Electrodeposition and Characterization of Poly 3-Amino-1,2,4-Triazole-5-Thiol Films on Brass Electrode in 0.1 M Methanol
Abstract
:1. Introduction
2. Experiment
2.1. Tested Monomer
2.2. Electropolymerization Electrolyte and Methods
- Chronoamperometry: Polarization of a constant potential.
- Cyclic voltammetry: Cyclic scanning of the potential.
2.3. Brass Samples
2.4. Electrochemical Cell
2.5. Electrochemical Techniques
2.6. Energy Dispersion of X-ray
2.7. Theoretical Calculation
3. Results and Discussion
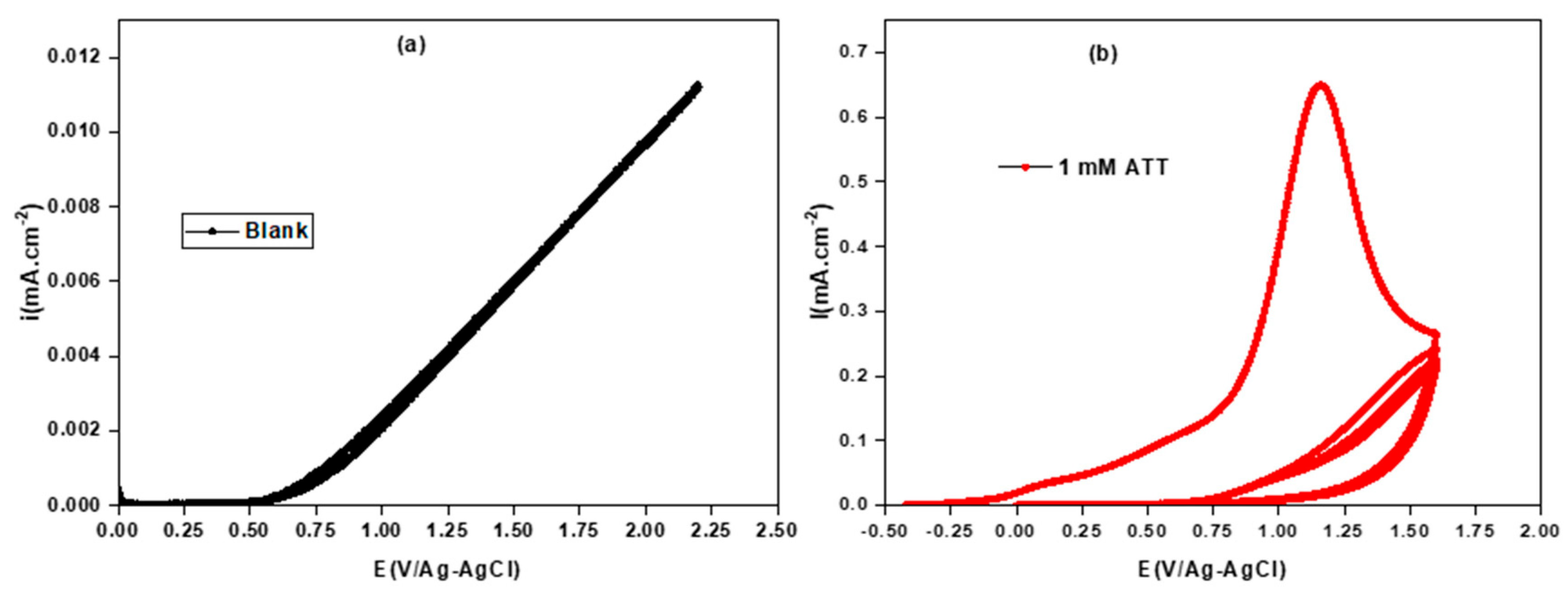
3.1. Oxidation of 3-Amino-1,2,4-triazole-5-thiol (ATT)
3.1.1. Poly-ATT Prepared by Cyclic Voltammetry
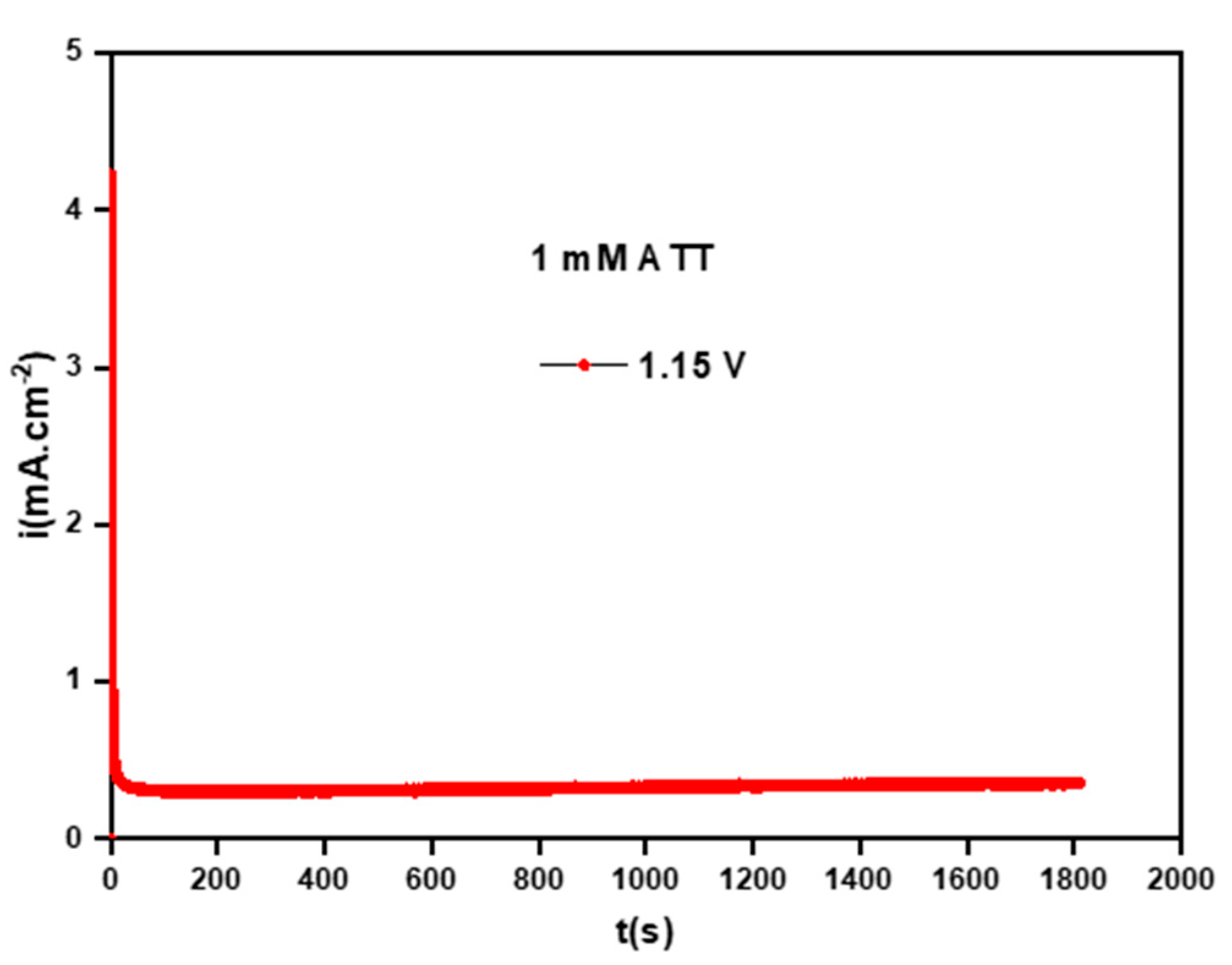
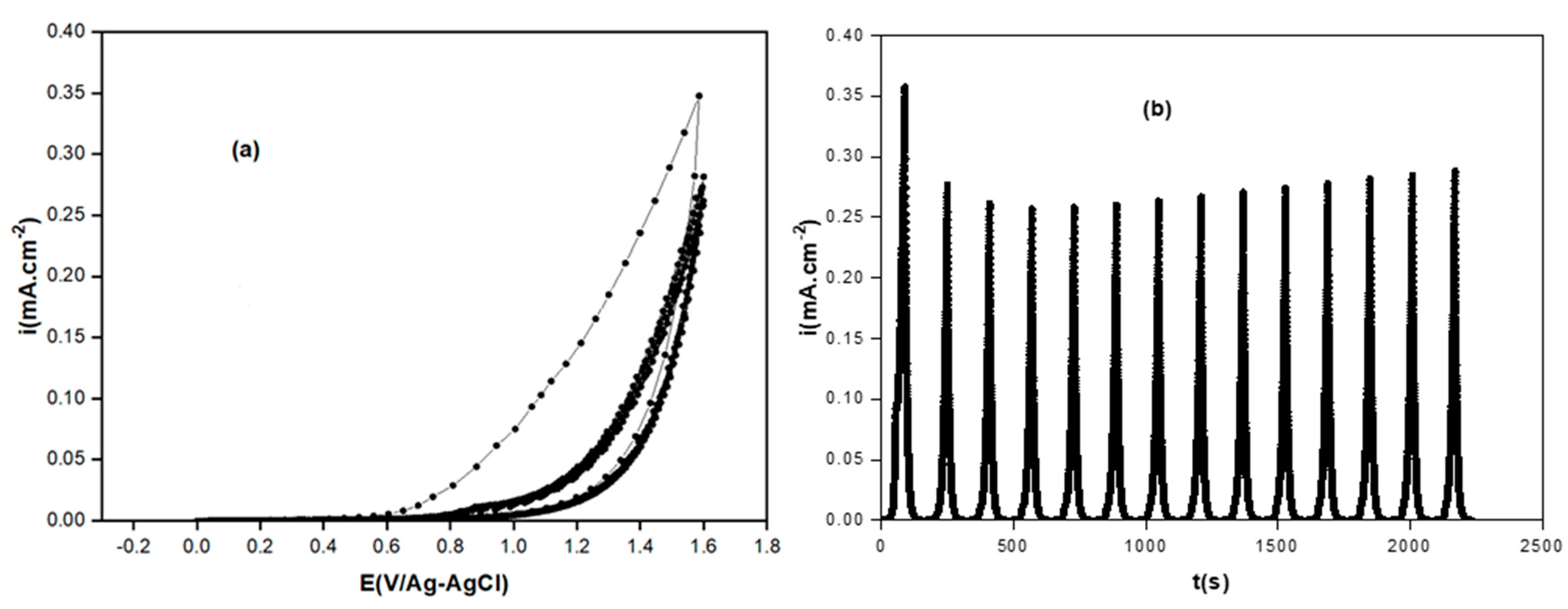
3.1.2. Poly-ATT Prepared by Chronoamperometry
3.2. Effect of Different Experimental Parameters
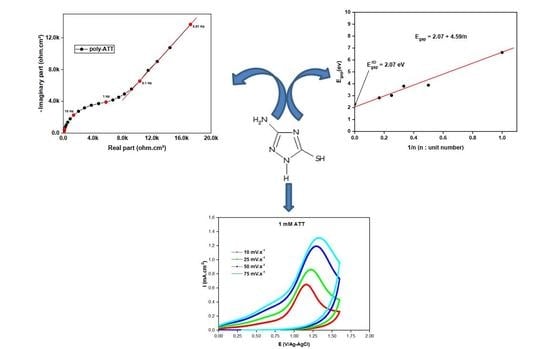
3.2.1. Effect of the Applied Potential
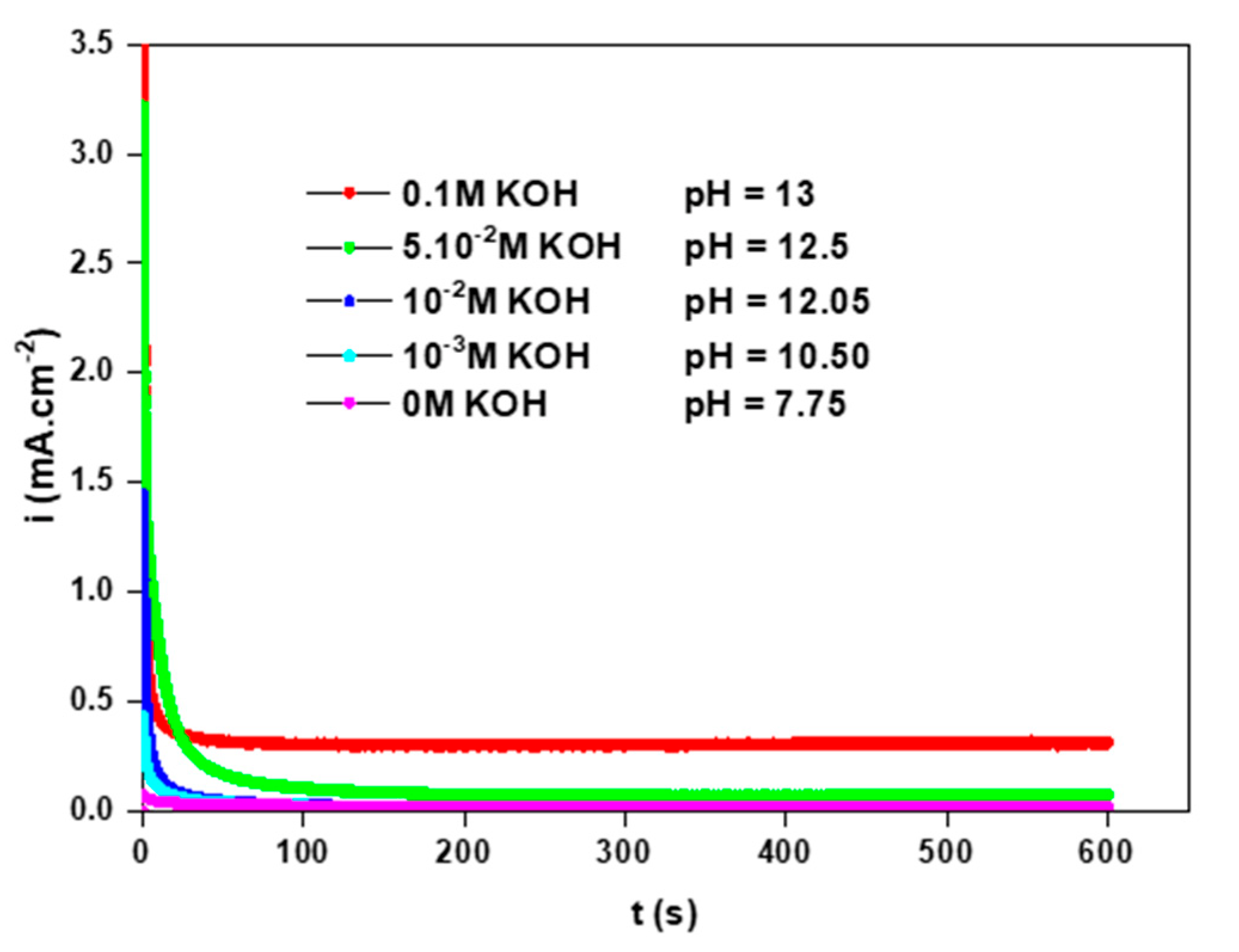
3.2.2. Effect of the pH of the Electrolyte Solution
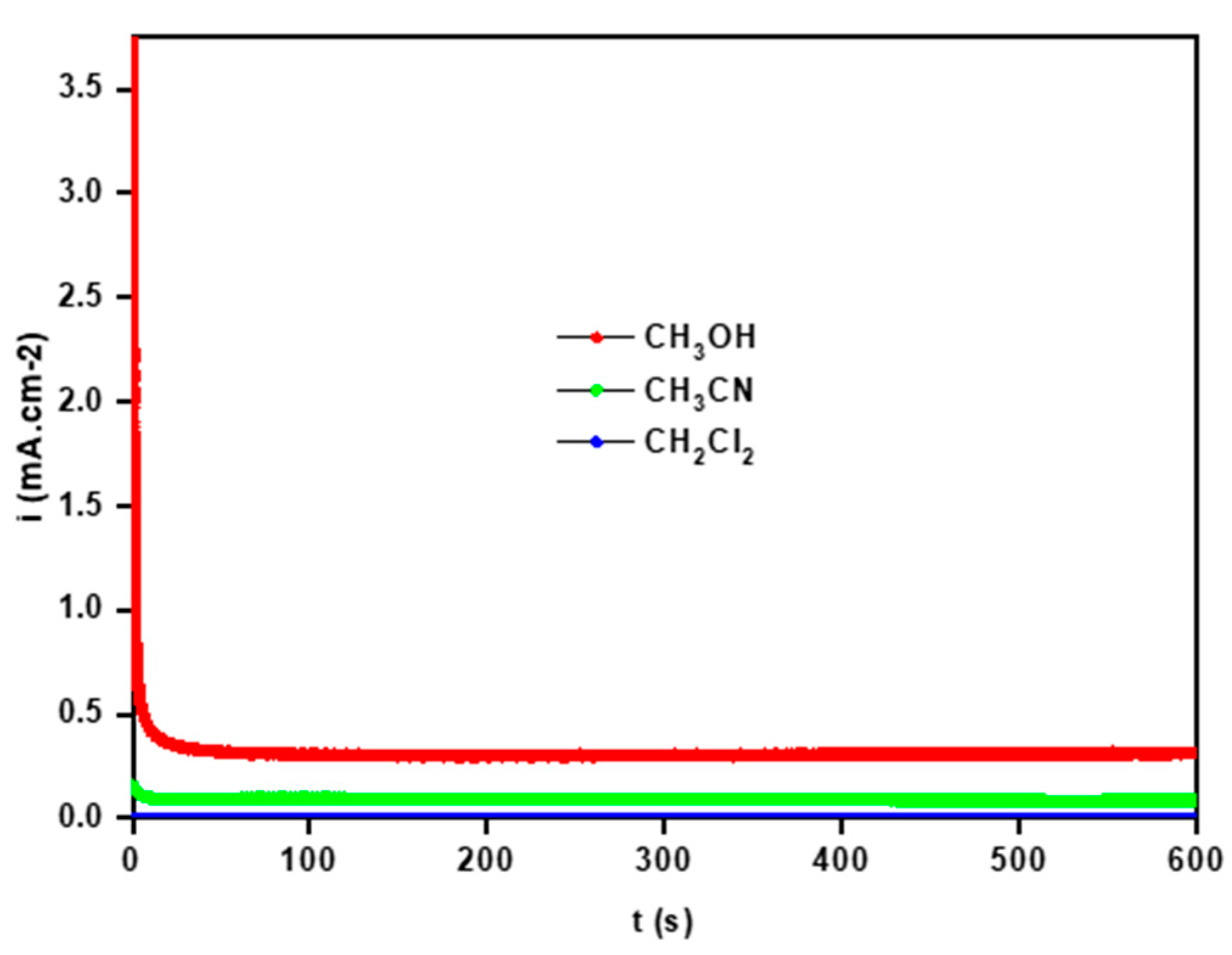
3.2.3. Solvent Effect
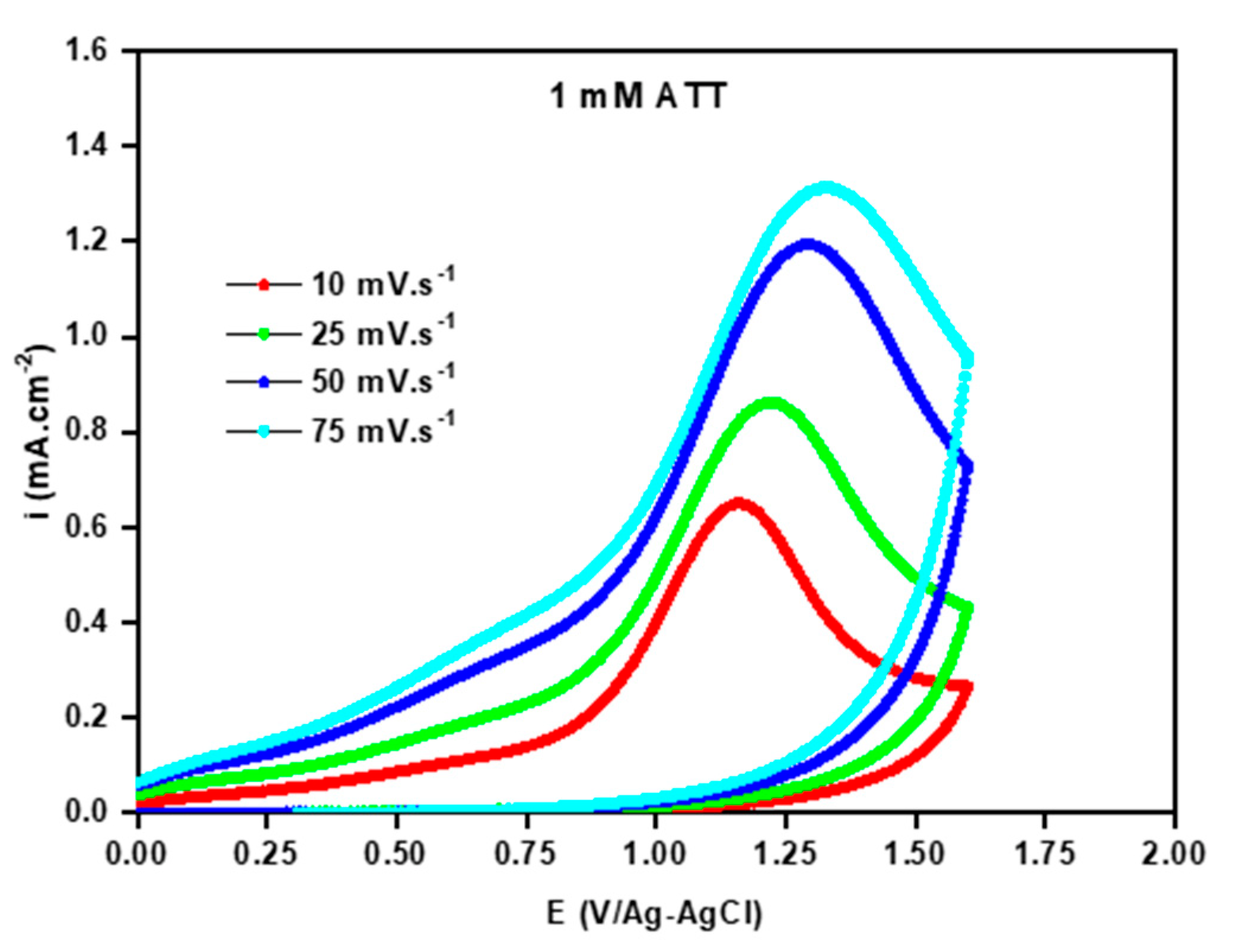
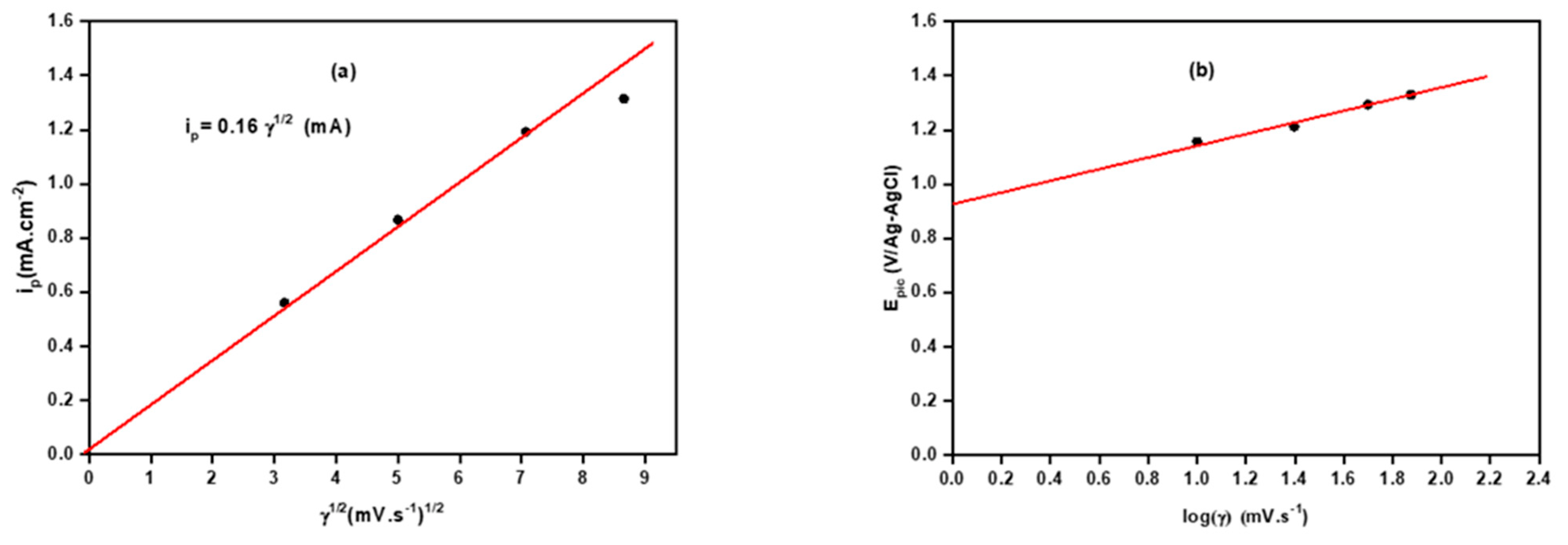
3.3. Kinetic Study of Electropolymerization of Poly-ATT
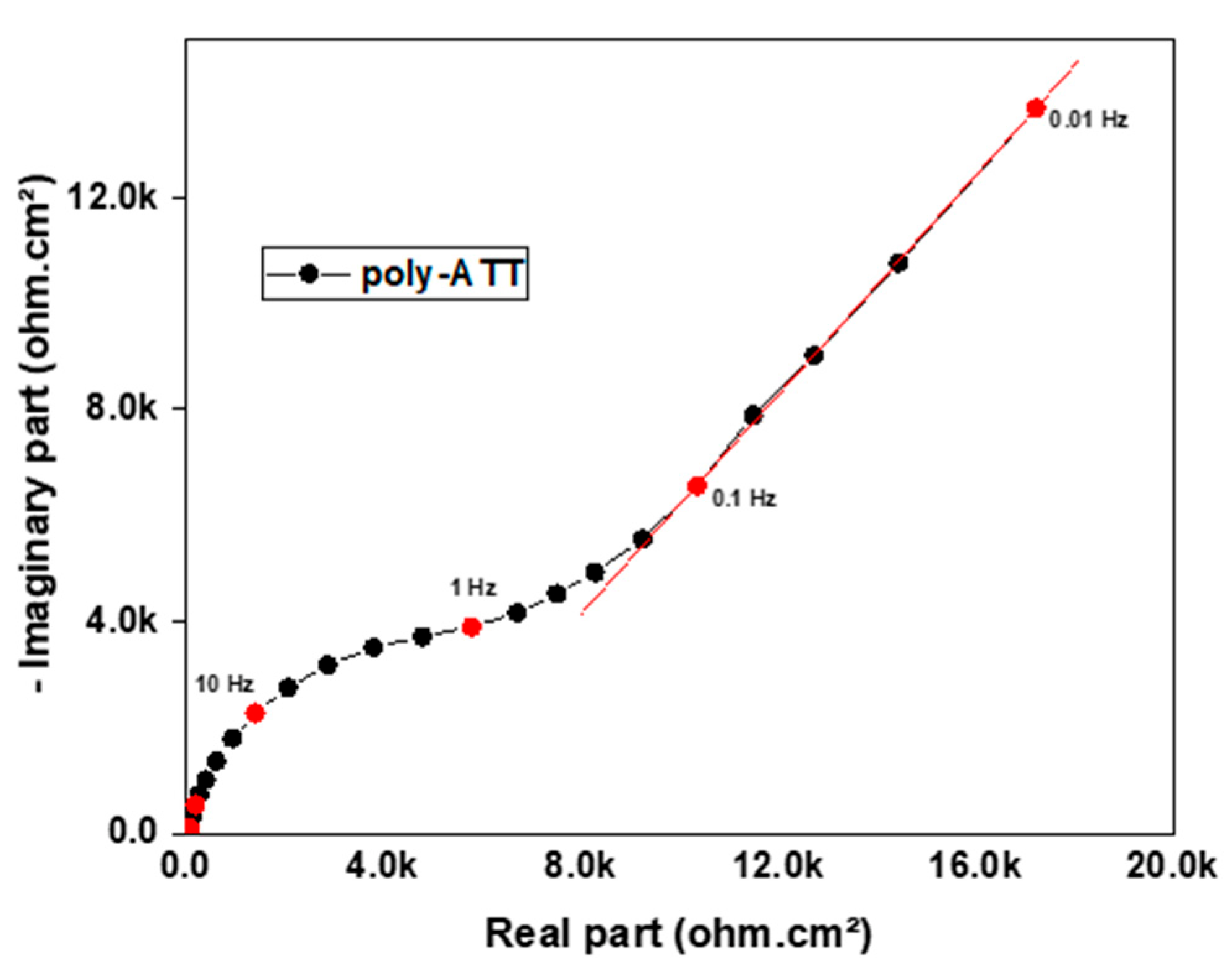
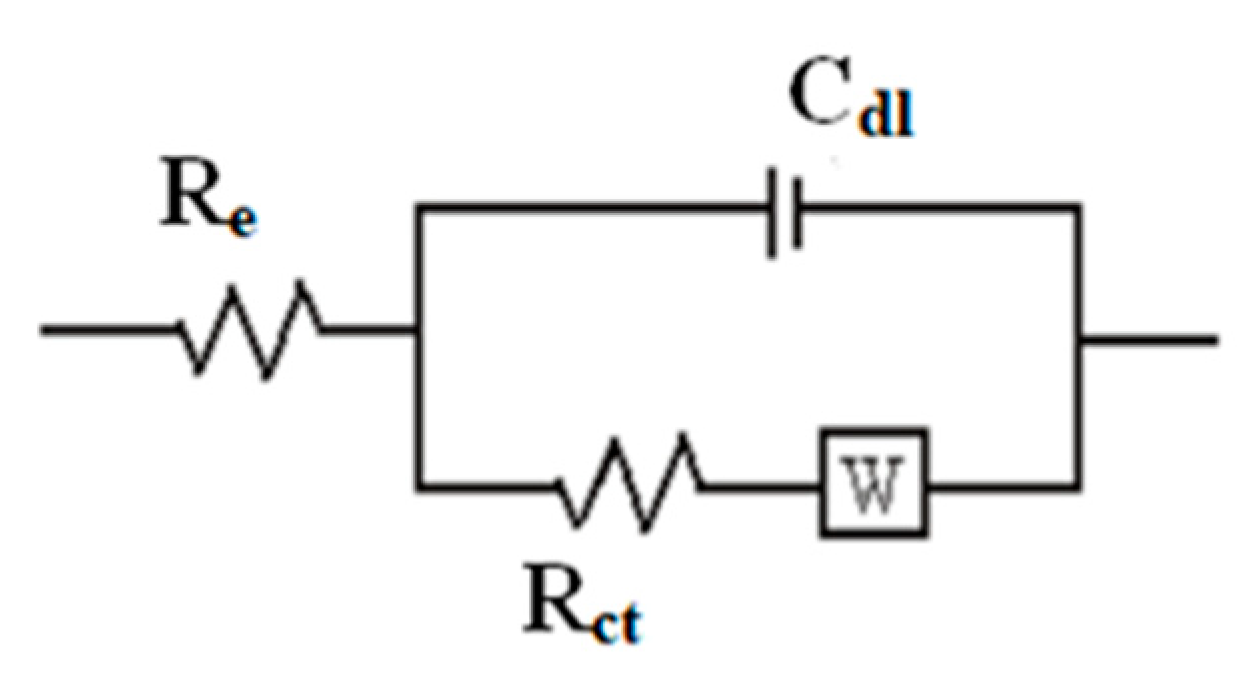
3.4. Electrochemical Impedance Spectroscopy
3.5. Surface Analysis by EDX
3.6. Theoretical Study
4. Conclusions
- -
- The cyclic voltammetry recordings show that the oxidation of the monomer exhibits a single oxidation peak corresponding to the oxidation of ATT at 1.15 V
