Effects of Y2O3 Addition on the Microstructure and Static Lead-Bismuth Eutectic Thermal Corrosion Behaviors of FeCrAlTiC-xY2O3 Laser Clade Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Powder and Coating
2.2. Micro-Hardness and 400 °C Wear Test
2.3. LBE Corrosion Test
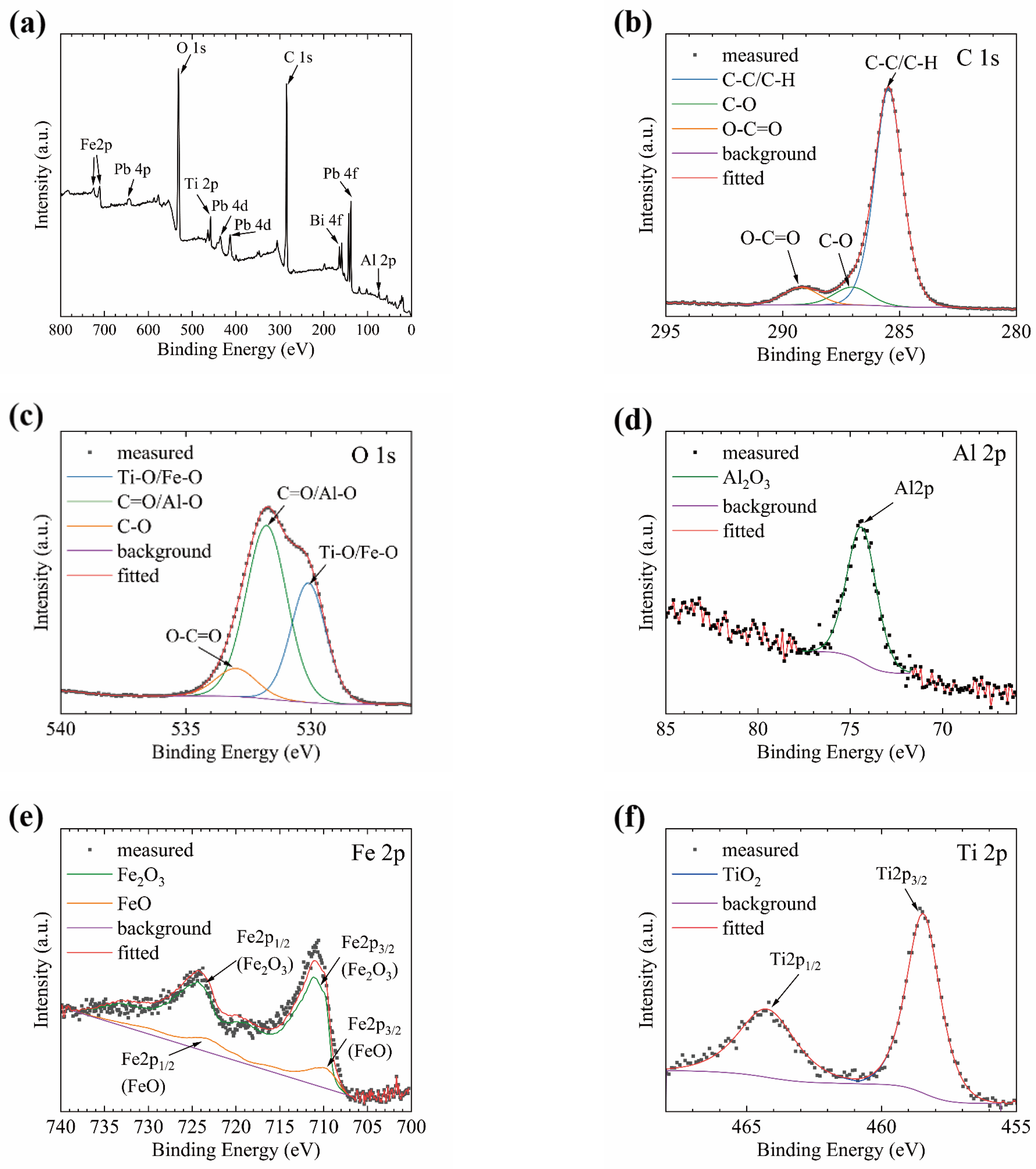
2.4. Characterization
3. Results and Discussions
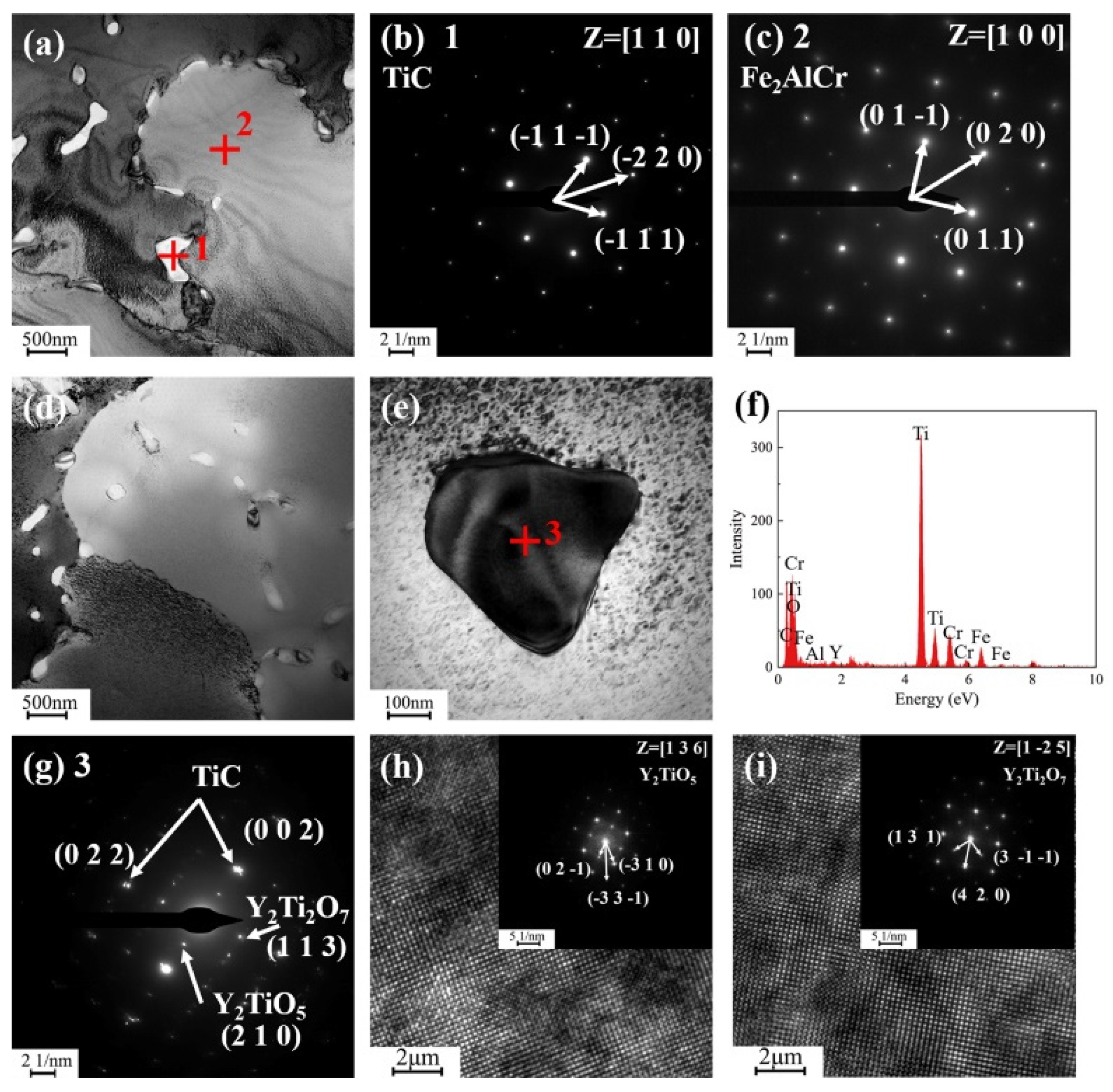
3.1. Phase Composition and Microstructures of Composite Coating
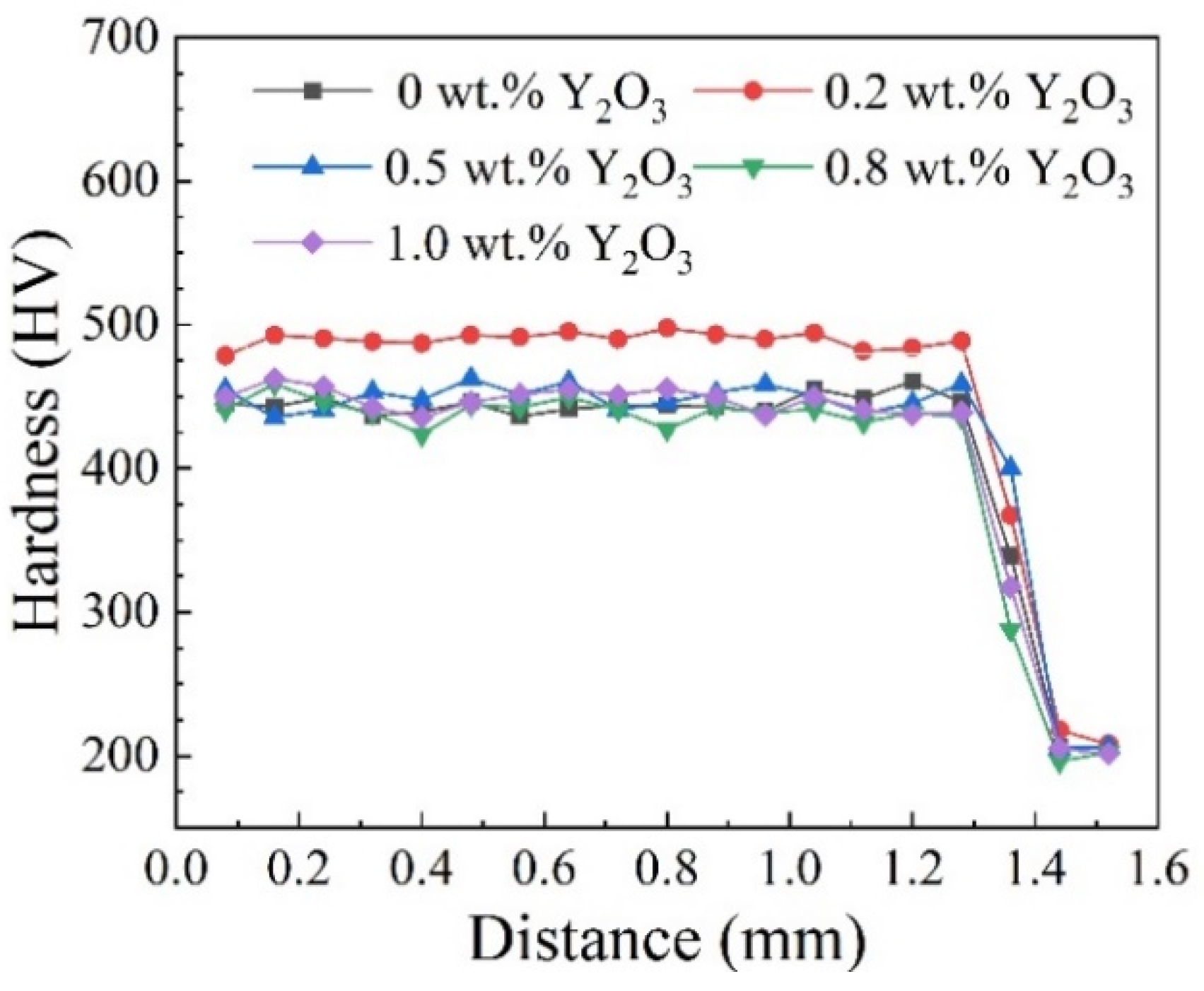
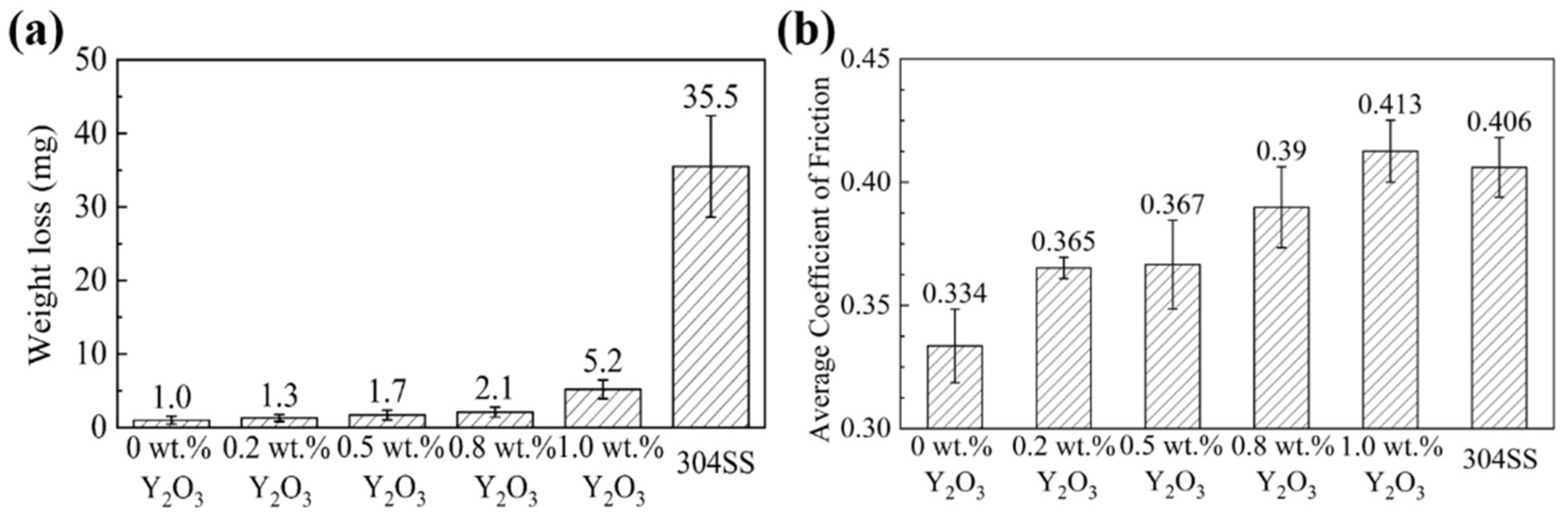
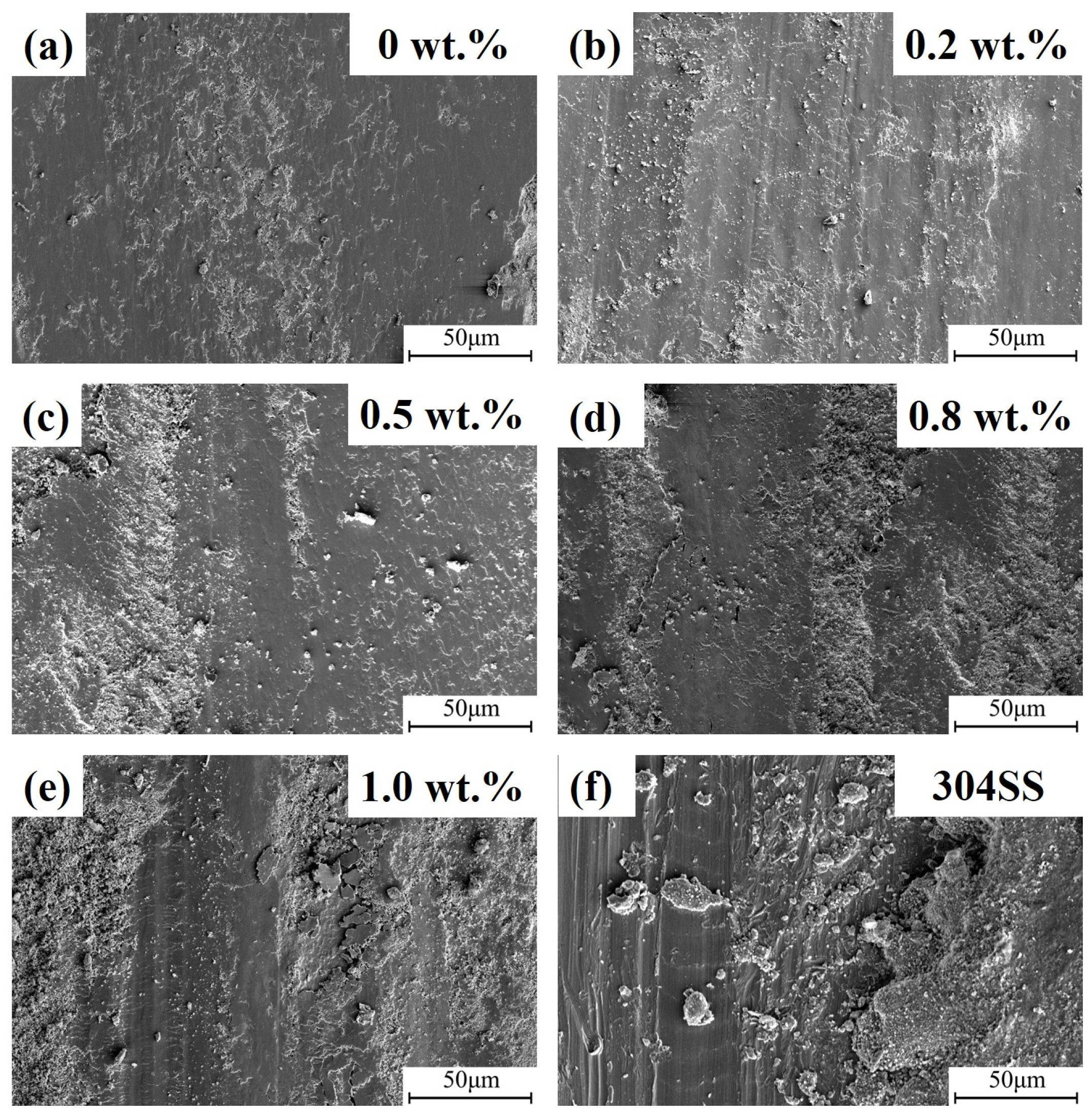
3.2. Micro-Hardness and 400 °C Wear Test
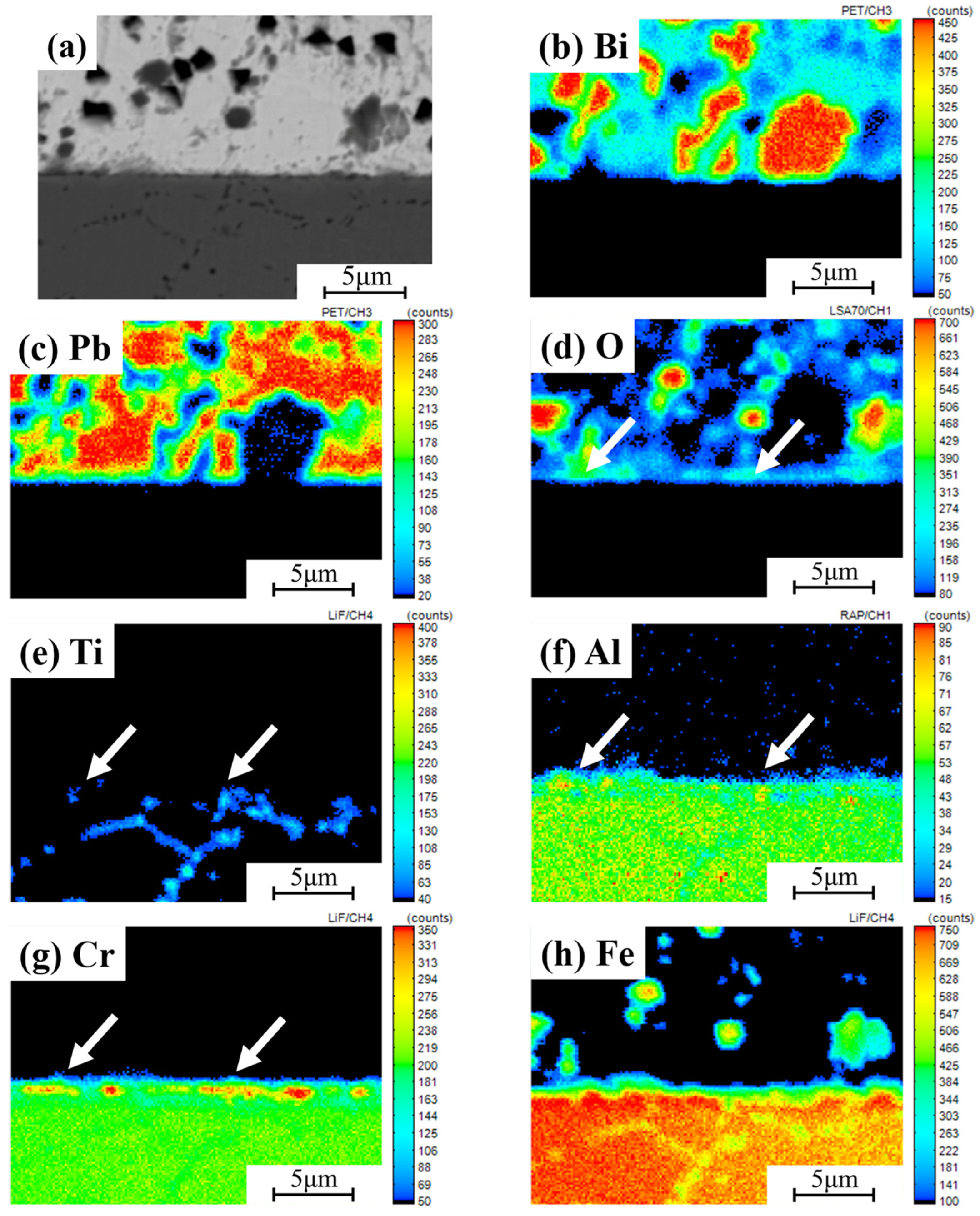
3.3. Corrosion Behavior in Static LBE at 500 °C
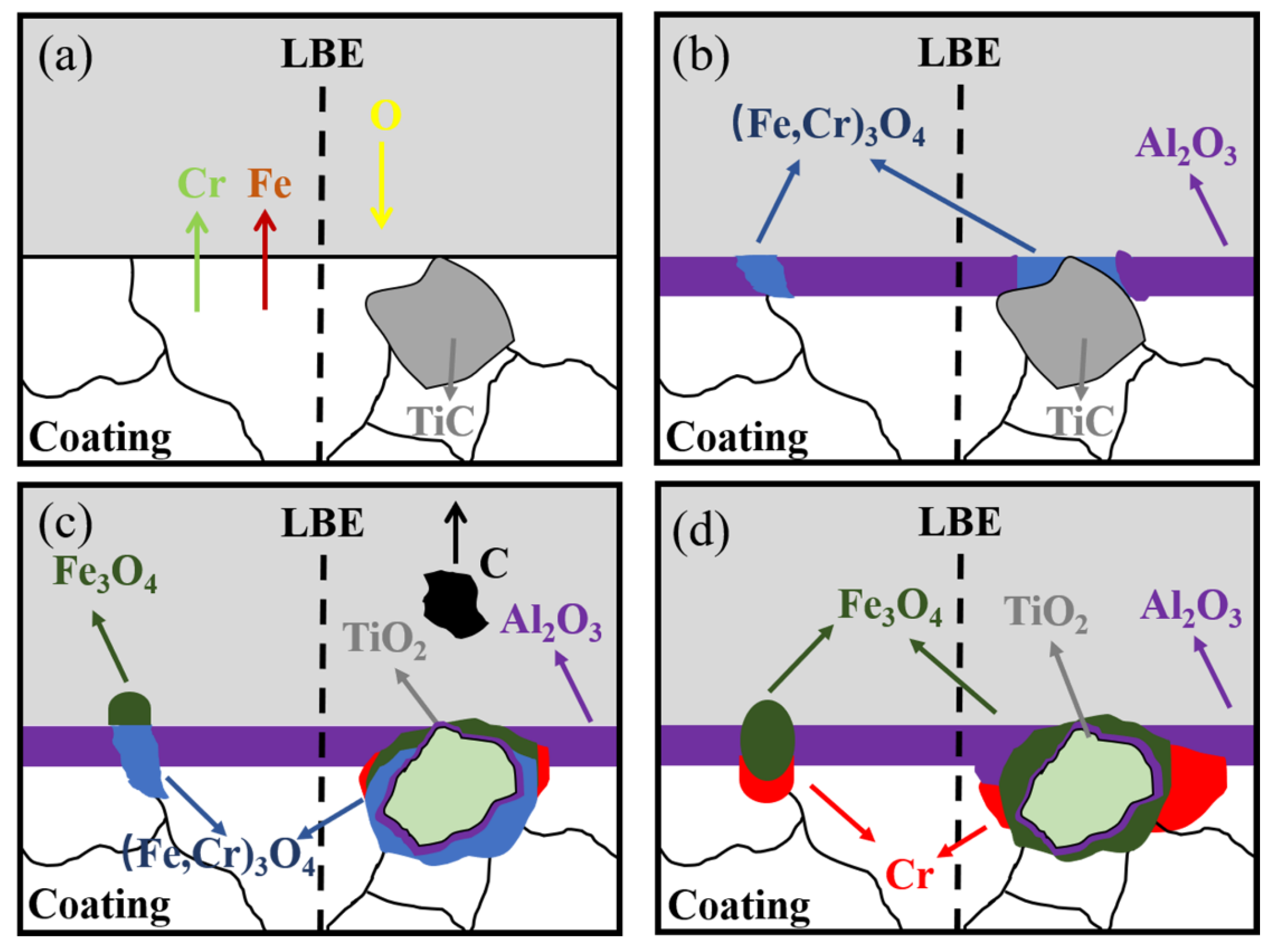
3.4. Corrosion Resistance Mechanism of Coating in Static LBE at 500 °C
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gong, X.; Short, M.P.; Auger, T.; Charalampopoulou, E.; Lambrinou, K. Environmental degradation of structural materials in liquid lead-and lead-bismuth eutectic-cooled reactors. Prog. Mater. Sci. 2022, 126, 100920. [Google Scholar] [CrossRef]
- Tian, S.J. Growth and Exfoliation Behavior of the Oxide scale on 316L and T91 in flowing liquid lead–bismuth eutectic at 480 °C. Oxid. Met. 2020, 93, 183–194. [Google Scholar] [CrossRef]
- Zhang, J. A review of steel corrosion by liquid lead and lead-bismuth. Corros. Sci. 2009, 51, 1207–1227. [Google Scholar] [CrossRef]
- Martinellia, L.; Ginestara, K.; Botton, V.; Delisle, C.; Balbaud-Célériera, F. Corrosion of T91 and pure iron in flowing and static Pb-Bi alloy between 450 °C and 540 °C: Experiments, modelling and mechanism. Corros. Sci. 2020, 176, 108897. [Google Scholar] [CrossRef]
