicate that PFA/FEP coatings have lower adhesion force with rubber than PTFE and other non-fluorine materials.
The results can also be analyzed according to the microstructure of the specimens. As the nano-ceramic coating and the bilayer waterborne PTFE coating have micro-nanostructures, rubber molecules can easily enter into the gaps of micro-nano structures under high temperature and high pressure in the process of rubber vulcanization. Then, the contact area between rubber and coating increases, and greater force is needed for demolding. The fibrous structure and gaps of the PTFE coating also increased the binding force between rubber and the coating surface, resulting in the increase of demolding force. It should also be noted that the PFA and FEP coatings had the largest surface roughness values Ra, but the lowest demolding forces. This indicates that the surface morphology structure of the coating is a more important factor for affecting the demolding force of rubber than the surface average roughness value, Ra. This is consistent with the conclusion in Sorgato et al. [
21]. Navabpour et al. [
19] studied the effect of surface roughness on the release force of coatings for an LDPE mold and found that, for coatings that showed near-zero release forces, surface roughness did not affect the LDPE adhesion; however, for coatings that showed significant adhesion to LDPE, the release force increased with increasing surface roughness. This may be the same for coatings of rubber molds, because the molten polymer can replicate the mold topography, resulting in mechanical interlocking between the polymer and the mold surfaces [
22]. Thus, for coatings which can adhere to rubber, the larger the Ra, the larger the demolding force.
Figure 7 shows the photos of the specimens from top-view after the vulcanization tests. The central area was in contact with rubber, and the edge area was the original coating. Compared to the edge area, the surface color of the central area for the uncoated specimen, nano-ceramic coating, and bilayer waterborne PTFE coating became darker, indicating that contaminants had adhered to the specimen surfaces. There was no obvious change for the other specimens.
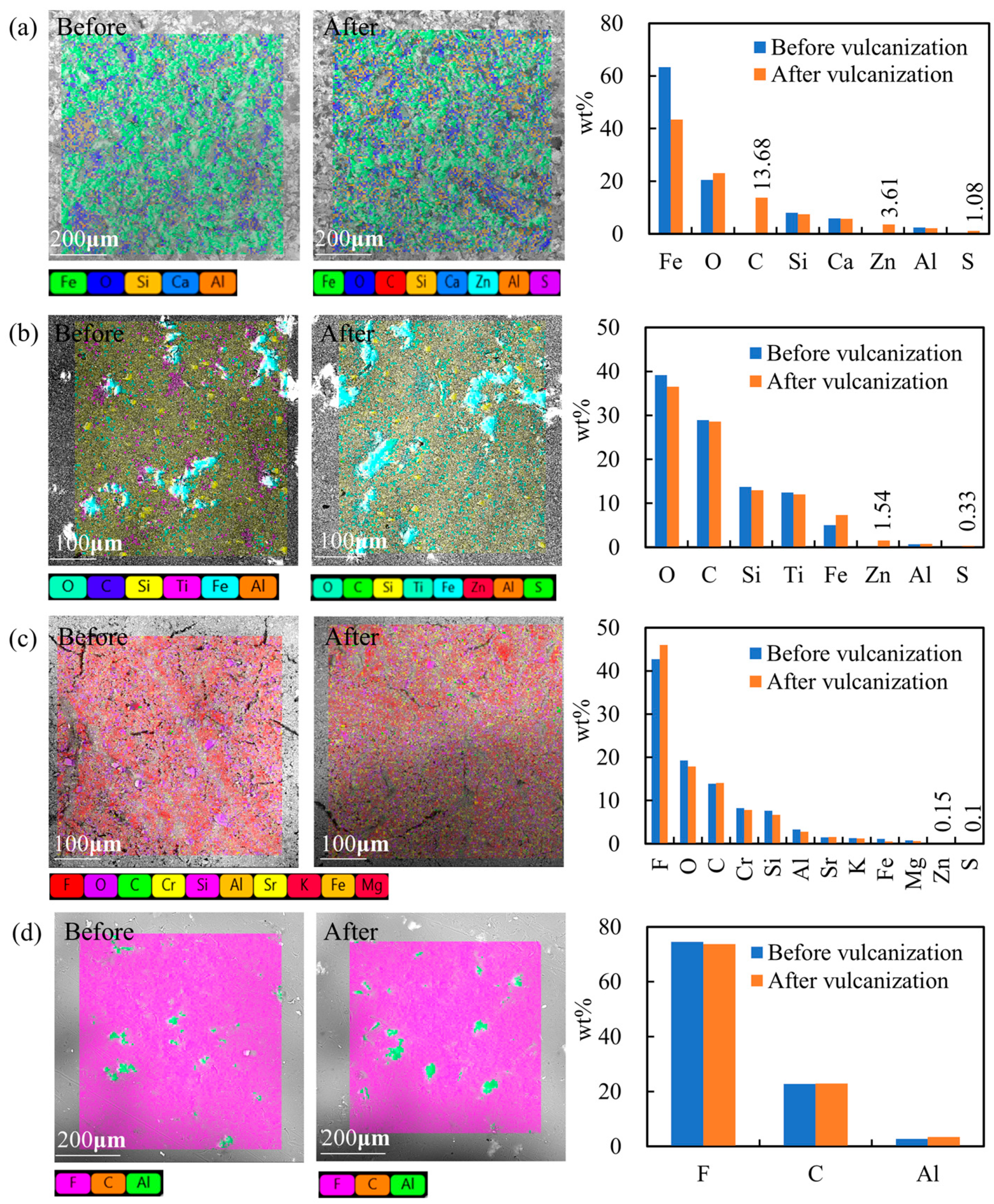
Figure 8 compares the surface microstructures and element compositions of the specimens before and after rubber vulcanization tests. Different colors in the SEM image represent different elements. In the histogram, the blue bar and the orange bar represent the element composition before and after vulcanization, respectively.