- -
- The voltammograms I = f(E) and I = f(t) enabled us to study the properties of the polymeric film as well as the optimal conditions of its formation (effect of applied potential, the pH of electrolytic solution, and solvent nature).
- -
- Electrochemical impedance measurements made it possible to highlight a charge transfer process followed by another purely diffusional one at low frequencies, showing that the synthesized polymer film is a semiconductor.
- -
- Surface analysis by EDX indicates the presence of an organic molecule in the form of a layer covering the surface of the brass electrode
- -
- The gap energy corresponding to poly-ATT is equal to 2.07 eV; this value shows that the polymer film synthesized is a semiconductor in nature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bouayadi, H.; Damej, M.; Molhi, A.; Lakbaibi, Z. Electrochemical and theoretical evaluation of thiocarbohydrazide as a brass (60/40) corrosion inhibitor in 3% NaCl solution and effect of temperature on this process. Int. J. Corros. Scale Inhib. 2022, 11, 1335–1354. [Google Scholar] [CrossRef]
- Rochdi, A.; Kassou, O.; Dkhireche, N.; Touir, R.; El Bakri, M.; Touhami, M.E.; Sfaira, M.; Mernari, B.; Hammouti, B. Inhibitive properties of 2,5-bis(n-methylphenyl)-1,3,4-oxadiazole and biocide on corrosion, biocorrosion and scaling controls of brass in simulated cooling water. Corros. Sci. 2014, 80, 442–452. [Google Scholar] [CrossRef]