- Gong, X.; Chen, H.; Wang, Z.; Peng, X.; Chen, Z.; Li, Y.; Du, J.; Yu, X.; Yin, Y. Effect of pre-exposure and simulated corrosion “pits” on liquid metal embrittlement of T91 steel exposed to liquid lead-bismuth eutectic at 350 °C. J. Nucl. Mater. 2022, 563, 153641. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, H.; Chang, H.; Sheng, Y.; Shen, T.; Zhu, Y.; Pang, L.; Cui, M.; Wei, K.; Xu, Y.; et al. Structure of surface oxides on martensitic steel under simultaneous ion irradiation and molten LBE corrosion. Corros. Sci. 2022, 195, 109953. [Google Scholar] [CrossRef]
- Gong, X.; Yang, Z.; Deng, Y.; Xiao, J.; Wang, H.; Yu, Z.; Yin, Y. Creep failure of a solution-annealed 15-15Ti steel exposed to stagnant lead-bismuth eutectic at 550 and 600 °C. Mater. Sci. Eng. A 2020, 798, 140230. [Google Scholar] [CrossRef]
- Serre, I.P.; Vogt, J.B. Liquid metal embrittlement sensitivity of the T91 steel in lead, in bismuth and in lead-bismuth eutectic. J. Nucl. Mater. 2020, 531, 152021. [Google Scholar] [CrossRef]
- Heinzel, A.; Müller, G.; Weisenburger, A. Corrosion behaviour of welds and Ta in liquid lead. J. Nucl. Mater. 2016, 469, 62–71. [Google Scholar] [CrossRef]
- Cairang, W.; Ma, S.; Gong, X.; Zeng, Y.; Yang, H.; Xue, D.; Qin, Y.; Ding, X.; Sun, H. Oxidation mechanism of refractory Molybdenum exposed to oxygen-saturated lead-bismuth eutectic at 600 °C. Corros. Sci. 2021, 179, 109132. [Google Scholar] [CrossRef]
- Rivai, A.K.; Takahashi, M. Corrosion investigations of Al-Fe-coated steels, high Cr steels, refractory metals and ceramics in lead alloys at 700 °C. J. Nucl. Mater. 2010, 398, 146–152. [Google Scholar] [CrossRef]
- Li, H.; Bai, P.; Lin, Z.; Zhang, J.; Tang, Q.; Pan, Y. Corrosion resistance in Pb-Bi alloy of 15-15Ti steel coated with Al2O3/SiC bilayer thin films by magnetron sputtering. Fusion Eng. Des. 2017, 125, 384–390. [Google Scholar] [CrossRef]