Figure 8a shows that before vulcanization, iron, oxygen, silicon, calcium, and aluminum are present on the surface of the uncoated specimen. After 10 rubber vulcanization tests, carbon, zinc, and sulfur were also detected. The weight concentrations of these eight elements were 43.34%, 23.03%, 7.42%, 5.67%, 2.17%, 13.68%, 3.61%, and 1.08%, respectively. The weight concentration of oxygen increased, and that of iron decreased. This illustrates that the contaminant of the rubber mold contained carbon, zinc, and sulfur.
Figure 8b shows that mainly oxygen, carbon, silicon, titanium, and iron were on the surface of the nano-ceramic specimen. It can be inferred that there may have been nano-SiO
2 or nano-TiO
2 in the nano-ceramic coating, which form the nanostructures on the substrate surface. Zinc and sulfur were also added on the surface of the nano-ceramic coating specimen after vulcanization, and their weight concentrations were 1.54% and 0.33%, respectively.
Figure 9 shows the distribution of elements of the nano-ceramic coating after vulcanization tests. Zinc and sulfur were distributed over the whole area, and their contents were slightly higher at the iron-rich areas. This indicates that the nano-ceramic coating could not resist the deposition of contaminants, but could reduce the deposition rate compared to the uncoated specimen.
Figure 8c shows that mainly fluorine, oxygen, carbon, chromium, silicon, and aluminum were on the surface of the bilayer waterborne PTFE coating. Pigments and fillers of the topcoat may be Cr
2O
3, SiO
2, and Al
2O
3. After vulcanization, zinc and sulfur were also added on the surface, but their weight concentrations were only 0.15% and 0.1%, respectively, much less than that of the nano-ceramic coating.
Figure 8d–g shows that the other four coatings mainly had carbon and fluorine on their surfaces, and the fluorine content was more than 74 wt.%. Their surface compositions changed little before and after rubber vulcanization tests, indicating that the coatings have good anti-fouling performance.
It can be seen that the nano-ceramic coating and bilayer waterborne PTFE coating have large water contact angles due to their micro-nanostructures, but they have large demolding force and poor anti-fouling performance. This indicates that surface wettability can not be used to evaluate the anti-sticking property of rubber molds. Similar results were shown in [
13]. Sánchez-Urbano et al. studied the critical sliding angle and release force of PUR from the coatings, and results showed that the PFA and ceramic coatings have similar critical sliding angles, but very different release forces.