- Gambirasi, A.; Cattarin, S.; Musiani, M.; Vázquez-Gómez, L.; Verlato, E. Electrochimica Acta Direct. Electrochim. Acta 2011, 56, 8582–8588. [Google Scholar] [CrossRef]
- Kalimuthu, P.; John, S.A. Nanostructured electropolymerized film of 5-amino-2-mercapto-1,3,4-thiadiazole on glassy carbon electrode for the selective determination of l-cysteine. Electrochem. Commun. 2009, 11, 367–370. [Google Scholar] [CrossRef]
- Kalimuthu, P.; John, S.A. Modification of electrodes with nanostructured functionalized thiadiazole polymer film and its application to the determination of ascorbic acid. Electrochim. Acta 2009, 55, 183–189. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Z.; Wan, M. Formation Mechanism of Self-Assembled Polyaniline Micro/Nanotubes. Langmuir 2002, 18, 917–921. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline Nanofibers: Facile Synthesis and Chemical Sensors. J. Am. Chem. Soc. 2002, 125, 314–315. [Google Scholar] [CrossRef]
- An, K.H.; Jeong, S.Y.; Hwang, H.R.; Lee, Y.H. Enhanced sensitivity of a gas sensor incorporating single-walled carbon nanotube–polypyrrole nanocomposites. Adv. Mater. 2004, 16, 1005–1009. [Google Scholar] [CrossRef]
- Nazari, M.; Agbolaghi, S.; Gheybi, H.; Abbaspoor, S.; Abbasi, F. A focus on the features of polyaniline nanofibres prepared via developing the single crystals of their block copolymers with poly (ethylene glycol). Bull. Mater. Sci. 2018, 41, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Chebabe, D.; Damej, M.; Dermaj, A.; Oubair, A.; Benassaoui, H.; Erramli, H.; Hajjaji, N.; Srhiri, A. Corrosion Inhibition of Brass in 3% NaCl Solution by Electrosynthesized Poly 4-amino-3-méthyl-1, 2, 4-triazole-5-thione. Anal. Bioanal. Chem. Res. 2020, 7, 389–401. [Google Scholar]