- Wu, Z.Y.; Zhao, X.; Liu, Y.; Cai, Y.; Li, J.Y.; Chen, H.; Wan, Q.; Yang, D.; Tan, J.; Liu, H.D.; et al. Lead-bismuth eutectic (LBE) corrosion behavior of AlTiN coatings at 550 and 600 °C. J. Nucl. Mater. 2020, 539, 152280. [Google Scholar] [CrossRef]
- Rivai, A.K.; Takahashi, M. Compatibility of surface-coated steels, refractory metals and ceramics to high temperature lead-bismuth eutectic. Prog. Nucl. Energy 2008, 50, 560–566. [Google Scholar] [CrossRef]
- Ferré, F.G.; Mairov, A.; Iadicicco, D.; Vanazzi, M.; Bassini, S.; Utili, M.; Tarantino, M.; Bragaglia, M.; Lamastra, F.R.; Nanni, F.; et al. Corrosion and radiation resistant nanoceramic coatings for lead fast reactors. Corros. Sci. 2017, 124, 80–92. [Google Scholar] [CrossRef]
- Han, J.C. Thermal shock resistance of ceramic coatings. Acta Mater. 2007, 55, 3573–3581. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, T.; Lin, J.; Qiao, L.; Tu, J.; Qin, S.; Cao, J.; Qi, J. Interaction between the third alloying element and the interfacial structure of AgCu-alloy brazed heterogeneous metal integration. J. Alloys Compd. 2021, 883, 160933. [Google Scholar] [CrossRef]
- Yan, Y.; Lin, J.; Liu, T.; Liu, B.; Wang, B.; Qiao, L.; Tu, J.; Cao, J.; Qi, J. Corrosion behavior of stainless steel-tungsten carbide joints brazed with AgCuX (X = In, Ti) alloys. Corros. Sci. 2022, 200, 110231. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Z.; Liu, X.; Wang, H.; Qin, B.; Lin, J.; Cao, J.; Qi, J.; Feng, J. Regulating the interfacial reaction of SC2W3O12/AgCuTi composite filler by introducing a carbon barrier layer. Carbon 2022, 191, 290–300. [Google Scholar] [CrossRef]
- Fetzer, R.; Weisenburger, A.; Jianu, A.; Müller, G. Oxide scale formation of modified FeCrAl coatings exposed to liquid lead. Corros. Sci. 2012, 55, 213–218. [Google Scholar] [CrossRef]