The good anti-fouling properties of PTFE, PFA, and FEP can be explained by their basic chemical structures. PTFE is polymerized from tetrafluoroethylene (TFE) by free radical methods [
24]. It is a linear polymer with only C and F elements. PFA is a copolymer of TFE and perfluoropropyl vinyl ether (PPVE) with a mole ratio of approximately 100:1 [
25]. Its structure is equivalent to PTFE, but with some fluorine atoms on the main chain being replaced by perfluoropropoxy groups. FEP is a copolymer of TFE and hexafluoropropylene (HFP). It is essentially PTFE with an occasionally trifluoromethyl (–CF
3) side group attached [
25,
26]. It can be seen that the carbon chains of the three fluororesins are surrounded by fluorine atoms, which is the most electro-negative and least-polarizable element known. The C–F bond is the strongest known single bond in organic chemistry (~450 kJ/mol), making the coating surface inert and very low intrinsic surface energy.
The FTIR reflection–absorption spectra of the PTFE, PFA, and FEP coatings are shown in
Figure 10. The spectra show characteristic absorption peaks at around 1206 cm
−1 and 1151 cm
−1 for the three kinds of coatings, which correspond to the anti-symmetric and symmetric stretching vibration peaks of –CF
2, respectively. Compared to the PTFE coating, the FEP and PFA coatings are characterized by an absorption peak at 981 cm
−1 that is assigned to the stretching vibration peak of –CF
3 [
27]. It can be seen from
Figure 10 that FEP and PFA have more –CF
3 side groups, while the –CF
3 group of PTFE only appears at the end of the molecular chain. The PTFE and PFA coatings have weak absorption peaks near 1645 cm
−1, corresponding to the –C=O stretching vibration [
28]. The –C=O group comes from the few unstable end groups such as –COOH and –COF that are generated during the preparation of fluororesin. The absorption band between 700–800 cm
−1 corresponds to the amorphous structure of fluororesin [
29]. The absorption intensity of the PFA and FEP coatings is higher than that of the PTFE coating, indicating that the crystallinity of PFA and FEP is lower than that of PTFE.
From
Figure 6 and
Figure 8, it is worth noting that although the PTFE coating has good anti-fouling performance, its demolding force is larger. This can be explained by the microstructures (
Figure 5d) and the molecular structures of PTFE. As the surface of PTFE resin is inert, the contaminants do not easily deposit on its surface, but the fibrous structure of PTFE increases the penetration of hot rubber material, causing large demolding force. PFA and FEP have lower crystallinity than PTFE, and their melt viscosities at 380 °C are 10
7–10
8 Pa·s and 10
3–10
5 Pa·s, respectively, which are much lower than that of PTFE, around 10
10 Pa·s [
24,
25,
26]. Thus, the surfaces of the PFA and FEP coatings are smoother than that of the PTFE coating, resulting in lower demolding force (as shown in
Figure 6).
To further investigate the elemental compositions and chemical states of the surface contaminants, XPS analysis was carried out for the uncoated specimen after 200 vulcanizations; the results of binding energy spectra are shown in
Figure 11.
Figure 11a is the survey spectrum, showing the presence of the Zn, S, C, and O peaks in the contaminants.
Figure 11b–d present core-level XPS spectra of Zn 2p, S 2p, and O 1s, respectively.
Figure 11b exhibits two peaks at the binding energies of 1022.1 eV and 1045.1 eV, attributed to Zn 2p
3/2 and Zn 2p
1/2 of Zn
2+, respectively.
Figure 11c shows two peaks at the binding energy of 161.5 eV and 162.8 eV, representing S 2p
1/2 and S 2p
3/2 of S
2−, respectively, which correspond to S–S and S–C bonds. In addition, there is a small peak at the binding energy of 169.0 eV attributed to the S–O bond. In
Figure 11d, the obvious peaks at the binding energies of 284.6 eV, 285.9 eV, and 288.4 eV are attributed to C–C/C=C, O–C=O/C=O, and C–H, respectively. To determine whether the contaminant contains ZnO or ZnS, the XPS spectra were compared with the results of Li et al. [
30]. We found that the XPS spectra of the contaminant were consistent with that of ZnS. Thus, Zn
2+ in the contaminant mainly exists in the form of ZnS. Bukhina [
3] reported that ZnS is the reaction product of the vulcanizing agent ZnO and sulphur in the process of rubber vulcanization. It adheres to the mold surface easily and can form a gray deposition layer. Along with the increase of vulcanization times, the low-molecular-weight components in the rubber will attach to the ZnS microcrystals, causing organic deposition.