- Trachli, B.; Keddam, M.; Takenouti, H.; Srhiri, A. Protective effect of electropolymerized 2-mercaptobenzimidazole upon copper corrosion. Prog. Org. Coat. 2002, 44, 17–23. [Google Scholar] [CrossRef]
- Elbakri, M.; Touir, R.; Touhami, M.E.; Srhiri, A.; Benmessaoud, M. Electrosynthesis of adherent poly(3-amino-1,2,4-triazole) films on brass prepared in nonaqueous solvents. Corros. Sci. 2008, 50, 1538–1545. [Google Scholar] [CrossRef]
- Maynor, B.W.; Filocamo, S.F.; Grinstaff, M.W.; Liu, J.J. Direct-Writing of Polymer Nanostructures: Poly(thiophene) Nanowires on Semiconducting and Insulating Surfaces. J. Am. Chem. Soc. 2001, 124, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, S.; Singha, N.; Khastgir, D. Effect of aromatic substitution in aniline on the properties of polyaniline. Eur. Polym. J. 2008, 44, 1763–1770. [Google Scholar] [CrossRef]
- Alvarez, S.; Manolache, S.; Denes, F. Synthesis of polyaniline using horseradish peroxidase immobilized on plasma-functionalized polyethylene surfaces as initiator. J. Appl. Polym. Sci. 2003, 88, 369–379. [Google Scholar] [CrossRef]
- Chou, C.; Yang, C.; Hsu, C.; Chen, T. Hybrid white-light emitting-LED based on luminescent polyfluorene polymer and quantum dots. J. Nanosci. Nanotechnol. 2007, 7, 2785–2789. [Google Scholar] [CrossRef] [PubMed]