- Lim, J.; Nam, H.O.; Hwang, I.S.; Kim, J.H. A study of early corrosion behaviours of FeCrAl alloys in liquid lead-bismuth eutectic environments. J. Nucl. Mater. 2010, 407, 205–210. [Google Scholar] [CrossRef]
- Weisenburger, A.; Heinzel, A.; Müller, G.; Muscher, H.; Rousanov, A. T91 cladding tubes with and without modified FeCrAlY coatings exposed in LBE at different flow, stress and temperature conditions. J. Nucl. Mater. 2008, 376, 274–281. [Google Scholar] [CrossRef]
- Giacco, M.D.; Weisenburger, A.; Jianu, A.; Lang, F.; Mueller, G. Influence of composition and microstructure on the corrosion behavior of different Fe-Cr-Al alloys in molten LBE. J. Nucl. Mater. 2012, 421, 39–46. [Google Scholar] [CrossRef]
- Hong, S.; Li, J.; Zhao, P.; Yu, Y.; Li, W. Evolution in wear and high-temperature oxidation resistance of laser-clad AlxMoNbTa refractory high-entropy alloys coatings with Al addition content. Coatings 2022, 12, 121. [Google Scholar] [CrossRef]
- Popovic, M.P.; Bolind, A.M.; Aussat, Y.; Gubser, A.J.; Hosemann, P. Oxidative passivation of Fe-Cr-Al steels in lead-bismuth eutectic under oxygen-controlled static conditions at 700 °C and 800 °C. J. Nucl. Mater. 2019, 523, 172–181. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, T.; Li, G. Research on wear and corrosion resistance of Ni60-WC coating fabricated by laser on preheated copper alloy. Coatings 2022, 12, 1537. [Google Scholar] [CrossRef]
- Li, A.; Xu, L.; Lin, N.; He, Y. Effect of WC and Mo2C additions on microstructure and corrosion resistance of Ti(C,N)-based cermet. J. Netshape Form. Eng. 2021, 13, 13–20. [Google Scholar] [CrossRef]
- Yue, K.; Lian, G.; Chen, C.; Feng, M. Effect of powder particle size of laser cladding on the forming control of cladding layer. J. Netshape Form. Eng. 2022, 14, 58–67. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Zhang, Q.; Liang, Y.; Ren, L.; Li, X. Fabrication of Fe-based composite coatings reinforced by TiC particles and its microstructure and wear resistance of 40Cr gear steel by low energy pulsed laser cladding. Opt. Laser Technol. 2019, 119, 105622. [Google Scholar] [CrossRef]