- Damej, M.; Chebabe, D.; Benmessaoud, M.; Dermaj, A.; Erramli, H.; Hajjaji, N.; Srhiri, A. Corrosion inhibition of brass in 3% NaCl solution by 3-methyl-1,2,4-triazol-5-thione. Corros. Eng. Sci. Technol. 2014, 50, 103–107. [Google Scholar] [CrossRef]
- Damej, M.; Benassaoui, H.; Chebabe, D.; Benmessaoud, M.; Erramli, H.; Dermaj, A.; Hajjaji, N.; Srhiri, A. Inhibition effect of 1,2,4-triazole-5-thione derivative on the corrosion of brass in 3% NaCl solution. J. Mater. Environ. Sci. 2016, 7, 738–745. [Google Scholar]
- Damej, M.; Chebabe, D.; Abbout, S.; Erramli, H.; Oubair, A.; Hajjaji, N. Corrosion inhibition of brass 60Cu–40Zn in 3% NaCl solution by 3-amino-1, 2, 4-triazole-5-thiol. Heliyon 2020, 6, e04026. [Google Scholar] [CrossRef]
- Chebabe, D.; Abdeddine, I.; Bariki, S.; Oubair, A.; Abbout, S.; Damej, M.; Makayssi, A. The Effect of the Chemical Structure of Benzopyridine and Benzimidazole on their Corrosion Inhibiting Action of Ordinary Steel in Acidic Medium. Anal. Bioanal. Electrochem. 2019, 11, 1701–1715. [Google Scholar]
- Damej, M.; Molhi, A.; Tassaoui, K.; El Ibrahimi, B.; Akounach, Z.; Addi, A.A.; El Hajjaji, S.; Benmessaoud, M. Experimental and Theoretical Study to Understand the Adsorption Process of p-Anisidine and 4-Nitroaniline for the Dissolution of C38 Carbon Steel in 1M HCl. ChemistrySelect 2022, 7, e202103192. [Google Scholar] [CrossRef]
- Damej, M.; Hsissou, R.; Berisha, A.; Azgaou, K.; Sadiku, M.; Benmessaoud, M.; Labjar, N.; El Hajjaji, S. New epoxy resin as a corrosion inhibitor for the protection of carbon steel C38 in 1M HCl. Experimental and theoretical studies (DFT, MC, and MD). J. Mol. Struct. 2022, 1254, 132425. [Google Scholar] [CrossRef]