- Mi, H.; Chen, T.; Deng, Z.; Li, S.; Liu, J.; Liu, D. Microstructure and mechanical properties of TiC/TiB composite ceramic coatings in-situ synthesized by ultrasonic vibration-assisted laser cladding. Coatings 2022, 12, 99. [Google Scholar] [CrossRef]
- Yue, H.; Lv, N.; Guo, C.; Zhao, L.; Li, Q.; Zhang, J.; Zhang, Y. Microstructure and mechanical properties of TiC/FeCrSiB coating by laser additive remanufacturing on shearer spiral blade. Surf. Coat. Technol. 2022, 431, 128043. [Google Scholar] [CrossRef]
- Zhu, H.; Geng, S.; Chen, G.; Wang, F. Ni-Mn3O4-CeO2 composite coating on ferritic stainless steel interconnects. Corros. Sci. 2022, 194, 109932. [Google Scholar] [CrossRef]
- Du, M.; Wang, L.; Gao, Z.; Yang, X.; Liu, T.; Zhan, X. Microstructure and element distribution characteristics of Y2O3 modulated WC reinforced coating on Invar alloys by laser cladding. Opt. Laser Technol. 2022, 153, 108205. [Google Scholar] [CrossRef]
- Ma, H.; Miao, Q.; Liang, W.; Liu, Y.; Liu, H.; Ma, H.; Zuo, S.; Xue, L. High temperature oxidation resistance of Y2O3 modified ZrB2-SiC coating for carbon/carbon composites. Ceram. Int. 2021, 47, 6728–6735. [Google Scholar] [CrossRef]
- London, A.J.; Santra, S.; Amirthapandian, S.; Panigrahi, B.K.; Sarguna, R.M.; Balaji, S.; Vijay, R.; Sundar, C.S.; Lozano-Perez, S.; Grovenor, C.R.M. Effect of Ti and Cr on dispersion, structure and composition of oxide nano-particles in model ODS alloys. Acta Mater. 2015, 97, 223–233. [Google Scholar] [CrossRef]
- Ribis, J.; Mouton, I.; Baumier, C.; Gentils, A.; Loyer-Prost, M.; Lunéville, L.; Siméone, D. Nano-structured materials under irradiation: Oxide dispersion-strengthened steels. Nanomaterials 2021, 11, 2590. [Google Scholar] [CrossRef]
- Karak, S.K.; Majumdar, J.D.; Witczak, Z.; Lojkowski, W.; Manna, I. Microstructure and mechanical properties of nano-Y2O3 dispersed ferritic alloys synthesized by mechanical alloying and consolidated by hydrostatic extrusion. Mater. Sci. Eng. A 2013, 580, 231–241. [Google Scholar] [CrossRef]
- Veyan, J.; Obaldia, E.; Alcantar-Peña, J.; Montes-Gutierrez, J.; Arellano-Jimenez, M.; Yacaman, M.; Auciello, O. Argon atoms insertion in diamond: New insights in the identification of carbon C 1s peak in X-ray photoelectron spectroscopy analysis. Carbon 2018, 134, 29–36. [Google Scholar] [CrossRef]
- Zhao, J.; Gao, F.; Pujari, S.; Zuilhof, H.; Teplyakov, A. universal calibration of computationally predicted N 1s binding energies for interpretation of XPS experimental measurements. Langmuir 2017, 33, 10792–10799. [Google Scholar] [CrossRef]
- Bagus, P.; Sousa, C.; Illas, F. XPS binding energy shifts as a function of bond distances: A case study of CO. J. Phys.: Condens. Matter 2022, 34, 154004. [Google Scholar] [CrossRef]
- Greczynski, G.; Hultman, L. X-ray photoelectron spectroscopy: Towards reliable binding energy referencing. Prog. Mater. Sci. 2020, 107, 100591. [Google Scholar] [CrossRef]
- Biesinger, M. Accessing the robustness of adventitious carbon for charge referencing (correction) purposes in XPS analysis: Insights from a multi-user facility data review. Appl. Surf. Sci. 2022, 597, 153681. [Google Scholar] [CrossRef]
- Straumal, B.B.; Korneva, A.; Kuzmin, A.; Lopez, G.A.; Rabkin, E.; Straumal, A.B.; Gerstein, G.; Gornakova, A.S. The grain boundary wetting phenomena in the Ti-containing high-entropy alloys: A review. Metals 2021, 11, 1881. [Google Scholar] [CrossRef]