- Abbout, S.; Zouarhi, M.; Chebabe, D.; Damej, M.; Berisha, A.; Hajjaji, N. Galactomannan as a new bio-sourced corrosion inhibitor for iron in acidic media. Heliyon 2020, 6, e03574. [Google Scholar] [CrossRef]
- Chafiq, M.; Chaouiki, A.; Damej, M.; Lgaz, H.; Salghi, R.; Ali, I.H.; Benmessaoud, M.; Masroor, S.; Chung, I.M. Bolaamphiphile-class surfactants as corrosion inhibitor model compounds against acid corrosion of mild steel. J. Mol. Liq. 2020, 309, 113070. [Google Scholar] [CrossRef]
- Molhi, A.; Hsissou, R.; Damej, M.; Berisha, A.; Thaçi, V.; Belafhaili, A.; Benmessaoud, M.; Labjar, N.; El Hajjaji, S. Contribution to the corrosion inhibition of C38 steel in 1 M hydrochloric acid medium by a new epoxy resin PGEPPP. Int. J. Corros. Scale Inhib. 2021, 10, 399–418. [Google Scholar] [CrossRef]
- Molhi, A.; Hsissou, R.; Damej, M.; Berisha, A.; Bamaarouf, M.; Seydou, M.; Benmessaoud, M.; El Hajjaji, S. Performance of two epoxy compounds against corrosion of C38 steel in 1 M HCl: Electrochemical, thermodynamic and theoretical assessment. Int. J. Corros. Scale Inhib. 2021, 10, 812–837. [Google Scholar] [CrossRef]
- Benassaoui, H.; Damej, M.; Benassaoui, E.; Dermaj, A.; Erramli, H.; Chebabe, D.; Hajjaji, N.; Srhiri, A. Electrosynthesis and Characterization of Adherent Poly (4-Amino-3 Methyl-1,2,4-Triazole-5-Thione) Films on B66 Bronze Electrode in Methanol. Port. Electrochim. Acta 2020, 38, 299–312. [Google Scholar] [CrossRef]