- Jiang, Y.; Smith, J.R.; Odette, G.R. Prediction of structural, electronic and elastic properties of Y2Ti2O7 and Y2TiO5. Acta Mater. 2010, 58, 1536–1543. [Google Scholar] [CrossRef]
- Gornakova, A.S.; Straumal, A.B.; Khodos, I.I.; Gnesin, I.B.; Mazilkin, A.A.; Afonikova, N.S.; Straumal, B.B. Effect of composition, annealing temperature, and high pressure torsion on structure and hardness of Ti–V and Ti–V–Al alloys. J. Appl. Phys. 2019, 125, 082522. [Google Scholar] [CrossRef]
- Hu, B.; Han, J.; Ge, S.; Hua, X.; Li, S.; Xing, H.; Wang, K.; Hu, P.; Fu, J.; Zhang, W.; et al. Secondary phases strengthening-toughening effects in the Mo-TiC-La2O3 alloys. Mater. Sci. Eng. A 2022, 831, 142271. [Google Scholar] [CrossRef]
- Zhuang, H.; Zhang, Q.; Zhang, D. Microstructure and tribological properties of Ni-based laser-clad coatings by rare earth modification. J. Therm. Spray Technol. 2021, 30, 1410–1431. [Google Scholar] [CrossRef]
- Chen, G.; Ju, N.; Lei, Y.; Wang, D.; Zhu, Q.; Li, T. Corrosion behavior of 410 stainless steel in flowing lead-bismuth eutectic alloy at 550°C. J. Nucl. Mater. 2019, 522, 168–183. [Google Scholar] [CrossRef]
- Wagner, C. Passivity and inhibition during the oxidation of metals at elevated temperature. Corros. Sci. 1965, 5, 751–764. [Google Scholar] [CrossRef]
- Hodge, J.D. Diffusion of chromium in magnetite as a function of oxygen partial pressure. J. Electrochem. Soc. 1978, 125, 55C–57C. [Google Scholar] [CrossRef]
- Cox, M.G.C.; McEnaney, B.; Scott, V.D. Phase interactions in the growth of thin oxide films on iron-chromium alloys. Philos. Mag. 1974, 29, 585–600. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Yan, W.; Sha, W.; Wang, W.; Shan, Y.Y.; Yang, K. Corrosion resistance of self-growing TiC coating on SIMP steel in LBE at 600 °C. Mater. Corros. 2016, 67, 1204–1212. [Google Scholar] [CrossRef]
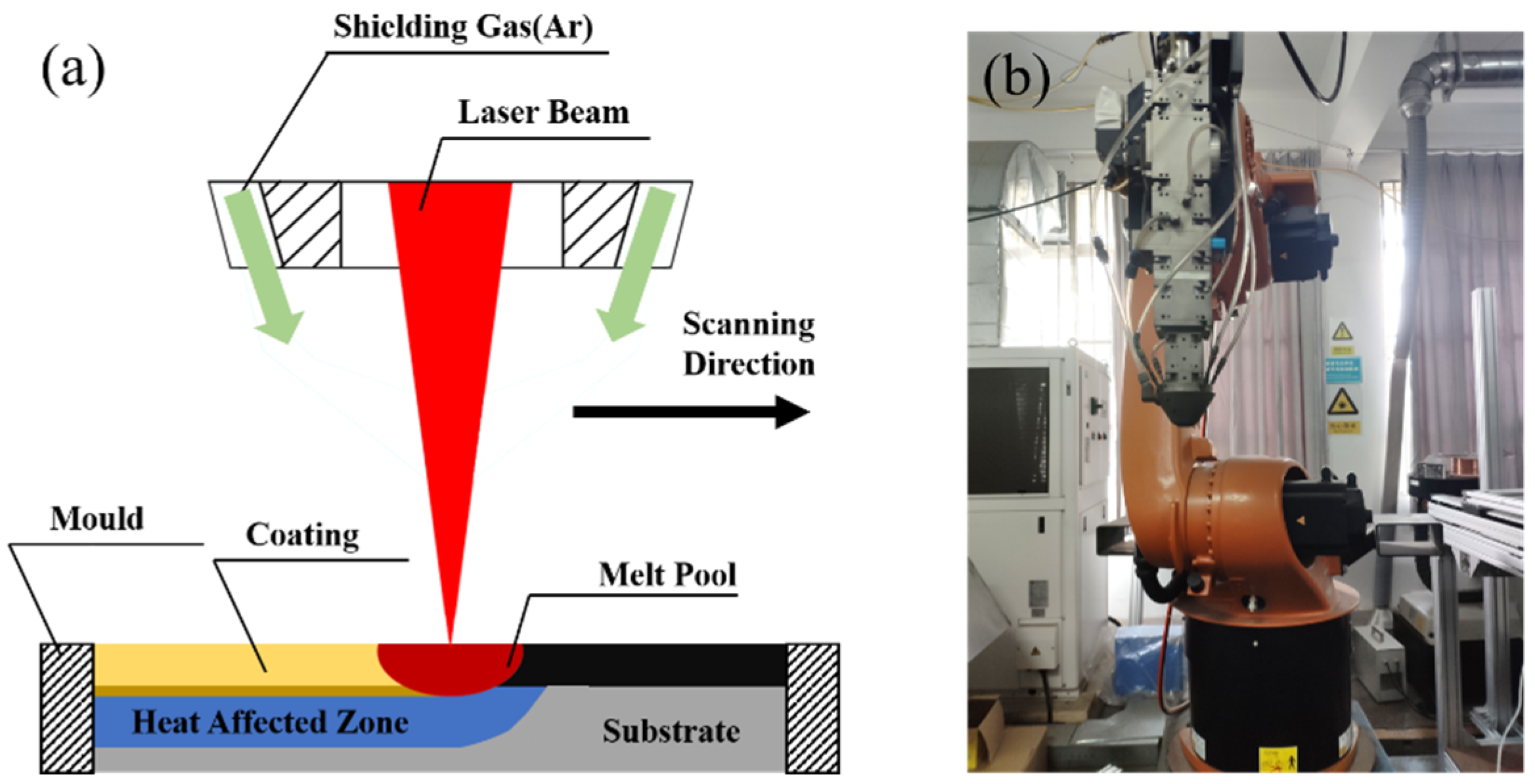