- Aghazadeh, M. Cathodic electrochemical deposition of nanostructured metal oxides/hydroxides and their composites for supercapacitor applications: A review. Anal. Bioanal. Electrochem. 2019, 11, 211–266. [Google Scholar]
- Kiya, Y.; Henderson, J.C.; Abruña, H.D. 4-Amino-4H-1,2,4-triazole-3,5-dithiol. J. Electrochem. Soc. 2007, 154, A844. [Google Scholar] [CrossRef]
- Arbizzani, C.; Mastragostino, M.; Meneghello, L. Polymer-based redox supercapacitors: A comparative study. Electrochim. Acta 1996, 41, 21–26. [Google Scholar] [CrossRef]
- Vorotyntsev, M.A.; Badiali, J.P.; Inzelt, G. Electrochemical impedance spectroscopy of thin films with two mobile charge carriers: Effects of the interfacial charging. J. Electroanal. Chem. 1999, 472, 7–19. [Google Scholar] [CrossRef]
- Garcia-Belmonte, G.; Fabregat-Santiago, F.; Bisquert, J.; Yamashita, M.; Pereira, E.C.; Castro-Garcia, S. Frequency dispersion in electrochromic devices and conducting polymer electrodes: A generalized transmission line approach. Ionics 1999, 5, 44–51. [Google Scholar] [CrossRef]
- Ayachi, S.; Ghomrasni, S.; Bouachrine, M.; Hamidi, M.; Alimi, K. Structure–property relationships of soluble poly(2,5-dibutoxyethoxy-1,4-phenylene-alt-2,5-thienylene) (PBuPT) for organic-optoelectronic devices. J. Mol. Struct. 2013, 1036, 7–18. [Google Scholar] [CrossRef]




| Synthesis of Poly-ATT | |||
|---|---|---|---|
| E (V/Ag-AgCl) | i0 (mA/cm−2) | if (mA/cm−2) | Q (mC) |
| 0.70 | 3.24 | 0.040 | 57.08 |
| 0.80 | 3.30 | 0.054 | 98.39 |
| 0.90 | 4.50 | 0.065 | 164.58 |