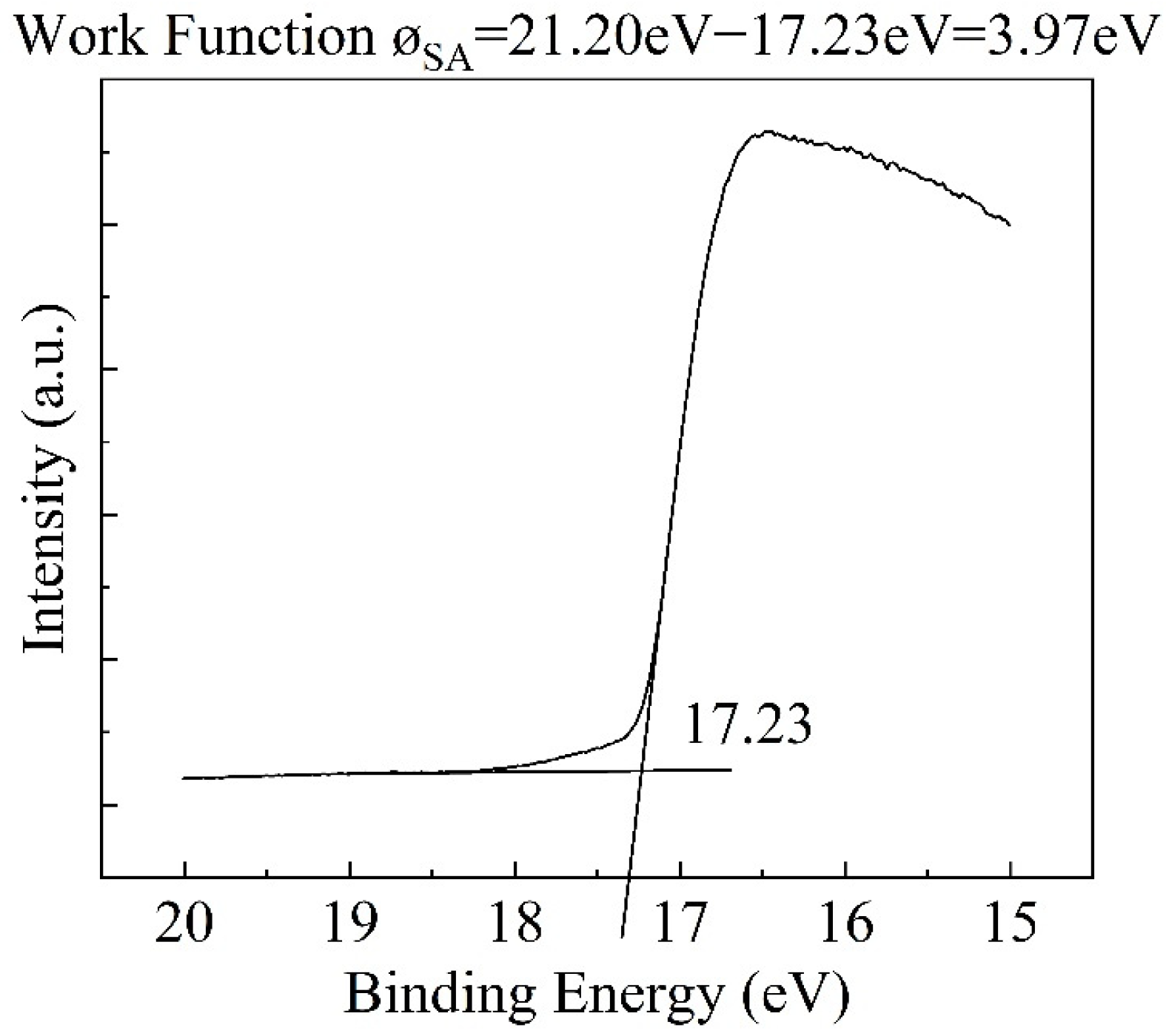
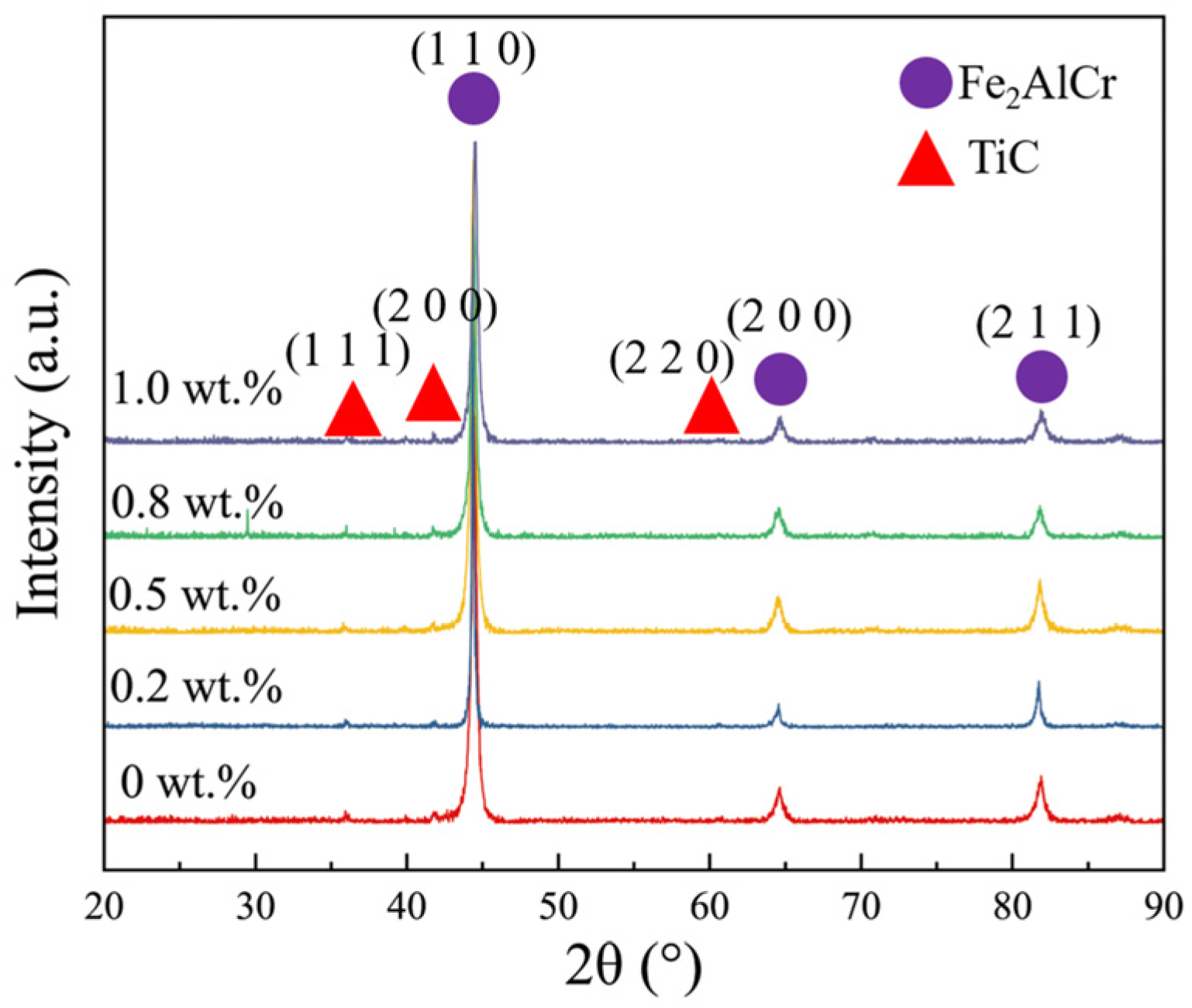
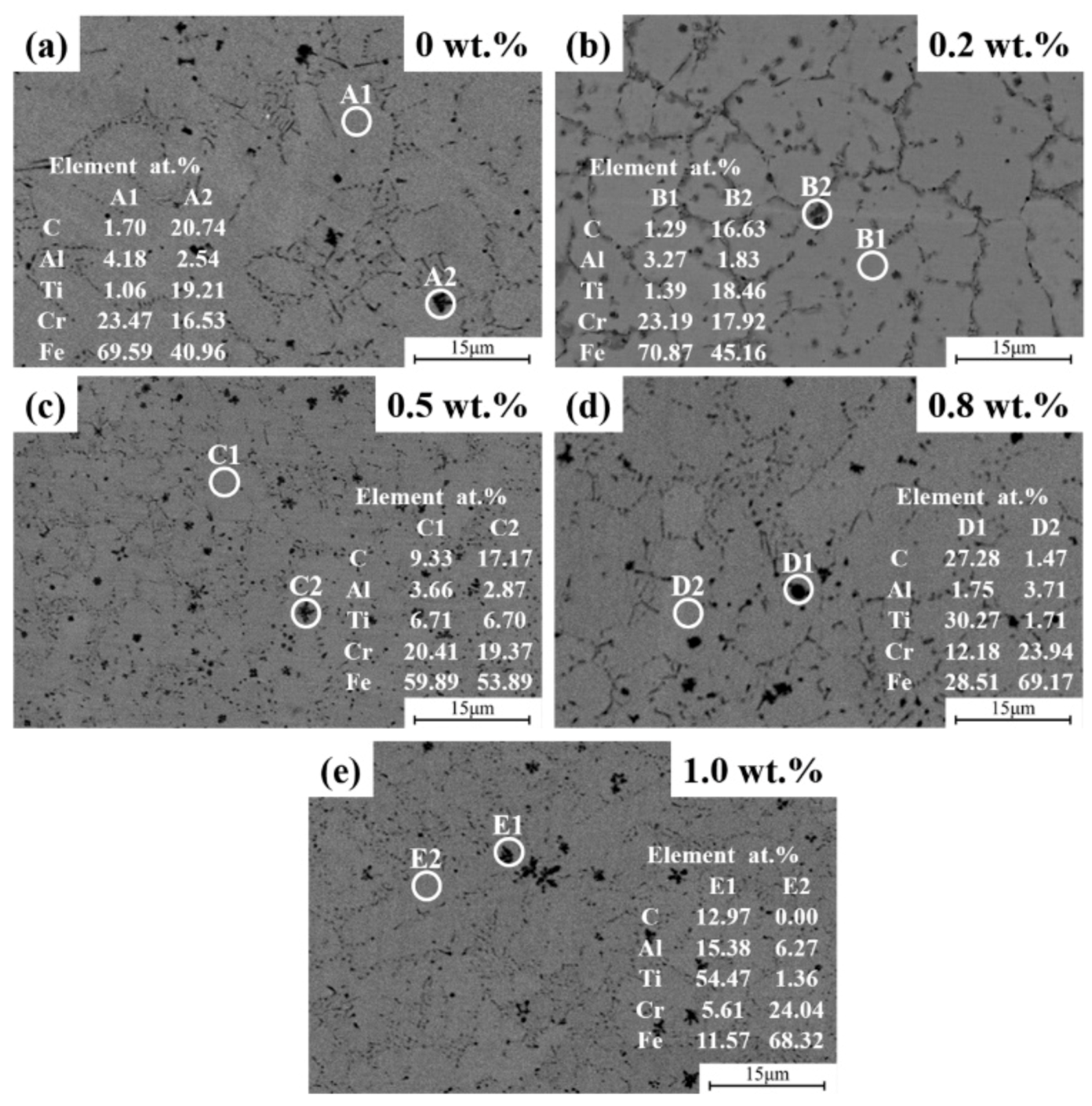

| No. | Cr | Al | Ti | C | Y2O3 | Fe |
|---|---|---|---|---|---|---|
| 1 | 24 | 20 | 20 | 2.8 | 0 | Bal. |
| 2 | 24 | 20 | 20 | 2.8 | 0.2 | Bal. |
| 3 | 24 | 20 | 20 | 2.8 | 0.5 | Bal. |
| 4 | 24 | 20 | 20 | 2.8 | 0.8 | Bal. |
| 5 | 24 | 20 | 20 | 2.8 | 1.0 | Bal. |
| Compound | (kJ/mol) | (J/mol·K) | (kJ/mol) |
|---|---|---|---|
| Al2O3 | −1115.8 | −207.6 | −995.3 |
| TiO2 | −941.0 | −178.6 | −802.9 |
| Cr2O3 | −750.4 | −169.8 | −619.1 |
| Fe3O4 | −547.1 | −153.1 | −428.7 |
| Fe2O3 | −541.1 | −168.7 | −410.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Zhao, X.; Wang, D.; Zhu, Q.; Li, T.; Lei, Y. Effects of Y2O3 Addition on the Microstructure and Static Lead-Bismuth Eutectic Thermal Corrosion Behaviors of FeCrAlTiC-xY2O3 Laser Clade Coatings. Coatings 2022, 12, 1759. https://doi.org/10.3390/coatings12111759
Jiang H, Zhao X, Wang D, Zhu Q, Li T, Lei Y. Effects of Y2O3 Addition on the Microstructure and Static Lead-Bismuth Eutectic Thermal Corrosion Behaviors of FeCrAlTiC-xY2O3 Laser Clade Coatings. Coatings. 2022; 12(11):1759. https://doi.org/10.3390/coatings12111759
Chicago/Turabian StyleJiang, Hongyi, Xinyi Zhao, Dan Wang, Qiang Zhu, Tianqing Li, and Yucheng Lei. 2022. "Effects of Y2O3 Addition on the Microstructure and Static Lead-Bismuth Eutectic Thermal Corrosion Behaviors of FeCrAlTiC-xY2O3 Laser Clade Coatings" Coatings 12, no. 11: 1759. https://doi.org/10.3390/coatings12111759
APA StyleJiang, H., Zhao, X., Wang, D., Zhu, Q., Li, T., & Lei, Y. (2022). Effects of Y2O3 Addition on the Microstructure and Static Lead-Bismuth Eutectic Thermal Corrosion Behaviors of FeCrAlTiC-xY2O3 Laser Clade Coatings. Coatings, 12(11), 1759. https://doi.org/10.3390/coatings12111759