| 1.00 | 1.00 | 0.300 | 500.66 |
| 1.10 | 1.2 | 0.580 | 624.25 |
| 1.20 | 1.5 | 0.300 | 463.78 |
| 1.30 | 5.60 | 0.110 | 162.29 |
| Scanning Speed γ (mV·s−1) | Epic (V/Ag-AgCl) | |
|---|---|---|
| 10 | 0.656 | 1.156 |
| 25 | 0.867 | 1.213 |
| 50 | 1.192 | 1.293 |
| 75 | 1.314 | 1.329 |
| Rct (kΩ·cm2) | Cdl (µF·cm−2) | N | |
|---|---|---|---|
| Poly-ATT | 10.2 | 31.2 | 0.5 |
| Unit Number | ELumo (eV) | EHomo (eV) | Egap (eV) |
|---|---|---|---|
| 1 | 3.2025 | −3.0084 | 6.2109 |
| 2 | −4.2169 | −6.7732 | 2.5563 |
| 3 | −3.5010 | −6.5998 | 3.0988 |
| 4 | −3.8468 | −6.6790 | 2.8322 |
| 5 | −4.4403 | −6.8708 | 2.4305 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damej, M.; Abouchane, M.; Doubi, M.; Erramli, H.; Benmessaoud, M.; Hajjaji, N. Electrodeposition and Characterization of Poly 3-Amino-1,2,4-Triazole-5-Thiol Films on Brass Electrode in 0.1 M Methanol. Coatings 2022, 12, 1784. https://doi.org/10.3390/coatings12111784
Damej M, Abouchane M, Doubi M, Erramli H, Benmessaoud M, Hajjaji N. Electrodeposition and Characterization of Poly 3-Amino-1,2,4-Triazole-5-Thiol Films on Brass Electrode in 0.1 M Methanol. Coatings. 2022; 12(11):1784. https://doi.org/10.3390/coatings12111784
Chicago/Turabian StyleDamej, Mohamed, Mohammed Abouchane, Mostafa Doubi, Hamid Erramli, Mohammed Benmessaoud, and Najat Hajjaji. 2022. "Electrodeposition and Characterization of Poly 3-Amino-1,2,4-Triazole-5-Thiol Films on Brass Electrode in 0.1 M Methanol" Coatings 12, no. 11: 1784. https://doi.org/10.3390/coatings12111784